Multimodal Investigation of Deep Gray Matter Nucleus in Patients with Multiple Sclerosis and Their Clinical Correlations: A Multivariate Pattern Analysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Procedures
2.1.1. Sample Collection
2.1.2. Clinical Neurological Evaluation
2.1.3. Research Design
2.2. MRI Protocols
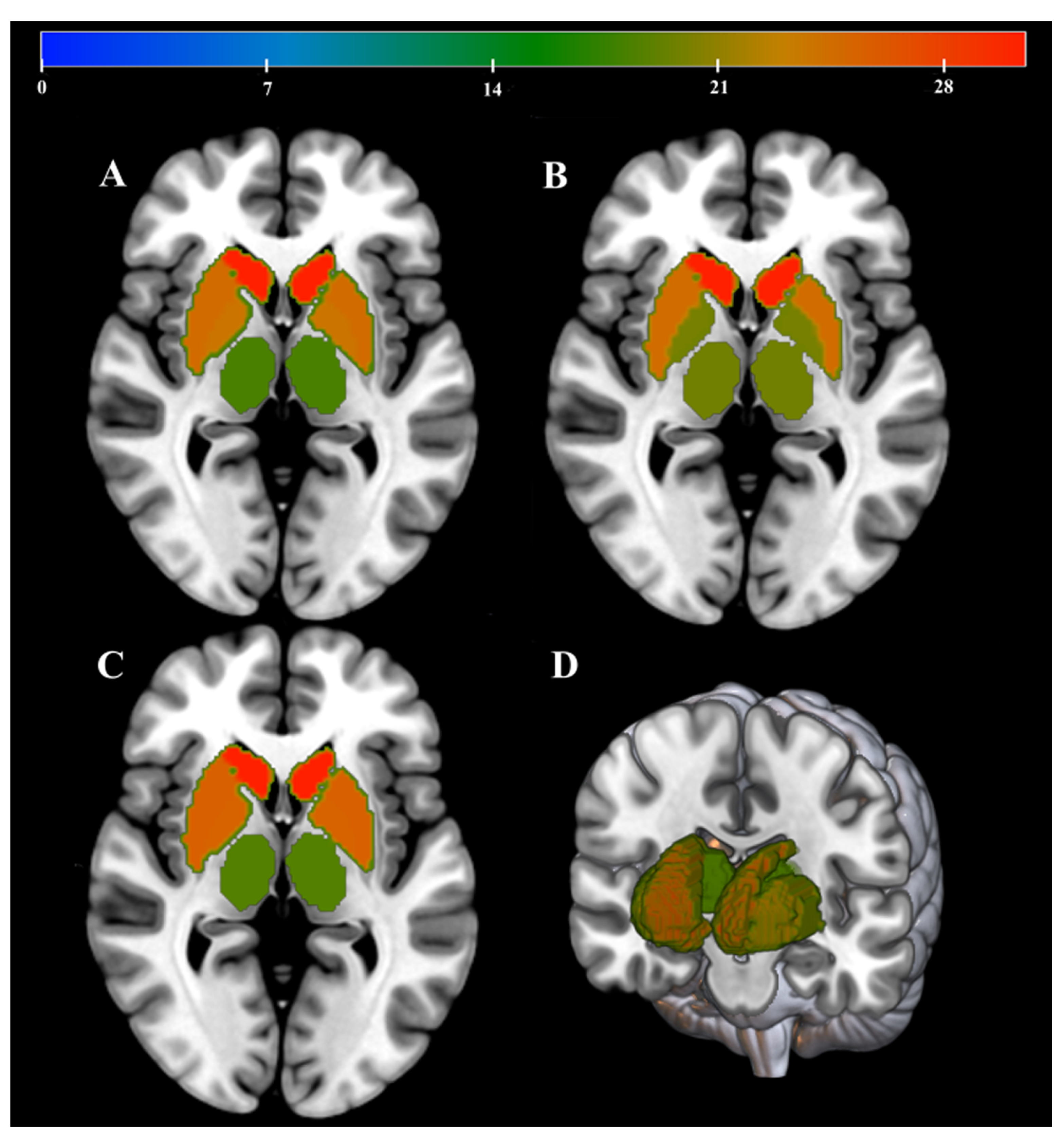
2.3. MRI Image Processing
2.3.1. Volumetric Measurements
2.3.2. QSM Reconstruction
2.3.3. DTI Constructions
2.3.4. Multivariate Pattern Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Neurological Scores of MS Patients and HC Groups in Study 1 and Study 2
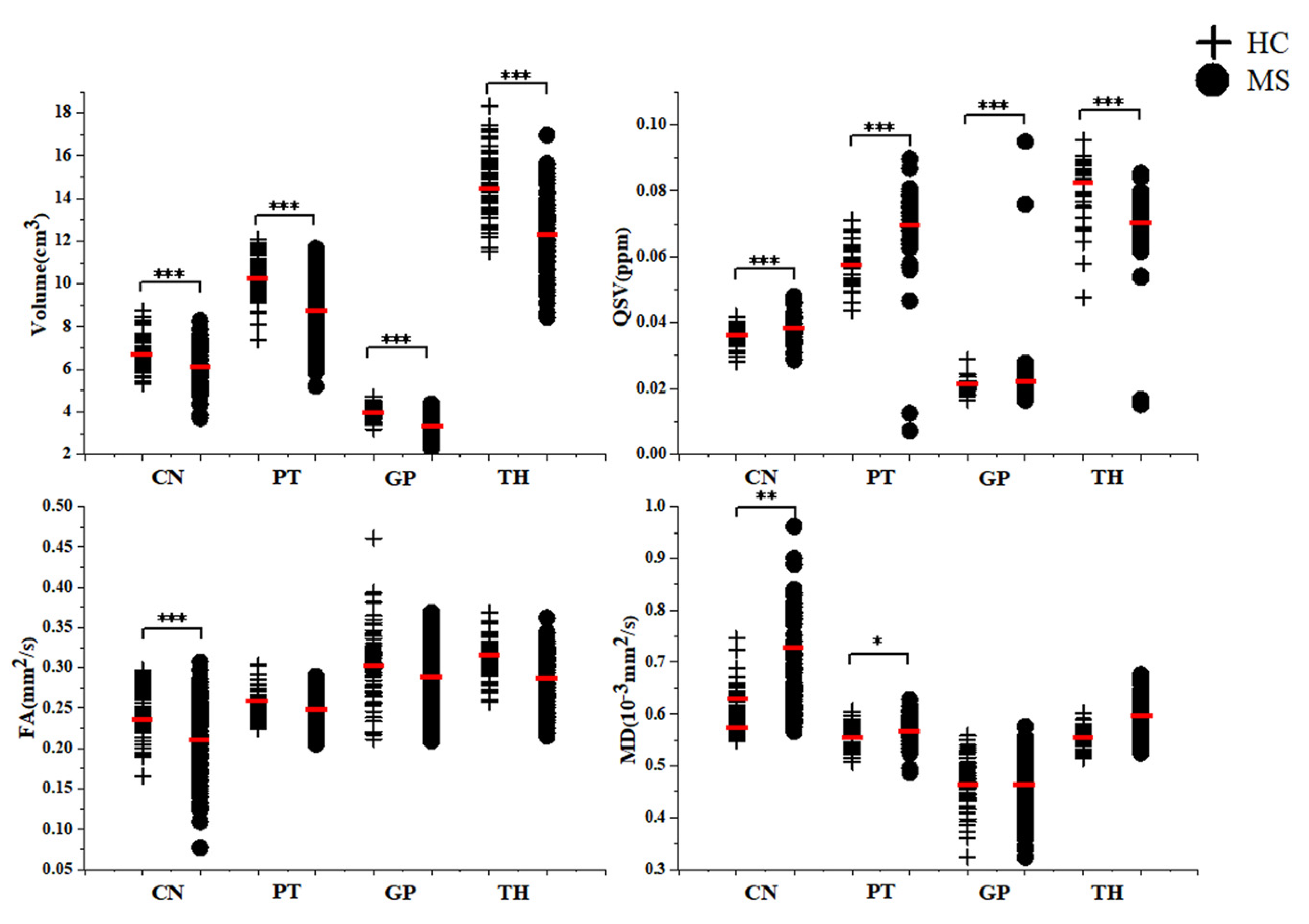
3.2. Comparison of MRI Quantitative Metrics between MS Patients and HC in Study 1 and Study 2
3.3. MVPA Evaluation
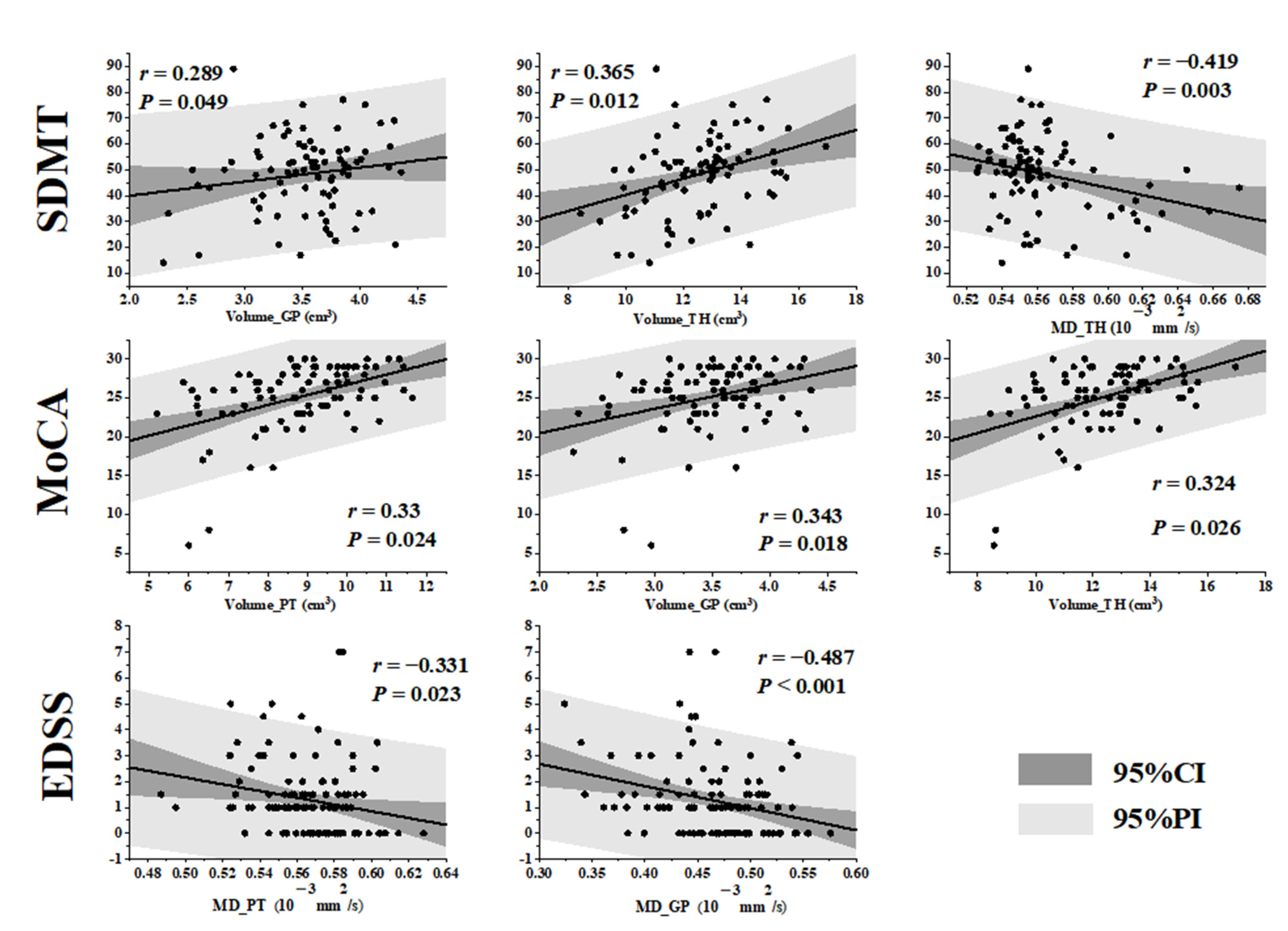
3.4. Correlations between MRI Quantitative Metrics and Clinical Neurological Scores in MS Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutzelnigg, A.; Lassmann, H. Cortical Lesions and Brain Atrophy in MS. J. Neurol. Sci. 2005, 233, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Czarnecki, D.; Shaikh, Z.A.; Priore, R.L.; Janardhan, V.; Kaliszky, Z.; Kinkel, P.R. Brain MRI Lesions and Atrophy Are Related to Depression in Multiple Sclerosis. NeuroReport 2000, 11, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic Neurodegeneration in Multiple Sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, G.; Petracca, M.; Monti, S.; Quarantelli, M.; Criscuolo, C.; Lanzillo, R.; Tedeschi, E.; Elefante, A.; Brescia Morra, V.; Brunetti, A.; et al. Unraveling Deep Gray Matter Atrophy and Iron and Myelin Changes in Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2021, 42, 1223–1230. [Google Scholar] [CrossRef]
- Schweser, F.; Deistung, A.; Reichenbach, J.R. Foundations of MRI Phase Imaging and Processing for Quantitative Susceptibility Mapping (QSM). Z. Für Med. Phys. 2016, 26, 6–34. [Google Scholar] [CrossRef]
- Schweser, F.; Raffaini Duarte Martins, A.L.; Hagemeier, J.; Lin, F.; Hanspach, J.; Weinstock-Guttman, B.; Hametner, S.; Bergsland, N.; Dwyer, M.G.; Zivadinov, R. Mapping of Thalamic Magnetic Susceptibility in Multiple Sclerosis Indicates Decreasing Iron with Disease Duration: A Proposed Mechanistic Relationship between Inflammation and Oligodendrocyte Vitality. NeuroImage 2018, 167, 438–452. [Google Scholar] [CrossRef]
- Tae, W.-S.; Ham, B.-J.; Pyun, S.-B.; Kang, S.-H.; Kim, B.-J. Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. J. Clin. Neurol. 2018, 14, 129–140. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion Tensor Imaging (DTI)-Based White Matter Mapping in Brain Research: A Review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Eshaghi, A.; Marinescu, R.V.; Young, A.L.; Firth, N.C.; Prados, F.; Cardoso, M.J.; Tur, C.; Angelis, F.D.; Cawley, N.; Brownlee, W.J.; et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain 2018, 141, 1665–1677. [Google Scholar] [CrossRef]
- Minagar, A.; Barnett, M.H.; Benedict, R.H.B.; Pelletier, D.; Pirko, I.; Sahraian, M.A.; Frohman, E.; Zivadinov, R. The Thalamus and Multiple Sclerosis: Modern Views on Pathologic, Imaging, and Clinical Aspects. Neurology 2013, 80, 210–219. [Google Scholar] [CrossRef]
- Kubilius, J.; Baeck, A.; Wagemans, J.; Op De Beeck, H.P. Brain-Decoding FMRI Reveals How Wholes Relate to the Sum of Parts. Cortex 2015, 72, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Takerkart, S.; Regragui, F.; Boussaoud, D.; Brovelli, A. Multivoxel Pattern Analysis for FMRI Data: A Review. Comput. Math. Methods Med. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Shi, K.; Pang, X.; Wang, Y.; Li, C.; Long, Q.; Zheng, J. Altered Interhemispheric Functional Homotopy and Connectivity in Temporal Lobe Epilepsy Based on FMRI and Multivariate Pattern Analysis. Neuroradiology 2021, 63, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.; Battista, P.; Castiglioni, I. Frontiers for the Early Diagnosis of AD by Means of MRI Brain Imaging and Support Vector Machines. Curr. Alzheimer Res. 2016, 13, 509–533. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Halicioglu, S.; Turkoglu, S.A. Role of Developmental Venous Anomalies in Etiopathogenesis of Demyelinating Diseases. Int. J. Neurosci. 2019, 129, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, H.S.; Gdeisat, M.A.; Burton, D.R.; Lalor, M.J.; Lilley, F.; Moore, C.J. Fast and Robust Three-Dimensional Best Path Phase Unwrapping Algorithm. Appl. Opt. 2007, 46, 6623. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-aron, B.; Sijbers, J.; Fieremans, E. Denoising of Diffusion MRI Using Random Matrix Theory. NeuroImage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-Ringing Artifact Removal Based on Local Subvoxel-Shifts: Gibbs-Ringing Artifact Removal. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef]
- Leemans, A.; Jones, D.K. The B-Matrix Must Be Rotated When Correcting for Subject Motion in DTI Data. Magn. Reson. Med. 2009, 61, 1336–1349. [Google Scholar] [CrossRef]
- Al-Radaideh, A.; Athamneh, I.; Alabadi, H.; Hbahbih, M. Cortical and Subcortical Morphometric and Iron Changes in Relapsing-Remitting Multiple Sclerosis and Their Association with White Matter T2 Lesion Load: A 3-Tesla Magnetic Resonance Imaging Study. Clin. Neuroradiol. 2019, 29, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Redlich, R.; Almeida, J.J.R.; Grotegerd, D.; Opel, N.; Kugel, H.; Heindel, W.; Arolt, V.; Phillips, M.L.; Dannlowski, U. Brain Morphometric Biomarkers Distinguishing Unipolar and Bipolar Depression: A Voxel-Based Morphometry–Pattern Classification Approach. JAMA Psychiatry 2014, 71, 1222. [Google Scholar] [CrossRef] [PubMed]
- Pravatà, E.; Rocca, M.A.; Valsasina, P.; Riccitelli, G.C.; Gobbi, C.; Comi, G.; Falini, A.; Filippi, M. Gray Matter Trophism, Cognitive Impairment, and Depression in Patients with Multiple Sclerosis. Mult. Scler. J. 2017, 23, 1864–1874. [Google Scholar] [CrossRef]
- Eshaghi, A.; Prados, F.; Brownlee, W.J.; Altmann, D.R.; Tur, C.; Cardoso, M.J.; De Angelis, F.; Van De Pavert, S.H.; Cawley, N.; De Stefano, N.; et al. Deep Gray Matter Volume Loss Drives Disability Worsening in Multiple Sclerosis: Deep Gray Matter Volume Loss. Ann. Neurol. 2018, 83, 210–222. [Google Scholar] [CrossRef]
- Cortese, R.; Battaglini, M.; Parodi, F.; Stromillo, M.L.; Portaccio, E.; Razzolini, L.; Giorgio, A.; Sormani, M.P.; Amato, M.P.; De Stefano, N. Mild Gray Matter Atrophy in Patients with Long-Standing Multiple Sclerosis and Favorable Clinical Course. Mult. Scler. J. 2022, 28, 154–159. [Google Scholar] [CrossRef]
- Du, S.; Sah, S.K.; Zeng, C.; Wang, J.; Liu, Y.; Xiong, H.; Li, Y. Iron Deposition in the Gray Matter in Patients with Relapse-Remitting Multiple Sclerosis: A Longitudinal Study Using Three-Dimensional (3D)-Enhanced T2*-Weighted Angiography (ESWAN). Eur. J. Radiol. 2015, 84, 1325–1332. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple Sclerosis Deep Grey Matter: The Relation between Demyelination, Neurodegeneration, Inflammation and Iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Al-Radaideh, A.; Athamneh, I.; Alabadi, H.; Hbahbih, M. Deep Gray Matter Changes in Relapsing-Remitting Multiple Sclerosis Detected by Multi-Parametric, High-Resolution Magnetic Resonance Imaging (MRI). Eur. Radiol. 2021, 31, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Taege, Y.; Hagemeier, J.; Bergsland, N.; Dwyer, M.G.; Weinstock-Guttman, B.; Zivadinov, R.; Schweser, F. Assessment of Mesoscopic Properties of Deep Gray Matter Iron through a Model-Based Simultaneous Analysis of Magnetic Susceptibility and R2*—A Pilot Study in Patients with Multiple Sclerosis and Normal Controls. NeuroImage 2019, 186, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, J.; Zivadinov, R.; Dwyer, M.G.; Polak, P.; Bergsland, N.; Weinstock-Guttman, B.; Zalis, J.; Deistung, A.; Reichenbach, J.R.; Schweser, F. Changes of Deep Gray Matter Magnetic Susceptibility over 2 Years in Multiple Sclerosis and Healthy Control Brain. NeuroImage Clin. 2018, 18, 1007–1016. [Google Scholar] [CrossRef]
- Sun, H.; Walsh, A.J.; Lebel, R.M.; Blevins, G.; Catz, I.; Lu, J.-Q.; Johnson, E.S.; Emery, D.J.; Warren, K.G.; Wilman, A.H. Validation of Quantitative Susceptibility Mapping with Perls’ Iron Staining for Subcortical Gray Matter. NeuroImage 2015, 105, 486–492. [Google Scholar] [CrossRef]
- Zhang, J.; Giorgio, A.; Vinciguerra, C.; Stromillo, M.L.; Battaglini, M.; Mortilla, M.; Tappa Brocci, R.; Portaccio, E.; Amato, M.P.; De Stefano, N. Gray Matter Atrophy Cannot Be Fully Explained by White Matter Damage in Patients with MS. Mult. Scler. J. 2021, 27, 39–51. [Google Scholar] [CrossRef]
- Zhou, F.; Zee, C.-S.; Gong, H.; Shiroishi, M.; Li, J. Differential Changes in Deep and Cortical Gray Matters of Patients with Multiple Sclerosis: A Quantitative Magnetic Resonance Imaging Study. J. Comput. Assist. Tomogr. 2010, 34, 431–436. [Google Scholar] [CrossRef]
- Solana, E.; Martinez-Heras, E.; Montal, V.; Vilaplana, E.; Lopez-Soley, E.; Radua, J.; Sola-Valls, N.; Montejo, C.; Blanco, Y.; Pulido-Valdeolivas, I.; et al. Regional Grey Matter Microstructural Changes and Volume Loss According to Disease Duration in Multiple Sclerosis Patients. Sci. Rep. 2021, 11, 16805. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Werring, D.J.; Wheeler–Kingshott, C.A.M.; Barker, G.J.; Parker, G.J.M.; Thompson, A.J.; Miller, D.H. Investigation of MS Normal-Appearing Brain Using Diffusion Tensor MRI with Clinical Correlations. Neurology 2001, 56, 926–933. [Google Scholar] [CrossRef]
- Hasan, K.M.; Halphen, C.; Boska, M.D.; Narayana, P.A. Diffusion Tensor Metrics, T2 Relaxation, and Volumetry of the Naturally Aging Human Caudate Nuclei in Healthy Young and Middle-Aged Adults: Possible Implications for the Neurobiology of Human Brain Aging and Disease. Magn. Reson. Med. 2008, 59, 7–13. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D.; DeLong, M.R. Chapter 6 Basal Ganglia-Thalamocortical Circuits: Parallel Substrates for Motor, Oculomotor, “Prefrontal” and “Limbic” Functions. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1991; Volume 85, pp. 119–146. ISBN 978-0-444-81124-0. [Google Scholar]
- Paulesu, E.; Perani, D.; Fazio, F.; Comi, G.; Pozzilli, C.; Martinelli, V.; Filippi, M.; Bettinardi, V.; Sirabian, G.; Passafiume, D.; et al. Functional Basis of Memory Impairment in Multiple Sclerosis: A [18F]FDG PET Study. NeuroImage 1996, 4, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.; Munro, P.M.G.; Youl, B.D.; Prineas, J.W.; Mcdonald, W.I. The longstanding MS lesion: A quantitative MRI and electron microscopic study. Brain 1991, 114, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Vigeveno, M.; Lopes, F.C.R.; Pouwels, P.J.W.; Polman, C.H.; Barkhof, F.; Geurts, J.J.G. Sex specific Extent and Severity of White Matter Damage in Multiple Sclerosis: Implications for Cognitive Decline. Hum. Brain Mapp. 2014, 35, 2348–2358. [Google Scholar] [CrossRef]
- Schoonheim, M.M.; Hulst, H.E.; Brandt, R.B.; Strik, M.; Wink, A.M.; Uitdehaag, B.M.J.; Barkhof, F.; Geurts, J.J.G. Thalamus Structure and Function Determine Severity of Cognitive Impairment in Multiple Sclerosis. Neurology 2015, 84, 776–783. [Google Scholar] [CrossRef]
- Shiee, N.; Bazin, P.-L.; Zackowski, K.M.; Farrell, S.K.; Harrison, D.M.; Newsome, S.D.; Ratchford, J.N.; Caffo, B.S.; Calabresi, P.A.; Pham, D.L.; et al. Revisiting Brain Atrophy and Its Relationship to Disability in Multiple Sclerosis. PLoS ONE 2012, 7, e37049. [Google Scholar] [CrossRef] [PubMed]
- Hagemeier, J. Gray Matter SWI-Filtered Phase and Atrophy Are Linked to Disability in MS. Front. Biosci. 2013, E5, 525–532. [Google Scholar] [CrossRef] [PubMed]



| HC (n = 71) | MS Patients (n = 115) | p | ||
|---|---|---|---|---|
| Sex (male/female) | 15/36 | 12/72 | 0.045 a | |
| Age (years) | 39.8 (30,49) | 32.87 (25.75,40.25) | <0.001 b | |
| Education (years) | 14 (12,16) | 14.43 (12,16) | 0.771 b | |
| DD (years) | - | 4.48 (1,7) | - | |
| EDSS scores | - | 1.29 (0,1.5) | - | |
| SDMT scores | 50.42 ± 15.21 | 48.17 (40,57.25) | 0.954 b | |
| MMSE scores | 29.14 (28,30) | 27.83 (27,29) | 0.001 b | |
| MOCA scores | 27.06 (25,29) | 25.38 (24,28) | 0.027 b | |
| Volume (cm3) | CN | 6.723 ± 0.725 | 6.012 ± 0.991 | <0.001 c |
| PT | 10.235 ± 0.961 | 8.820 ± 1.510 | <0.001 c | |
| GP | 3.958 ± 0.328 | 3.493 ± 0.457 | <0.001 c | |
| TH | 14.461 ± 1.448 | 12.333 ± 1.823 | <0.001 c | |
| TIV | 1425.024 ± 125.437 | 1395.152 ± 111.560 | 0.108 d | |
| QSV (ppm) | CN | 0.035 (0.035,0.038) | 0.037 (0.035,0.038) | <0.001 e |
| PT | 0.057 (0.053,0.064) | 0.071 (0.068,0.078) | <0.001 e | |
| GP | 0.020 (0.019,0.021) | 0.025 (0.023,0.026) | <0.001 e | |
| TH | 0.094 (0.078,0.087) | 0.706 (0.068,0.076) | <0.001 e | |
| FA (mm2/s) | CN | 0.250 (0.228,0.271) | 0.209 ± 0.048 | <0.001 e |
| PT | 0.253 ± 0.01 | 0.250 ± 0.018 | 0.214 e | |
| GP | 0.306 ± 0.046 | 0.289 ± 0.040 | 0.940 e | |
| TH | 0.311 ± 0.024 | 0.285 ± 0.032 | 0.072 e | |
| MD (10−3 mm2/s) | CN | 0.616 (0.578,0.598) | 0.666 (0.602,0.713) | 0.007 e |
| PT | 0.568 (0.553,0.581) | 0.566 ± 0.002 | 0.020 e | |
| GP | 0.471 ± 0.006 | 0.465 (0.436,0.498) | 0.075 e | |
| TH | 0.554 ± 0.002 | 0.570 (0.546,0.580) | 0.189 e | |
| HC (n = 53) | MS (n = 53) | p | ||
|---|---|---|---|---|
| Sex (male/female) | 15/38 | 18/35 | 0.529 a | |
| Age (years) | 36.53 ± 9.852 | 35.81 ± 10.077 | 0.712 b | |
| Education (years) | 14.17 (12,16) | 14.52 (15,16) | 0.870 c | |
| DD (years) | - | 4.8173 (1.00,8.00) | - | |
| Volume (cm3) | CN | 6.739 ± 0.758 | 5.939 ± 1.050 | <0.001 d |
| PT | 10.267 (9.460, 10.906) | 8.89 (7.700, 10.181) | <0.001 d | |
| GP | 3.919 (3.751, 3.968) | 3.516 (3.083, 3.867) | <0.001 d | |
| TH | 14.404 ± 1.351 | 12.233 ± 1.983 | <0.001 d | |
| TIV | 1411.808 (1310.134, 1480.254) | 1387.844 ± 17.732 | 0.048 e | |
| QSV (ppm) | CN | (0.0347,0.0377) | (0.0357,0.0386) | 0.016 f |
| PT | 0.058 ± 0.007 | 0.0718 ± 0.008 | <0.001 f | |
| GP | 0.020 ± 0.002 | 0.0239 ± 0.002 | <0.001 f | |
| TH | (0.0766,0.0867) | (0.0678,0.0780) | 0.008 f | |
| FA (mm2/s) | CN | (0.222,0.269) | 0.202 ± 0.006 | 0.015 f |
| PT | 0.250 ± 0.017 | 0.254 ± 0.018 | 0.335 f | |
| GP | 0.301 ± 0.0414 | 0.296 ± 0.036 | 0.552 f | |
| TH | 0.313 ± 0.0229 | 0.282 ± 0.032 | 0.229 f | |
| MD (10−3 mm2/s) | CN | (0.586,0.651) | (0.608,0.577) | 0.031 f |
| PT | (0.559,0.582) | (0.542,0.577) | 0.021 f | |
| GP | (0.439,0.498) | (0.415,0.487) | 0.268 f | |
| TH | (0.548,0.571) | (0.545,0.601) | 0.075 f | |
| Region | Weights (%) | Cluster Size (vox) | |
|---|---|---|---|
| QSV | CN | 30.53 | 1666 |
| PT | 25.54 | 2061 | |
| GP | 25.33 | 573 | |
| TH | 18.6 | 2083 | |
| FA | CN | 31.38 | 1666 |
| PT | 25.34 | 573 | |
| GP | 24.58 | 2061 | |
| TH | 18.7 | 2083 | |
| MD | CN | 32.21 | 1666 |
| PT | 25.89 | 573 | |
| GP | 21.11 | 2083 | |
| TH | 20.79 | 2061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Yan, Z.; Li, Y.; Ding, S.; Wang, X.; Shi, Z.; Feng, J.; Du, S.; Tan, Z.; Zeng, C. Multimodal Investigation of Deep Gray Matter Nucleus in Patients with Multiple Sclerosis and Their Clinical Correlations: A Multivariate Pattern Analysis Study. J. Pers. Med. 2023, 13, 1488. https://doi.org/10.3390/jpm13101488
Yin F, Yan Z, Li Y, Ding S, Wang X, Shi Z, Feng J, Du S, Tan Z, Zeng C. Multimodal Investigation of Deep Gray Matter Nucleus in Patients with Multiple Sclerosis and Their Clinical Correlations: A Multivariate Pattern Analysis Study. Journal of Personalized Medicine. 2023; 13(10):1488. https://doi.org/10.3390/jpm13101488
Chicago/Turabian StyleYin, Feiyue, Zichun Yan, Yongmei Li, Shuang Ding, Xiaohua Wang, Zhuowei Shi, Jinzhou Feng, Silin Du, Zeyun Tan, and Chun Zeng. 2023. "Multimodal Investigation of Deep Gray Matter Nucleus in Patients with Multiple Sclerosis and Their Clinical Correlations: A Multivariate Pattern Analysis Study" Journal of Personalized Medicine 13, no. 10: 1488. https://doi.org/10.3390/jpm13101488
APA StyleYin, F., Yan, Z., Li, Y., Ding, S., Wang, X., Shi, Z., Feng, J., Du, S., Tan, Z., & Zeng, C. (2023). Multimodal Investigation of Deep Gray Matter Nucleus in Patients with Multiple Sclerosis and Their Clinical Correlations: A Multivariate Pattern Analysis Study. Journal of Personalized Medicine, 13(10), 1488. https://doi.org/10.3390/jpm13101488





