Abstract
The post-percutaneous coronary intervention (post-PCI) fractional flow reserve (FFR) can detect suboptimal PCI or residual ischemia and potentially lead to fewer adverse clinical outcomes. We sought to investigate the predictive value of the angiography-derived FFR for adverse cardiovascular events in patients after PCI. We conducted a comprehensive search of electronic databases, MEDLINE, EMBASE, and the Cochrane Library, for studies published until March 2023 that investigated the prognostic role of angiography-derived fractional flow reserve values after PCI. We investigated the best predictive ability of the post-PCI angiography-derived FFR and relative risk (RR) estimates with 95% confidence intervals (CIs) between post-PCI angiography-derived FFR values and adverse events. Thirteen cohort studies involving 6961 patients (9719 vascular lesions; mean follow-up: 2.2 years) were included in this meta-analysis. The pooled HR of the studies using specific cut-off points for post-PCI angiography-derived FFR was 4.13 (95% CI, 2.92–5.82) for total cardiovascular events, while the pooled HRs for target vessel revascularization, cardiac death, target vessel myocardial infarction, and target lesion revascularization were 6.87 (95% CI, 4.93–9.56), 6.17 (95% CI, 3.52–10.80), 3.98 (95% CI, 2.37–6.66) and 6.27 (95% CI, 3.08–12.79), respectively. In a sensitivity analysis of three studies with 1789 patients assessing the predictive role of the post-PCI angiography-derived FFR as a continuous variable, we found a 58% risk reduction for future adverse events per 0.1 increase in the post-PCI angiography-derived FFR value. In conclusion, post-PCI angiography-derived FFR is an effective tool for predicting adverse cardiovascular events and could be potentially used in decision making, both during PCI and in the long-term follow-up.
1. Introduction
Coronary artery disease (CAD) is a leading cause of death worldwide. Specifically, 126 million individuals globally suffer from ischemic heart disease (1655 per 100,000), representing approximately 2% of the worldwide population in 2017 [1]. Percutaneous coronary intervention (PCI), depending on the specific clinical setting in which it is performed (in acute coronary syndrome or chronic coronary syndromes), has been shown improve quality of life and prognosis [2,3] In most large-scale trials, physiology-guided PCI using a pressure wire to assess the fractional flow reserve (FFR) has been shown to be superior to angiography-only guided PCI and is thus currently advocated by guidelines for decision making during PCI [4,5,6]. Despite the benefit of FFR-guided revascularization, the increased time and cost of the physiology assessment result in its underutilization, according to real-world data [7]. As a result, a wealth of new software assessing the angiography-derived FFR have emerged.
The angiography-derived FFR can assess the hemodynamic severity of coronary stenosis by combining fluid dynamics computation and a 3D anatomical vessel reconstruction based on angiographical views without the involvement of coronary vessel instrumentation with a pressure wire and the administration of vasodilator agents. One method, the quantitative flow ratio (QFR), has shown good correlation and diagnostic accuracy compared to the FFR [7,8,9]. Recently, FAVOR III China described that QFR-guided PCI was associated with improved 1- and 2-year clinical outcomes compared to the standard coronary angiography-guided PCI [10,11].
Although both PCI techniques and equipment have rapidly evolved in recent years, a significant proportion of patients undergoing an angiographically successful PCI suffer from adverse events, such as recurrent angina or silent ischemia [12]. A post-PCI functional assessment can detect suboptimal PCI or residual ischemia, leading to possible efforts to optimize the final result to reduce further the risk of adverse clinical outcomes. The immediate post-stenting measurement of the FFR could aid in optimizing revascularization results and potentially improve outcomes as it is well established that suboptimal PCI is an independent predictor of major cardiac adverse events [13,14,15]. Recent studies demonstrated the role of the post-PCI angiography-derived FFR as a predictor of a vessel- or patient-oriented outcomes in patients with stable CAD, acute coronary syndromes, or in-stent restenosis [16,17,18,19,20,21,22,23,24,25,26,27,28]. The primary objective of this meta-analysis was to evaluate whether the post-PCI angiography-derived FFR predicts coronary adverse events in CAD patients undergoing PCI. Second, we sought to investigate whether publication bias could have affected our results. Third, we evaluated the effects of several demographic and angiographic factors on the possible predictive role of the post-PCI angiography-derived FFR to identify the phenotype of the patient that would benefit most from such an assessment.
2. Materials and Methods
The systematic review and meta-analysis were conducted in accordance with the PRISMA 2020 checklist [29] (see also Supplementary Material). The outcomes of interest were: (1) total CV events (including vessel-oriented composite endpoint (VOCE), defined as the composite of cardiac death, vessel-related myocardial infarction (MI), or ischemia-driven target vessel revascularization (TVR) or major adverse cardiovascular events (MACE) or target lesion failure (TLF)), (2) TVR, (3) cardiac death, (4) target vessel MI and (5) TLR (target lesion revascularization). TLR was defined as revascularization post-stenting within the stent or within the 5 mm borders adjacent to the stent.
2.1. Data Sources and Research
For this systematic review and meta-analysis, a systematic search of the literature was performed in the PubMed, Cochrane, and Embase databases for cohort studies published until March 2023 that investigated the prognostic role of the post-PCI angiography-derived FFR. The following search terms were used: QFR, quantitative flow ratio, quantitative flow ratio AND coronary artery disease, QFR AND coronary artery disease AND (POST AND QFR AND prognosis, QFR AND prognosis, POST AND quantitative flow ratio AND prognosis, angiography-derived FFR. angiography-derived Fractional Flow Reverse AND vFFR. The search was not restricted to any language. Data sources were also identified by manually searching the references of articles, reviews and meta-analyses. We subsequently searched online resources such as the abstracts for major cardiovascular conventions and clinicaltrials.gov (accessed on 4 May 2023) to ensure the identification of all published and unpublished studies.
2.2. Study Selection
Studies were considered eligible if they met the following criteria: (1) were full-length publications in peer-reviewed journals; (2) were randomized-controlled studies, case studies, or cohort studies, either retrospective or prospective; (3) included patients with CAD who underwent PCI; (4) recorded the post-PCI angiography-derived FFR value; (5) reported a VOCE, defined as the composite of cardiac death, vessel-related MI, or TVR or MACE or TLR; and (6) had a minimum follow-up period of up to 6 months. No restriction criteria were imposed regarding the size of the population studied or the type of the population (chronic or acute coronary syndromes).
2.3. Data Extraction and Quality Assessment
Two reviewers (K.-P.G. and D.O.) independently conducted the data extraction, study selection and evaluation for the risk bias of the studies. Disagreements were resolved via consensus. The same 2 reviewers independently extracted data regarding the study population, intervention types, sample size, mean age, gender, follow-up period, indications of procedures, method of assessing the angiography-derived FFR, the cut-off point of the angiography-derived FFR and the studies’ outcomes and results. The quality of each study was evaluated via the Newcastle–Ottawa Scale (NOS) [30]. The NOS scale evaluates the quality of research by assessing the study population, comparability and outcome. This scale allocates up to 9 points for the lowest risk of bias in 4 domains: the selection of study groups (4 points), the comparability of the groups (2 points) and the ascertainment of exposure and outcomes (3 points). A study’s quality was considered poor if its Newcastle–Ottawa score was below 7.
2.4. Data Synthesis and Analysis
Each study described the risk estimates as hazard ratios (HRs), relative risks (RRs), odds ratios or dichotomous frequency data. We managed HRs as RRs. Fully adjusted RRs were selected over crude estimates, whenever available, as provided by the authors in multivariable regression models. We investigated the prognostic value of the post-PCI angiography-derived FFR by extracting and pooling RRs for the following outcomes from each study: (1) VOCE, including cardiac death, vessel-related MI and ischemia-driven TVR; (2) MACE; and (3) TLR. Moderate to significant heterogeneity existed among the studies, and a random-effects model was subsequently implemented. To test whether the true effect in all studies was the same (i.e., heterogeneity), we used the I-squared measure (I2), which permits the quantification of discrepancy among studies. Forest plots were created for a graphical representation of the individual studies’ RRs and confidence intervals (CIs).
We also performed a sensitivity analysis of three studies in which the RR for the post-PCI angiography-derived FFR were described as a continuous variable and calculated the adjusted, pooled RR per 0.1 increase in the post-PCI angiography-derived FFR value for total cardiovascular events.
The contribution of continuous study moderators to the overall heterogeneity was assessed via a meta-regression analysis with fixed-effects estimates. Publication bias was illustrated graphically via funnel plots, and its associations with our results were evaluated via the Duval and Tweedie trim-and-fill method and the classic fail-safe N method, as introduced by Rosenthal.
All analyses were performed with comprehensive meta-analysis version 2 (Biostat, Englewood, NJ, USA). We deemed statistical significance to be p < 0.05.
3. Results
3.1. Literature Search Results
Our initial systematic search of the literature retrieved 270 studies, 13 of which were suitable for the analysis (Figure 1). In total, 249 articles were excluded from this meta-analysis after reading the titles and the abstracts because they were irrelevant to the research purpose. Specifically, 28 studies were systematic review articles, 8 studies were editorials, letters or commentaries, 2 studies were case reports, 30 studies reported the pre-PCI angiography-derived FFR, 79 studies investigated the coronary CT angiography-derived FFR, 64 studies had no measurement of the post-PCI angiography-derived FFR and 38 studies had no relevant clinical outcome reported. Finally, eight articles were excluded after a full review for the following reasons: one study had a population similar to an included study [31], five studies did not report the data necessary for this analysis [16,32,33,34,35] and two did refer to other indicators but not to the post-PCI angiography-derived FFR [36,37].

Figure 1.
Flow diagram of study selection procedure.
3.2. Study Characteristics
Our meta-analysis included 13 original articles published since 2019. The included studies investigated 6961 patients (9719 vascular lesions; mean follow-up: 2.2 years). Several populations, such as patients with chronic ischemic heart disease, acute coronary syndrome and in-stent restenosis after PCI-DES, were contained in this meta-analysis. For the analysis of the total coronary adverse events, all but one study reported the HR without using a cut-off point [21]. Details of the individual studies regarding the association of the angiography-derived FFR with coronary artery events are provided in Table 1. The sample sizes ranged from 169 to 1805 individuals. Almost all studies examined age, sex and other cardiovascular risk factors.

Table 1.
Overview of studies on the association of angiography-derived FFR and cardiovascular events.
3.3. The Effect of the Post-PCI Angiography-Derived FFR on Total Cardiovascular Events
The magnitude of risk for cardiovascular events in subjects with post-PCI angiography-derived FFR values below the cut-off provided by each study (12 studies in total, in which 11 studies reported VOCE and 1 study MACE) was significantly higher compared with the risk of individuals with higher post-PCI angiography-derived FFR values. Patients with lower angiography-derived FFR values after PCI experienced a four times higher risk of cardiovascular events during the follow-up period. Specifically, the total RR value was 4.13 (95% CI, 2.92–5.82) (Figure 2A).
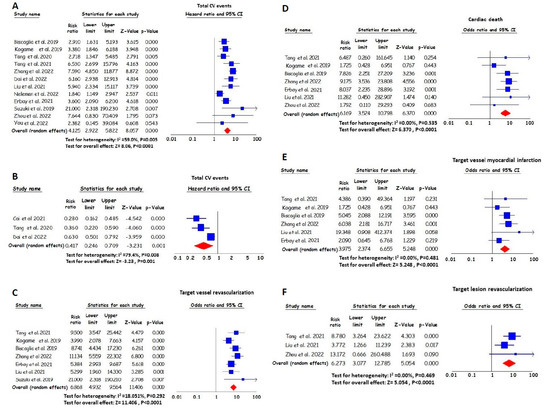
Figure 2.
RR and 95% CI for lower angiography-derived FFR values and total cardiovascular (CV) events ((A) based on cut-off values [16,17,18,19,20,22,23,24,25,26,27,28]; (B) per 0.1 increase [19,20,21]), target vessel revascularization (C) [16,18,22,23,24,25,26], cardiac death (D) [16,18,22,23,24,25,28], target vessel myocardial infarction (E) [16,18,22,23,24,25] and target lesion revascularization (F) [16,22,28]. The squares’ sizes show the weight of each study, and the lines illustrate the 95% CI for individual studies with a lower and upper limit. The diamonds and their width represent the combined results of the meta-analysis.
In three studies, the post-PCI angiography-derived FFR value was considered a continuous variable. An increase per 0.1 in the pooled post-PCI angiography-derived FFR value resulted in a 58% reduction in the risk of cardiovascular events (HR 0.42 [95% CI, 0.25–0.71], per 0.1 increase) (Figure 2B).
3.4. The Effect of the Post-PCI Angiography-Derived FFR on TVR
The magnitude of risk for TVR in individuals with lower post-PCI angiography-derived FFR values was significantly higher compared with the magnitude of risk for individuals with higher values. Specifically, patients with lower post-PCI angiography-derived FFR values had an approximately seven times greater pooled RR for TVR during the follow-up period. The RR value was 6.87 (95% CI, 4.93–9.56) (Figure 2C).
3.5. The Effect of the Post-PCI Angiography-Derived FFR on Cardiac Death
Patients with lower post-PCI angiography-derived FFR values experienced higher levels of risk for cardiac death compared to those with higher post-PCI angiography-derived FFR values. Specifically, the pooled RR value was 6.17 (95% CI, 3.52–10.80) (Figure 2D).
3.6. The Effect of the Post-PCI Angiography-Derived FFR on Target Vessel MI
The magnitude of risk for target vessel MI in individuals with lower post-PCI angiography-derived FFR values was significantly higher when compared with the risk of individuals with higher values. Specifically, the pooled HR for the lower angiography-derived FFR was 3.98 (95% CI, 2.37–6.66) for target vessel MI (Figure 2E).
3.7. The Effect of the Post-PCI Angiography-Derived FFR on TLR
During the follow-up period, the magnitude of risk for TLR in individuals with lower post-PCI angiography-derived FFR values was significantly higher compared with the risk of individuals with higher values. The pooled HR for the lower angiography-derived FFR was 6.27 (95% CI, 3.08–12.79) for TLR (Figure 2F).
3.8. Publication Bias
The funnel plots demonstrate an almost symmetrical distribution of the included studies around the average (Figure 3). The imputed HR values based on the trim-and-fill method were 3.92 (95% CI, 2.79–5.50), 6.01 (95% CI, 4.22–8.56), 6.17 (95% CI, 3.52–10.80) and 3.80 (95% CI, 2.28–6.31) for total CV events, TVR, cardiac death and target vessel MI, respectively, which are not lower than our original risk estimates but are still significant. Regarding the fail-safe N test, the number of missing studies that would need to be added to the analysis to give a statistically nonsignificant overall effect was 497, 282, 50, and 22, respectively. Importantly, it is less likely that there are >41 (497/12 = 41.4), >40 (282/7 = 40.3), 7 (50/7 = 7.1) and 6 (33/6 = 5.5) unpublished studies for every 1 study that we found for total CV events, TVR, cardiac death and target vessel MI, respectively. These findings indicate that the apparent publication bias is inadequate to influence our results or interpretations in a meaningful way.
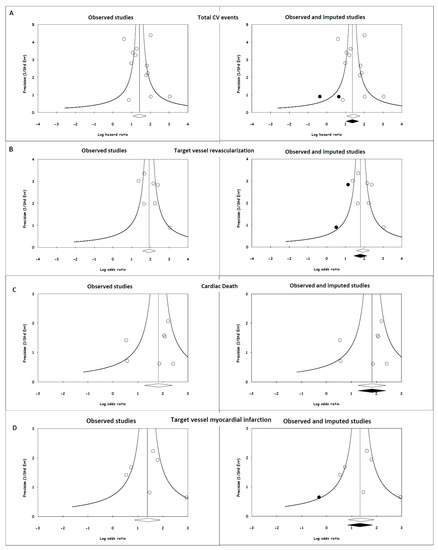
Figure 3.
Publication bias for endpoints and their potential effects. (A) Total CV events; (B) target vessel revascularization; (C) cardiac death; and (D) target vessel myocardial infarction. The open circles in the left and right plots depict individual studies relating the lower value of the angiography-derived FFR with cardiovascular events, and the open diamonds are the HR and 95% CI for the meta-analysis. The solid circles in the right side of the figure represent imputed studies, and the solid diamonds are the HR and 95% CI for the meta-analysis after adjusting for publication bias.
3.9. Meta-Regression Analysis
The duration of follow-up was the strongest predictor of the size of the log HR in patients with lower post-PCI angiography-derived FFR values, and it was inversely related to the prognostic role of the post-PCI angiography-derived FFR for total cardiovascular events (p = 0.001, Figure 4B). Age at the enrollment indicated inverse associations with the predictive value of the post-PCI angiography-derived FFR value (p = 0.02, Figure 4A). The cut-off point of the angiography-derived FFR was not a predictor (p = 0.26, Figure 4C), while the percentage of diabetic patients in each study demonstrated a non-statistically significant positive trend (p = 0.07, Figure 4D). The percentage of smokers in each study and the percentage of LAD vessels showed positive associations with the predictive role of the post-PCI angiography-derived FFR (p = 0.02). The percentage of patients with acute coronary syndrome illustrated a negative association with the predictive value of the angiography-derived FFR after PCI (p = 0.03) (Figure 4E–G).
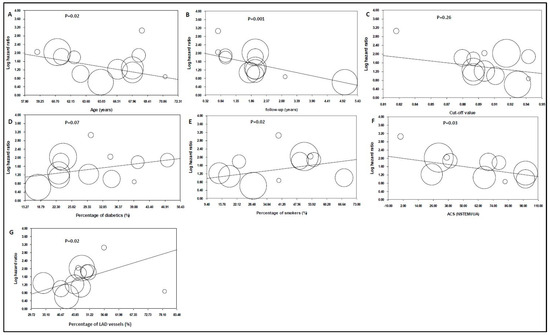
Figure 4.
Hazard ratios (HRs) of total cardiovascular events in patients with lower angiography-derived FFR values as an impact of (A) age (data from 12 studies [16,17,19,20,22,23,24,25,26,27,28,36]); (B) the study’s population follow-up period (data from 12 studies [16,17,19,20,22,23,24,25,26,27,28,36]); (C) cut-off value (data from 12 studies [16,17,19,20,22,23,24,25,26,27,28,36]); (D) the percentage of the study population with diabetes mellitus (data from 12 studies [16,17,19,20,22,23,24,25,26,27,28,36]); (E) the percentage of the study population, who were smokers (data from 11 studies [16,17,19,20,22,24,25,26,27,28,36]); (F) the percentage of patients presenting with ACS (data from 10 studies [16,17,18,19,20,21,22,23,24,25]); and (G) the percentage of LAD vessels (data from 12 studies [16,17,19,20,21,22,23,24,25,27,28,36]). Each circle represents one study that shows the actual coordinates for that study. The weight of each study is proportional to the size of each circle. The center line shows the values predicted via fixed-effects meta-regression. The vertical axis is on a log scale.
4. Discussion
This systematic review and meta-analysis investigated the relationship of the post-PCI angiography-derived FFR with cardiovascular adverse events. We pooled data from thirteen published studies, including approximately 7000 patients who underwent PCI, and investigated adverse outcomes after a mean follow-up period of more than 2 years. Our study is the first meta-analysis to show that lower post-PCI angiography-derived FFR values increase the risk for future cardiovascular adverse events, including TVR, cardiac death, target vessel MI and TLR. Our main finding is that patients with an impaired post-PCI angiography-derived FFR value experienced a four times higher risk of adverse coronary events during the follow-up period. Also, patients with lower post-PCI angiography-derived FFR values presented with seven-, six-, four-, and sixfold higher risks for TVR, cardiac death, target vessel MI and TLR, respectively. According to a sensitivity analysis of three of the included studies, we found that a 0.1 increase in the post-PCI angiography-derived FFR was associated with a risk reduction of 58% for future coronary adverse events. In addition, our study identified that age, clinical presentation and time of follow-up could influence the predictive ability of the post-PCI angiography-derived FFR.
A recent meta-analysis including studies with both post-PCI invasive FFR/iFR and post-PCI angiography-derived FFR values showed that impaired post-PCI physiology assessment values are related to increased adverse cardiac events [38]. Using the same primary outcome as in our study, the authors reported a twofold increase in adverse cardiovascular events in patients with lower post-PCI invasive or angiography-derived FFR values, whereas our study showed a fourfold higher risk in patients with lower angiography-derived FFR values. This difference could be attributed firstly to the inclusion of neutral FFR studies in the meta-analysis by Griffioen et al. [38]. Secondly, in contrast to Griffioen et al. we included two large QFR studies that reported significantly higher risks of adverse cardiovascular events in patients with lower post-PCI QFR values [18,19]. Accordingly, a recent meta-analysis of post-PCI invasive FFR studies reported similar results, supporting the prognostic value of post-PCI physiology [39,40].
The studies used in our meta-analysis included patients with both stable coronary disease and acute coronary syndromes, as well as different clinical scenarios such as in-stent restenosis lesions, indicating that lower post-PCI angiography-derived FFR values are predictive of adverse events in a wide spectrum of coronary artery disease. In most of the included studies, the QFR was used as the method of FFR estimation via angiography [16,17,18,19,20,21,22,23,24,25,26,27,28]. The QFR is a well-studied index of coronary physiology. In a large, randomized trial, QFR-guided PCI showed improved clinical outcomes compared to angiography-guided PCI [10,11]. According to the results of our study, post-PCI guidance using the QFR could offer additional clinical benefits.
The presence of residual ischemia after revascularization can lead to adverse events. Common reasons for residual ischemia are diffuse stenosis beyond the margins of the stent, untreated lesions, or marginal-to-stent coronary artery dissection [24,41]. Intracoronary imaging could be helpful in identifying the underlying pathology. IVUS has shown promising results in revascularization guidance [42,43] and could be used adjacent to coronary physiology indices. Another non-invasive modality that has shown good correlations with the invasive FFR and QFR is fractional flow reserve–computed tomography (FFR-CT), which combines computational fluid dynamics and the coronary artery tree demonstration from coronary computed tomographic angiography [44,45,46,47,48,49]. The prognostic role of the post-PCI FFR-CT needs further investigation.
Microvascular dysfunction predicts adverse cardiac events independently from the fractional flow reserve and successful epicardial coronary revascularization [50]. The index of microcirculatory resistance (IMR) is the gold standard method for coronary microvascular assessment. Based on recent studies, the post-PCI QFR, in either an acute or elective setting, has been incorporated into algorithms for assessing microvascular dysfunction. Many studies have investigated the prognostic role of these angiography-derived indexes of microvascular dysfunction, such as IMRangio and non-hyperaemic IMRangio, providing promising results that are comparable to the ones achieved via the actual measurement of the IMR [51,52,53,54,55]. The additional post-PCI prognostic information that the QFR can offer via post-PCI IMR estimation provides an advantage to this method.
4.1. Clinical Implications
The post-PCI angiography-derived FFR had better predictive value in patients with some specific characteristics. These characteristics include a younger age, smoking, presentation with chronic coronary artery disease and coronary artery disease located in the LAD. Also, the post-PCI angiography-derived FFR mainly predicts short-term coronary adverse events because adverse events occurring shortly after the procedure are related to residual stenosis. The cut-off point does not affect its predictive value as long as it ranges from 0.88 to 0.94.
According to previous studies, lower post-PCI QFR values can predict vessel-oriented composite endpoints during follow-up [24]. A higher post-PCI QFR value in patients with three-vessel disease who underwent PCI was associated with a lower risk for vessel-oriented clinical outcomes [25]. Another clinical implication is in patients with in-stent restenosis after DES implantation. Recent studies described that lower QFR values after drug-coated balloon angioplasty were related to worse clinical outcomes during follow-up [16,22]. Patients presenting with acute coronary syndrome may benefit from QFR measurements immediately after the culprit lesion is stented but also from the assessment of non-culprit vessels [20,23].
4.2. Strengths and Limitations
A limitation of this meta-analysis is that all studies apart from one were retrospective cohort studies. Also, the currently included studies had small population sizes. However, when combined, the studies provide a substantial sample size for providing conclusive results. Furthermore, clinically relevant outcomes such as heart failure and emergency hospitalizations could not be investigated due to a lack of reporting in the available studies. However, data on revascularization and MI rates imply a higher number of elective and emergency hospitalizations in patients with low post-PCI angiography-derived FFR values.
5. Conclusions
Post-PCI angiography-derived FFR assessment is a predictor for cardiovascular events during the follow-up period. When performed in patients with a high risk of recurrent events, such as diabetics, smokers and patients undergoing PCI in the LAD, it could provide an effective tool to estimate patient risk in the catheterization laboratory in a wire-free manner and without any medications to guide both acute (in the catheterization laboratory) and long-term (secondary prevention) management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13081251/s1. Prisma checklist.
Author Contributions
All of the authors contributed with the writing, review, and editing of the study. Development of the systematic review: K.-P.G. and D.O.; collaboration in carrying out the systematic review and the verification of results K.A., D.T.-P. and I.D.; development of the manuscript: D.T.-P., K.-P.G. and V.G.; meta-analysis and statistical analysis: D.T.-P., C.V. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this meta-analysis are available from the corresponding author upon reasonable request.
Conflicts of Interest
This manuscript has not been published and is not under consideration for publication elsewhere. We have no conflicts of interest to disclose, and all authors have approved the manuscript, agreeing with its submission.
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Chacko, L.; Howard, J.; Rajkumar, C.; Nowbar, A.N.; Kane, C.; Mahdi, D.; Foley, M.; Shun-Shin, M.; Cole, G.; Sen, S.; et al. Effects of Percutaneous Coronary Intervention on Death and Myocardial Infarction Stratified by Stable and Unstable Coronary Artery Disease: A Meta-Analysis of Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006363. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, S.P.; Hartigan, P.M.; Teo, K.K.; Maron, D.J.; Spertus, J.A.; Mancini, G.J.; Kostuk, W.; Chaitman, B.R.; Berman, D.; Lorin, J.D.; et al. Effect of PCI on Long-Term Survival in Patients with Stable Ischemic Heart Disease. N. Engl. J. Med. 2015, 373, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- van Nunen, L.X.; Zimmermann, F.M.; Tonino, P.A.; Barbato, E.; Baumbach, A.; Engstrøm, T.; Klauss, V.; MacCarthy, P.A.; Manoharan, G.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015, 7, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.; Fearon, W.F.; Barbato, E.; Tonino, P.A.; Engstrøm, T.; Kääb, S.; Dambrink, J.H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Terentes-Printzios, D.; Oikonomou, D.; Gkini, K.-P.; Gardikioti, V.; Aznaouridis, K.; Dima, I.; Tsioufis, K.; Vlachopoulos, C. Angiography-based estimation of coronary physiology: A frame is worth a thousand words. Trends Cardiovasc. Med. 2022, 32, 366–374. [Google Scholar] [CrossRef]
- Buono, A.; Mühlenhaus, A.; Schäfer, T.; Trieb, A.-K.; Schmeißer, J.; Koppe, F.; Münzel, T.; Anadol, R.; Gori, T. QFR Predicts the Incidence of Long-Term Adverse Events in Patients with Suspected CAD: Feasibility and Reproducibility of the Method. J. Clin. Med. 2020, 9, 220. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, S.H.; Lee, J.M.; Hwang, D.; Zhang, J.; Kim, J.; Im, S.Y.; Kim, H.K.; Nam, C.W.; Doh, J.H.; et al. Clinical relevance and prognostic implications of contrast quantitative flow ratio in patients with coronary artery disease. Int. J. Cardiol. 2021, 325, 23–29. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.A.; Chen, Y.; Pu, J.; et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef]
- Song, L.; Xu, B.; Tu, S.; Guan, C.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.A.; Chen, Y.; et al. 2-Year Outcomes of Angiographic Quantitative Flow Ratio-Guided Coronary Interventions. J. Am. Coll. Cardiol. 2022, 80, 2089–2101. [Google Scholar] [CrossRef]
- De Luca, L.; Rosano, G.M.; Spoletini, I. Post-percutaneous coronary intervention angina: From physiopathological mechanisms to individualized treatment. Cardiol. J. 2022, 29, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Kasula, S.; Hacioglu, Y.; Ahmed, Z.; Uretsky, B.F.; Hakeem, A. Utilizing Post-Intervention Fractional Flow Reserve to Optimize Acute Results and the Relationship to Long-Term Outcomes. JACC Cardiovasc. Interv. 2016, 9, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Piroth, Z.; Toth, G.G.; Tonino, P.A.; Barbato, E.; Aghlmandi, S.; Curzen, N.; Rioufol, G.; Pijls, N.H.; Fearon, W.F.; Jüni, P.; et al. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2017, 10, e005233. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Huang, J.; Westra, J.; Cohen, D.J.; Chen, Y.; Andersen, B.K.; Holm, N.R.; Xu, B.; Tu, S.; Wijns, W. Immediate post-procedural functional assessment of percutaneous coronary intervention: Current evidence and future directions. Eur. Heart J. 2021, 42, 2695–2707. [Google Scholar] [CrossRef]
- Liu, L.; Ding, F.; Gutiérrez-Chico, J.L.; Zhu, J.; Zhu, Z.; Du, R.; Yang, Z.; Hu, J.; Tu, S.; Zhang, R. Prognostic value of post-procedural μQFR for drug-coated balloons in the treatment of in-stent restenosis. Cardiol. J. 2023, 30, 167–177. [Google Scholar] [CrossRef]
- You, W.; Zhou, Y.; Wu, Z.; Meng, P.; Pan, D.; Yin, D.; Yang, S.; Wu, X.; Ye, F. Post-PCI quantitative flow ratio predicts 3-year outcome after rotational atherectomy in patients with heavily calcified lesions. Clin. Cardiol. 2022, 45, 558–566. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, S.; Yuan, S.; Guan, C.; Zou, T.; Qiao, Z.; Xie, L.; Wang, H.; Song, L.; Xu, B.; et al. Effects of diabetes mellitus on post-intervention coronary physiological assessment derived by quantitative flow ratio in patients with coronary artery disease underwent percutaneous coronary intervention. Diabetes Res. Clin. Pract. 2022, 186, 109839. [Google Scholar] [CrossRef]
- Dai, N.; Yuan, S.; Dou, K.; Zhang, R.; Hu, N.; He, J.; Guan, C.; Zou, T.; Qiao, Z.; Duan, S.; et al. Prognostic Implications of Prestent Pullback Pressure Gradient and Poststent Quantitative Flow Ratio in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2022, 11, e024903. [Google Scholar] [CrossRef]
- Tang, J.; Chu, J.; Hou, H.; Lai, Y.; Tu, S.; Chen, F.; Yao, Y.; Ye, Z.; Gao, Y.; Mao, Y.; et al. Clinical implication of QFR in patients with ST-segment elevation myocardial infarction after drug-eluting stent implantation. Int. J. Cardiovasc. Imaging 2021, 37, 755–766. [Google Scholar] [CrossRef]
- Cai, X.; Tian, F.; Jing, J.; Jin, Q.; Zhou, S.; Yin, W.; Chen, Y.; Wu, Q.; Fu, Z.; Chen, Y. Prognostic value of quantitative flow ratio measured immediately after drug-coated balloon angioplasty for in-stent restenosis. Catheter. Cardiovasc. Interv. 2021, 97, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hou, H.; Chu, J.; Chen, F.; Yao, Y.; Gao, Y.; Ye, Z.; Zhuang, S.; Lai, Y.; Liu, X. Clinical implication of quantitative flow ratio to predict clinical events after drug-coated balloon angioplasty in patients with in-stent restenosis. Clin. Cardiol. 2021, 44, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Erbay, A.; Penzel, L.; Abdelwahed, Y.S.; Klotsche, J.; Heuberger, A.; Schatz, A.S.; Steiner, J.; Haghikia, A.; Sinning, D.; Fröhlich, G.M.; et al. Prognostic Impact of Pancoronary Quantitative Flow Ratio Assessment in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes. Circ. Cardiovasc. Interv. 2021, 14, e010698. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Tebaldi, M.; Brugaletta, S.; Cerrato, E.; Erriquez, A.; Passarini, G.; Ielasi, A.; Spitaleri, G.; Di Girolamo, D.; Mezzapelle, G.; et al. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc. Interv. 2019, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Kogame, N.; Takahashi, K.; Tomaniak, M.; Chichareon, P.; Modolo, R.; Chang, C.C.; Komiyama, H.; Katagiri, Y.; Asano, T.; Stables, R.; et al. Clinical Implication of Quantitative Flow Ratio After Percutaneous Coronary Intervention for 3-Vessel Disease. JACC Cardiovasc. Interv. 2019, 12, 2064–2075. [Google Scholar] [CrossRef]
- Suzuki, N.; Nishide, S.; Kimura, T.; Aoyagi, T.; Kanamori, K.; Shiratori, Y.; Hayami, N.; Murakawa, Y.; Kozuma, K. Relationship of quantitative flow ratio after second-generation drug-eluting stent implantation to clinical outcomes. Heart Vessels 2020, 35, 743–749. [Google Scholar] [CrossRef]
- Neleman, T.; Scoccia, A.; Masdjedi, K.; Tomaniak, M.; Ligthart, J.M.; Witberg, K.T.; Vermaire, A.; Wolff, Q.; Visser, L.; Cummins, P.; et al. The prognostic value of angiography-based vessel fractional flow reserve after percutaneous coronary intervention: The FAST Outcome study. Int. J. Cardiol. 2022, 15, 14–19. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, B.; Fan, F.; Yang, F.; Fang, S.; Wang, Z.; Qiu, L.; Gong, Y.; Huo, Y. Prognostic Value of Coronary Angiography-Derived Fractional Flow Reserve Immediately After Stenting. Front. Cardiovasc. Med. 2022, 21, 834553. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Erriquez, A.; Uretsky, B.F.; Brugaletta, S.; Spitaleri, G.; Cerrato, E.; Quadri, G.; Manfrini, M.; Pompei, G.; Scancarello, D.; Trichilo, M.; et al. Impact of trans-stent gradient on outcome after PCI: Results from a HAWKEYE substudy. Int. J. Cardiovasc. Imaging 2022, 38, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.N.; Liu, B.; Li, L.B.; Yin, D.L.; Zhang, H.; Pan, D.F.; You, W.; Wu, Z.M.; Wu, X.Q.; Zhao, L.; et al. Cut-off values of lesion and vessel quantitative flow ratio in de novo coronary lesion post-drug-coated balloon therapy predicting vessel restenosis at mid-term follow-up. Chin. Med. J. 2021, 134, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhang, R.; Yuan, S.; Hu, N.; Guan, C.; Zou, T.; Qiao, Z.; He, J.; Duan, S.; Xie, L.; et al. Prognostic Implications of Quantitative Flow Ratio-Derived Physiological 2-Dimensional Residual Disease Patterns After Stenting. JACC Cardiovasc. Interv. 2022, 15, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, Q.; Zhong, J.; Chen, L.; Chen, L.; Ye, M.; Yan, Y.; Chen, L.; Luo, Y. Impact of diabetes on coronary physiology evaluated by quantitative flow ratio in patients who underwent percutaneous coronary intervention. J. Diabetes Investig. 2022, 12, 1203–1212. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, Q.; Chen, L.; Ye, Z.; Chen, H.; Sun, J.; Hong, J.; Ye, M.; Yan, Y.; Chen, L.; et al. Physiological benefits evaluated by quantitative flow ratio in patients with reduced left ventricular ejection fraction who underwent percutaneous coronary intervention. BMC Cardiovasc. Disord. 2020, 20, 523. [Google Scholar] [CrossRef]
- Zhang, R.; Dou, K.; Guan, C.; Zou, T.; Zhang, M.; Yuan, S.; Qiao, Z.; Xie, L.; Sun, Z.; Song, L.; et al. Outcomes of quantitative flow ratio-based percutaneous coronary intervention in an all-comers study. EuroIntervention 2022, 17, 1240–1251. [Google Scholar] [CrossRef]
- Kirigaya, H.; Okada, K.; Hibi, K.; Maejima, N.; Iwahashi, N.; Matsuzawa, Y.; Minamimoto, Y.; Kosuge, M.; Ebina, T.; Tamura, K.; et al. Post-procedural quantitative flow ratio gradient and target lesion revascularization after drug-coated balloon or plain-old balloon angioplasty. J. Cardiol. 2022, 80, 511–517. [Google Scholar] [CrossRef]
- Griffioen, A.M.; Oord, S.C.H.V.D.; Teerenstra, S.; Damman, P.; Van Royen, N.; Van Geuns, R.J.M. Clinical Relevance of Impaired Physiological Assessment After Percutaneous Coronary Intervention: A Meta-analysis. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100448. [Google Scholar]
- Andersen, B.K.; Ding, D.; Mogensen, L.J.H.; Tu, S.; Holm, N.R.; Westra, J.; Wijns, W. Predictive value of post-percutaneous coronary intervention fractional flow reserve: A systematic review and meta-analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 99–108. [Google Scholar] [CrossRef]
- Hwang, D.; Koo, B.K.; Zhang, J.; Park, J.; Yang, S.; Kim, M.; Yun, J.P.; Lee, J.M.; Nam, C.W.; Shin, E.S.; et al. Prognostic Implications of Fractional Flow Reserve After Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2022, 5, e2232842. [Google Scholar] [CrossRef]
- Chung, J.H.; Ann, S.H.; Koo, B.K.; Nam, C.W.; Doh, J.H.; Singh, G.B.; Kim, H.I.; Shin, E.S. Assessment of stent edge dissections by fractional flow reserve. Int. J. Cardiol. 2015, 15, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.W.; Yoon, H.J.; Cho, Y.K.; Park, H.S.; Kim, H.; Hur, S.H.; Kim, Y.N.; Chung, I.S.; Koo, B.K.; Tahk, S.J.; et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: Fractional flow reserve-guided versus intravascular ultrasound-guided. JACC Cardiovasc. Interv. 2010, 3, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Kaziród-Wolski, K.; Sielski, J.; Gąsior, M.; Bujak, K.; Hawranek, M.; Pyka, Ł.; Gierlotka, M.; Pawłowski, T.; Siudak, Z. Factors affecting short- and long-term survival of patients with acute coronary syndrome treated invasively using intravascular ultrasound and fractional flow reserve: Analysis of data from the Polish Registry of Acute Coronary Syndromes 2017–2020. Kardiol. Pol. 2023, 81, 265–272. [Google Scholar] [CrossRef]
- Modi, B.N.; Sankaran, S.; Kim, H.J.; Ellis, H.; Rogers, C.; Taylor, C.A.; Rajani, R.; Perera, D. Predicting the Physiological Effect of Revascularization in Serially Diseased Coronary Arteries. Circ. Cardiovasc. Interv. 2019, 12, e007577. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Rudziński, P.N.; Demkow, M.; Kępka, C. Is the Majority Benefitting at the Costs of the Minority Among Patients Undergoing CTA as the First-Line Diagnostic in Highly Suspected Coronary Artery Disease? JACC Cardiovasc. Imaging 2019, 12, 944. [Google Scholar] [CrossRef]
- Tang, C.X.; Qiao, H.Y.; Zhang, X.L.; Di Jiang, M.; Schoepf, U.J.; Rudziński, P.N.; Giovagnoli, D.P.; Lu, M.J.; Li, J.H.; Wang, Y.N.; et al. Functional CAD-RADS using FFRCT on therapeutic management and prognosis in patients with coronary artery disease. Eur. Radiol. 2022, 32, 5210–5221. [Google Scholar] [CrossRef]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Apostolou, I.; Papathanasiou, L.; Papagikas, P.; Hamilos, M.; Aggeli, C.; Spanos, A.; Milkas, A. Noninvasive estimation of Fraction Flow Reserve (FFR): The first real-time FFRangio™ application in Greece. Hell. J. Cardiol. 2019, 60, 324–326. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, M.; Xiang, R.; Han, B.; Chang, J.; Zuo, Z.; Wu, F.; Ma, K. Diagnostic performance of computed tomography-based fraction flow reserve in identifying myocardial ischemia caused by coronary artery stenosis: A meta-analysis. Hell. J. Cardiol. 2022, 63, 1–7. [Google Scholar] [CrossRef]
- Nishi, T.; Murai, T.; Ciccarelli, G.; Shah, S.V.; Kobayashi, Y.; Derimay, F.; Waseda, K.; Moonen, A.; Hoshino, M.; Hirohata, A.; et al. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ. Cardiovasc. Interv. 2019, 12, e007889. [Google Scholar] [CrossRef]
- Geng, Y.; Wu, X.; Liu, H.; Zheng, D.; Xia, L. Index of microcirculatory resistance: State-of-the-art and potential applications in computational simulation of coronary artery disease. J. Zhejiang Univ. B 2022, 23, 123–140. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Oxford Acute Myocardial Infarction (OXAMI) Study Investigators; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Takahashi, T.; Rios, S.A.; Latib, A.; Lee, J.M.; Fearon, W.F.; Kobayashi, Y. Diagnostic performance and prognostic impact of coronary angiography-based Index of Microcirculatory Resistance assessment: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2022, 99, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Terentes-Printzios, D.; Shanmuganathan, M.; Marin, F.; Scarsini, R.; Bradley-Watson, J.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.; Kharbanda, R.K.; et al. Long-Term Clinical Outcomes in Patients With an Acute ST-Segment-Elevation Myocardial Infarction Stratified by Angiography-Derived Index of Microcirculatory Resistance. Front. Cardiovasc. Med. 2021, 8, 717114. [Google Scholar] [CrossRef]
- Sans-Roselló, J.; Fernández-Peregrina, E.; Duran-Cambra, A.; Carreras-Mora, J.; Sionis, A.; Álvarez-García, J.; García-García, H.M. Prognostic Value of Microvascular Resistance at Rest in Patients With Takotsubo Syndrome. JACC Cardiovasc. Imaging 2022, 15, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).