Ferric Carboxymaltose in Patients with Acute Decompensated Heart Failure and Iron Deficiency: A Real-Life Study
Abstract
1. Introduction
Aim of the Study
2. Methods
2.1. Outcomes
2.2. Statistical Analysis
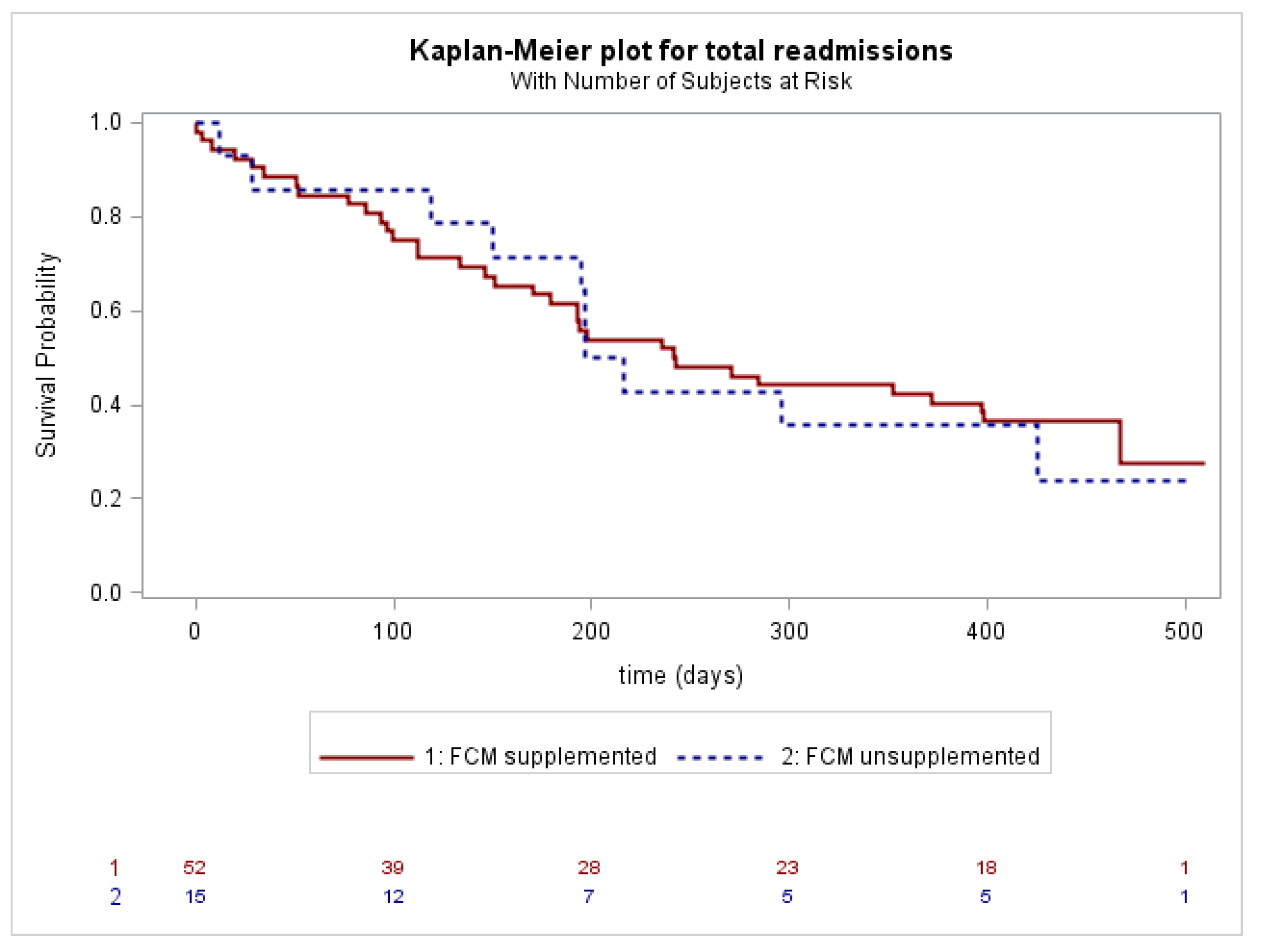
3. Results
Baseline Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Hearth J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Gremmler, U.; Krumm, M.; Mibach, F.; Schön, N.; Taggeselle, J.; Dahm, J.B.; Angermann, C.E. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: The PrEP Registry. Clin. Res. Cardiol. 2017, 106, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Hearth J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; von Haehling, S.; Doehner, W.; Banasiak, W.; et al. Iron Deficiency Predicts Impaired Exercise Capacity in Patients with Systolic Chronic Heart Failure. J. Card. Fail. 2011, 17, 899–906. [Google Scholar] [CrossRef]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Taher, A.T. Iron deficiency beyond erythropoiesis: Should we be concerned? Curr. Med. Res. Opin. 2018, 34, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; Van Veldhuisen, D.J.; De Boer, R.A.; Van Der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef]
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.J.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients with Symptomatic Chronic Heart Failure and Iron Deficiency: FERRIC-HF: A Randomized, Controlled, Observer-Blinded Trial. J. Am. Coll. Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ponikowski, P.; Van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef]
- Beck-Da-Silva, L.; Piardi, D.; Soder, S.; Rohde, L.E.; Pereira-Barretto, A.C.; de Albuquerque, D.; Bocchi, E.; Vilas-Boas, F.; Moura, L.Z.; Montera, M.W.; et al. IRON-HF study: A randomized trial to assess the effects of iron in heart failure patients with anemia. Int. J. Cardiol. 2013, 168, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, D.J.; Ponikowski, P.; Van Der Meer, P.; Metra, M.; Böhm, M.; Doletsky, A.; Voors, A.A.; MacDougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of Ferric Carboxymaltose on Exercise Capacity in Patients with Chronic Heart Failure and Iron Deficiency. Circulation 2017, 136, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.M.; Januzzi, J.L.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Kirwan, B.-A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Hearth Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef]
- Wienbergen, H.; for the RAID-HF (Registry Analysis of Iron Deficiency-Heart Failure) Study Group; Pfister, O.; Hochadel, M.; Fach, A.; Backhaus, T.; Bruder, O.; Remppis, B.A.; Maeder, M.T.; von Scheidt, W.; et al. Long-term effects of iron deficiency in patients with heart failure with or without anemia: The RAID-HF follow-up study. Clin. Res. Cardiol. 2019, 108, 93–100. [Google Scholar] [CrossRef]
- Ponikowski, P.; Kirwan, B.-A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2018, 73, 115–123. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. 1965, 14, 61–65. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- AHA Medical/Scientific Statement. Revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation 1994, 90, 644–645. [Google Scholar]
- Mahler, D.A.; Wells, C.K. Evaluation of Clinical Methods for Rating Dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nutritional Anaemias. Report of a WHO Group of Experts; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1972; Volume 503, pp. 1–29.
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.; Sindone, A.; Van Der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: Putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur. J. Hearth Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef]


| (n = 90) | With ID (n = 73) | Without ID (n = 17) | p Value | |
|---|---|---|---|---|
| Sex, n (%) | 0.2120 | |||
| Male | 57 (63.3) | 44 (60.3) | 13 (76.5) | |
| Female | 33 (36.7) | 29 (39.7) | 4 (23.5) | |
| Age (years), mean ± SD | 82.7 ± 9.7 | 82.9 ± 8.9 | 82.1 ± 12.7 | 0.7722 |
| BMI, kg/m2, mean ± SD | 27.1 ± 5.9 | 26.9 ± 5.7 | 28.2 ± 6.9 | 0.4822 |
| Barthel index, mean ± SD | 48.5 ± 25.5 | 49.1 ± 27.0 | 45.9 ± 18.6 | 0.6447 |
| CCI, n (%) | 0.6928 | |||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 1 | 12 (13.5) | 9 (12.5) | 3 (17.7) | |
| 2+ | 77 (86.5) | 63 (87.5) | 14 (82.4) | |
| Comorbidities, n (%) | ||||
| Coronary artery disease | 46 (51.1) | 39 (53.4) | 7 (41.2) | 0.3629 |
| Hypertension | 72 (80.0) | 57 (78.1) | 15 (88.2) | 0.5068 |
| COPD | 45 (50.0) | 35 (48.0) | 10 (58.8) | 0.4191 |
| Diabetes Mellitus | 35 (38.9) | 28 (38.4) | 7 (41.2) | 0.8299 |
| Malignancy | 24 (26.7) | 20 (27.4) | 4 (23.5) | 1.0000 |
| AF/AFL | 21 (23.3) | 17 (23.3) | 4 (23.5) | 1.0000 |
| Chronic Kidney Disease | 16 (17.8) | 12 (16.4) | 4 (23.5) | 0.4925 |
| Medications, n (%) | ||||
| ACEi/ARB | 41 (45.6) | 34 (46.6) | 7 (41.2) | 0.6873 |
| Betablockers | 55 (61.1) | 46 (63.0) | 9 (52.9) | 0.4429 |
| Loop diuretics | 63 (70.0) | 52 (71.2) | 11 (64.7) | 0.5969 |
| MRAs | 27 (30.0) | 21 (28.8) | 6 (35.3) | 0.5969 |
| ARNi | 2 (2.2) | 0 (0.0) | 2 (11.8) | 0.0340 |
| Digoxin | 3 (3.3) | 3 (4.7) | 0 (0.0) | 1.0000 |
| Antiplatelets | 35 (38.9) | 30 (41.1) | 5 (29.4) | 0.3735 |
| Anticoagulants | 52 (57.8) | 41 (56.2) | 11 (64.7) | 0.5215 |
| NYHA, n (%) | 0.2427 | |||
| 1 | 3 (3.5) | 2 (2.8) | 1 (6.3) | |
| 2 | 15 (17.2) | 12 (16.9) | 3 (18.8) | |
| 3 | 34 (39.1) | 31 (43.7) | 3 (18.8) | |
| 4 | 35 (40.2) | 26 (36.6) | 9 (56.3) | |
| mMRC, n (%) | 0.7758 | |||
| 1 | 1 (1.2) | 1 (1.5) | 0 (0.0) | |
| 2 | 3 (3.7) | 3 (4.5) | 0 (0.0) | |
| 3 | 6 (7.4) | 6 (9.0) | 0 (0.0) | |
| 4 | 16 (19.8) | 13 (19.4) | 3 (21.4) | |
| 5 | 38 (46.9) | 29 (43.3) | 9 (64.3) | |
| 6 | 17 (21.0) | 15 (22.4) | 2 (14.3) | |
| LVEF, n (%) | 0.5573 | |||
| >50% | 46 (53.5) | 39 (55.7) | 7 (43.8) | |
| 40–50% | 15 (17.5) | 13 (18.6) | 2 (12.5) | |
| 30–40% | 15 (17.4) | 11 (15.7) | 4 (25.0) | |
| <30% | 10 (11.6) | 7 (10.0) | 3 (18.8) | |
| LVEF, n (%) | 0.3867 | |||
| >50% | 46 (53.5) | 39 (55.7) | 7 (43.8) | |
| ≤50% | 40 (46.5) | 31 (44.3) | 9 (56.3) | |
| Hb (g/L), mean ±SD | 116.1 ± 20.3 | 113.4 ± 20.1 | 127.9 ± 17.0 | 0.0071 |
| Hb < 120 (F) o <130 (M) g/L, n (%) | 60 (66.7) | 51 (69.9) | 9 (52.9) | 0.1825 |
| BNP, ng/L, median (IQR) | 786 (458, 1500) | 801 (484, 1511) | 545 (275, 1463) | 0.1199 |
| eGFR, ml/min/1.73 m2, median (IQR) | 50.5 (37.0, 65.4) | 49.8 (36.3, 64.0) | 64.0 (46.8, 81.6) | 0.1504 |
| HbA1c, mmol/mol, median (IQR) | 44.0 (39.0, 52.0) | 45.0 (39.0, 52.0) | 42.5 (37.0, 48.0) | 0.2913 |
| TSH, mIU/L, median (IQR) | 1.53 (0.91, 2.33) | 1.44 (0.88, 2.38) | 1.69 (1.20, 2.09) | 0.7245 |
| Ferritin, ug/L, median (IQR) | 70.0 (34.0, 225.0) | 58.0 (27.0, 116) | 469.0 (299.0, 684) | <0.0001 |
| Serum iron, umol/L, median (IQR) | 6.1 (4.3, 9.2) | 6.0 (4.2, 8.0) | 10.7 (7.1, 11.2) | 0.0037 |
| TSAT, %, median (IQR) | 10.3 (7.1, 16.3) | 9.6 (6.0, 12.5) | 18.5 (12.3, 22.8) | 0.0005 |
| (n = 73) | FCM Supplemented (n = 55) | FCM Unsupplemented (n = 18) | p Value | |
|---|---|---|---|---|
| Sex, n (%) | 0.2327 | |||
| Male | 44 (60.3) | 31 (56.4) | 13 (72.2) | |
| Female | 29 (39.7) | 24 (43.6) | 5 (27.8) | |
| Age (years), mean ± SD | 82.9 ± 8.9 | 83.0 ± 8.4 | 82.6 ± 10.7 | 0.8618 |
| BMI, kg/m2, mean ± SD | 26.9 ± 5.7 | 27.3 ± 6.0 | 25.9 ± 4.9 | 0.3873 |
| Barthel index, mean ± SD | 49.1 ± 27.0 | 45.4 ± 25.6 | 60.9 ± 28.6 | 0.0377 |
| CCI, n (%) | 1.0000 | |||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 1 | 9 (12.5) | 7 (12.7) | 2 (11.8) | |
| 2+ | 63 (87.5) | 48 (87.3) | 15 (88.2) | |
| Comorbidities, n (%) | ||||
| Coronary artery disease | 39 (53.4) | 28 (50.9) | 11 (61.1) | 0.4513 |
| Hypertension | 57 (78.1) | 43 (78.2) | 14 (77.8) | 1.0000 |
| COPD | 35 (48.0) | 30 (34.6) | 5 (27.8) | 0.0485 |
| Diabetes Mellitus | 28 (38.4) | 23 (41.8) | 5 (27.8) | 0.2876 |
| Malignancy | 20 (27.4) | 15 (27.3) | 5 (27.8) | 1.0000 |
| AF/AFL | 17 (23.3) | 14 (25.5) | 3 (16.7) | 0.5361 |
| Chronic Kidney Disease | 12 (16.4) | 8 (14.6) | 4 (22.2) | 0.4744 |
| Medications, n (%) | ||||
| ACEi/ARB | 34 (46.6) | 26 (47.3) | 8 (44.4) | 0.8346 |
| Betablockers | 46 (63.0) | 37 (67.3) | 9 (50.0) | 0.1876 |
| Loop diuretics | 52 (71.2) | 42 (76.4) | 10 (55.6) | 0.0905 |
| MRAs | 21 (28.8) | 16 (29.1) | 5 (27.8) | 0.9149 |
| ARNi | 0 (0.0) | 0 (0.0) | 0 (0.0) | -- |
| Digoxin | 3 (4.7) | 3 (6.0) | 0 (0.0) | 1.0000 |
| NYHA, n (%) | 0.7788 | |||
| 1 | 2 (2.8) | 1 (1.9) | 1 (5.9) | |
| 2 | 12 (16.9) | 9 (16.7) | 3 (17.7) | |
| 3 | 31 (43.7) | 24 (44.4) | 7 (41.2) | |
| 4 | 26 (36.6) | 20 (37.0) | 6 (35.3) | |
| mMRC, n (%) | 0.6897 | |||
| 1 | 1 (1.5) | 1 (2.0) | 0 (0.0) | |
| 2 | 3 (4.5) | 2 (3.9) | 1 (6.3) | |
| 3 | 6 (9.0) | 5 (9.8) | 1 (6.3) | |
| 4 | 13 (19.4) | 8 (15.7) | 5 (31.3) | |
| 5 | 29 (43.3) | 24 (47.1) | 5 (31.3) | |
| 6 | 15 (22.4) | 11 (21.6) | 4 (25.0) | |
| EF, n (%) | 0.1234 | |||
| >50% | 39 (55.7) | 31 (58.5) | 8 (47.1) | |
| 40–50% | 13 (18.6) | 9 (17.0) | 4 (23.5) | |
| 30–40% | 11 (15.7) | 10 (18.9) | 1 (5.9) | |
| <30% | 7 (10.0) | 3 (5.7) | 4 (23.5) | |
| EF, n (%) | 0.4090 | |||
| >50% | 39 (55.7) | 31 (58.5) | 8 (47.1) | |
| ≤50% | 31 (44.3) | 22 (41.5) | 9 (52.9) | |
| Hb (g/L), mean ± SD | 113.4 ± 20.1 | 110.1 ± 20.7 | 123.4 ± 14.5 | 0.0136 |
| Hb < 120 (F) o < 130 (M) g/L, n (%) | 51 (69.9) | 41 (74.6) | 10 (55.6) | 0.1275 |
| BNP, ng/L, median (IQR) | 801 (484, 1511) | 798 (463, 1442) | 878 (526, 3775) | 0.2207 |
| eGFR, ml/min/1.73 m2, median (IQR) | 49.8 (36.3, 64.0) | 49.3 (35.9, 64.8) | 50.3 (41.2, 63.2) | 0.7460 |
| HbA1c, %, median (IQR) | 45.0 (39.0, 52.0) | 44.0 (39.0, 53.0) | 46.0 (42.0, 49.0) | 0.8174 |
| TSH, mIU/L, median (IQR) | 1.44 (0.88, 2.38) | 1.35 (0.86, 2.19) | 1.89 (0.97, 3.00) | 0.2871 |
| Ferritin, µg/L, median (IQR) | 58.0 (27.0, 116) | 51.5 (27.0, 104) | 93.0 (39.0, 143) | 0.2937 |
| Serum iron, umol/L, median (IQR) | 6.0 (4.2, 8.0) | 5.8 (4.0, 6.8) | 8.1 (5.9, 10.3) | 0.0308 |
| TSAT, %, median (IQR) | 9.6 (6.0, 12.5) | 8.7 (5.0, 11.6) | 12.8 (10.6, 19.6) | 0.0019 |
| With ID (n = 67) | Without ID (n = 15) | p Value | FCM Supplemented (n = 52) | FCM Unsupplemented (n = 15) | p Value | |
|---|---|---|---|---|---|---|
| Death, n (%) | 13 (19.4) | 2 (13.3) | 0.7228 | 10 (19.2) | 3 (20.0) | 1.0000 |
| All-cause readmissions, n (%) | 44 (65.7) | 11 (73.3) | 0.8150 | 34 (65.4) | 10 (66.7) | 0.9266 |
| Readmissions for heart failure or cardiovascular events, n (%) | 31 (46.3) | 8 (53.3) | 0.7882 | 26 (50.0) | 5 (33.3) | 0.2541 |
| LOS (days), median (Q1, Q3) | 9 (7, 13) | 9 (7, 13) | 0.4017 | 11 (8, 15) | 6 (5, 9) | 0.0002 |
| Mean Differences within Group | Mean Differences between Groups (Follow-Up) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCM Supplemented | FCM Unsupplemented | ||||||||||
| Admission (SE) | Follow-Up (SE) | Mean Difference (SE) | p Value | Admission (SE) | Follow-Up (SE) | Mean Difference (SE) | p Value | Mean Difference (SE) | p Value § | Effect Size d | |
| Ferritin, ug/L | 143.8 (10.7) | 222.7 (17.4) | 78.9 (20.8) | 0.0015 | 180.7 (14.6) | 56.7 (22.4) | −124.0 (27.1) | <0.0001 | −166.1 (28.9) | <0.0001 | 0.63 |
| TSAT, % | 10.7 (0.7) | 19.4 (1.2) | 8.7 (1.3) | <0.0001 | 12.8 (1.0) | 16.6 (1.6) | 3.7 (1.8) | 0.1576 | 2.8 (2.0) | 0.0268 | 0.65 |
| Hb, g/L | 114.4 (1.5) | 128.3 (2.4) | 13.8 (3.0) | <0.0001 | 118.2 (2.0) | 120.9 (3.2) | 2.74 (3.9) | 0.8925 | 10.1 (3.3) | 0.0255 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capone, F.; Cipriani, A.; Molinari, L.; Noale, M.; Gusella, B.; Lucente, F.; Savino, S.; Bertomoro, A.; Saller, A.; Giannini, S.; et al. Ferric Carboxymaltose in Patients with Acute Decompensated Heart Failure and Iron Deficiency: A Real-Life Study. J. Pers. Med. 2023, 13, 1250. https://doi.org/10.3390/jpm13081250
Capone F, Cipriani A, Molinari L, Noale M, Gusella B, Lucente F, Savino S, Bertomoro A, Saller A, Giannini S, et al. Ferric Carboxymaltose in Patients with Acute Decompensated Heart Failure and Iron Deficiency: A Real-Life Study. Journal of Personalized Medicine. 2023; 13(8):1250. https://doi.org/10.3390/jpm13081250
Chicago/Turabian StyleCapone, Federico, Alberto Cipriani, Leonardo Molinari, Marianna Noale, Beatrice Gusella, Fabrizio Lucente, Sandro Savino, Antonella Bertomoro, Alois Saller, Sandro Giannini, and et al. 2023. "Ferric Carboxymaltose in Patients with Acute Decompensated Heart Failure and Iron Deficiency: A Real-Life Study" Journal of Personalized Medicine 13, no. 8: 1250. https://doi.org/10.3390/jpm13081250
APA StyleCapone, F., Cipriani, A., Molinari, L., Noale, M., Gusella, B., Lucente, F., Savino, S., Bertomoro, A., Saller, A., Giannini, S., & Vettor, R. (2023). Ferric Carboxymaltose in Patients with Acute Decompensated Heart Failure and Iron Deficiency: A Real-Life Study. Journal of Personalized Medicine, 13(8), 1250. https://doi.org/10.3390/jpm13081250








