1. Introduction
Bipolar disorder (BD), a devastating mental illness affecting over 2.5% of the population, is characterized by mood fluctuations ranging from mania or hypomania to depression with significant subsyndromal symptoms that commonly present between major mood episodes [
1]. In the course of bipolar illness, the bipolar depressed phase (BDD) is challenging to manage and, if undertreated, it can lead to worse outcomes, including higher levels of cognitive and functional impairment [
2] and increased suicidal risk as compared to patients experiencing hypomania or mania [
3,
4]. Treatment-resistant bipolar depression (TRBDD) is defined as failed attempts to achieve remission after 8 weeks of at least two separate monotherapeutic treatments or one monotherapy with one combination treatment [
5]. The subjective nature in diagnosing BD highlights the unmet need for accurate biomarkers to support the diagnosis, monitor and predict clinical outcomes, and ultimately help arrest the neuroprogressive course of the disease.
Dysregulation of the immune system has emerged as a major contributor to the pathophysiology of several affective disorders, including BD, with evidence based on elevated levels of circulating immune markers, notably altered release of cytokines, and inflammatory changes in the central nervous system [
6,
7,
8,
9]. Chronic inflammation has been correlated to treatment refractoriness in BD, BDD, MDD, and schizophrenia through several biological mechanisms related to cytokine and proinflammatory mediators, alterations in neurotrophins, microglial function, and increased oxidative stress [
8,
9,
10,
11,
12,
13]. A prospective theory linking inflammation and treatment resistance stems from the reconceptualized nature of BD as a neuroprogressive disorder [
8,
9,
14].
A few clinical trials have targeted the immune system in BD subjects through administering treatment with adjunctive inflammatory modulation via the cyclooxygenase-2 (COX-2) inhibitor, celecoxib (CBX), with promising findings of accelerated treatment response due to the constitutional expression of COX-2 in the brain [
15,
16]. Regarding other psychiatric illnesses, CBX, in addition to mainstay treatment, has shown evidence of improved treatment response and remission in MDD [
17] and marked improvement in positive and negative symptoms in schizophrenia [
18]. In our main clinical study, we demonstrated the efficacy of CBX add-on therapy in TRBDD where the patient group treated with CBX in addition to the antidepressant, ESC, experienced significantly higher rates of treatment response and remission compared to the group receiving ESC and a placebo add-on in a randomized trial [
10]. The present follow-up study was undertaken to further characterize the observed improvement in treatment response in TRBDD patients treated with add-on CBX by means of the Systemic Immune-Inflammation Index (SII) and to seek possible correlations with select inflammatory biomarkers, which may also reflect, at least in part, the presumptive neuroinflammatory process occurring in the CNS.
The SII is a composite biomarker calculated from the product of absolute neutrophils and platelets, divided by the number of lymphocytes available in the complete blood count (CBC with differential). Developed in 2014 by Hu et al. to predict the prognosis of patients with hepatocellular carcinoma, the SII has been shown to have prognostic value for several solid malignancies [
19,
20] and cardiovascular disease [
21,
22], while its use in psychiatric disorders has remained largely under-explored. Precursor indices to the SII, such as the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR), have been studied in BD, wherein the NLR was significantly elevated in BD versus healthy controls [
11,
23]. Studies in MDD and BDD have linked higher SII levels to severity of depression and/or mania [
23,
24,
25]. Demir et al. and Kinoshita et al. similarly found a direct relationship between depression severity and the NLR [
26,
27]. Zhou et al. reported that patients with MDD have a higher SII level compared with healthy controls [
24]. Dionisie et al. found the SII to be significantly higher in BDD patients compared to unipolar depression (and healthy controls), as well as higher SII and NLR in the manic phase of BDD [
23,
28]. The SII is a peripheral inflammation index by extrapolation and may reflect biological processes relevant to the neuroprogression of bipolar depression, such as oxidative stress by way of the neutrophil component, for example [
12].
Despite the aforementioned relationships between BDD and peripheral inflammation, to our knowledge, no studies have explored SII in the context of TRBDD. In the associated clinical study, we demonstrated improved response and remission rates in TRBDD patients receiving ESC + CBX compared to ESC + PBO [
10]. In this secondary biomarker study, our aim was to characterize the relationship of the SII with the clinical response to treatment in the clinical study. We hypothesized that (1) elevated SII at baseline will discriminate TRBDD from HC subjects; (2) baseline SII is associated with abnormal levels of circulating immune-metabolic biomarkers; and (3), baseline SII is associated with post-treatment clinical outcomes by treatment arm.
2. Materials and Methods
2.1. Study Population
Males and females aged between 21 and 65 with a diagnosis of BD I or II based on the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) and who met criteria for TRBDD with a minimum score of 18 on the 17-item Hamilton Depression Scale (HAMD-17) were considered for the study. The sample (
N = 69) included 65.2% female, 65.2% white, mean age 42 years (SD = 12.7). Study participants did not have any other medical diagnosis. A comorbid psychiatric diagnosis, with the exception of anxiety disorder, was an exclusionary criterion. To be classified as treatment resistant, participants had to have previously failed two adequate trials of antidepressants and/or a mood stabilizer or atypical antipsychotic medication, as outlined by the Maudsley Staging Method (MSM) [
29]. This is in accord with the definition of TRBD-De, as described in the review article by Fornaro et al. [
30]. Patients had to be clinically stable on either a mood stabilizer and/or antipsychotic medication for at least two weeks before entering the study.
A history of substance use or dependence within 12 months preceding the screening visit was exclusionary. Patients were excluded in the presence of any abnormal routine laboratory examinations, a pain condition including fibromyalgia, history of peptic ulcer, uncontrolled hypertension, anemia, liver disease, kidney disease, arthritis, recurrent migraines, epilepsy, stroke, gum disease, autoimmune disease, pregnant or lactating females, and females taking oral contraceptives. Concurrent use of stimulants, anticoagulant agents, nicotine-containing substances, corticosteroids, or lithium was exclusionary. Celecoxib has the potential to increase lithium blood levels, leading to toxicity [
31]. Routine blood analyses were conducted to ensure normal ranges in the CBC, complete metabolic panel, lipid profile, and thyroid function. Urinalysis and urine drug screening were conducted to further exclude participants with underlying infection and/or drug use. Known allergies or hypersensitivities to the study medications and concomitant pharmacologic contraindications were additional exclusion criteria. During the initial screening visit, the study protocol was detailed to potential participants, and written informed consent was obtained as approved by the Institutional Review Board (IRB) of Loyola University Medical Center (LUMC).
2.2. Healthy Controls
The original RCT design for evaluating primary clinical outcomes did not include a healthy control (HC) group precisely matched to the patient group for key demographic parameters [
10]. For supplemental molecular analyses, an HC group was utilized from our database (included in the Supplemental Information) to compare against TRBDD subjects. Recruitment for HC subjects was conducted via flyers on the Loyola University Medical Center campus. Volunteers were required to provide written informed consent approved by the IRB before the screening process. Screening and exclusions criteria were similar to the TRBDD groups, with the key difference being a negative history of or concurrent mental illness. Subjects were excluded if they had any current medical conditions or significant history thereof. Regarding mental illness, HCs were excluded if there was any personal or family history in first-degree relatives for substance use and/or mental illness. HAMD-17 scores were required to be less than 5 on the rating scale. Blood samples were obtained once at the initial screening, as HCs did not receive any intervention. Based on our experience, measured values are stable, barring any intercurrent illness. HC subjects were enrolled if their routine laboratory tests fell within the normal range.
2.3. Study Design of Clinical Study
Full details of the study design and study flow chart can be found in our primary study [
10]. For ease of reference, the necessary details will be provided here. This was a 10-week, randomized, double-blind, placebo-controlled, two-arm study of TRBDD patients using escitalopram (ESC) in combination with an anti-inflammatory medication, Celecoxib (CBX). It included an initial screening visit, a 2-week minimum washout phase, a 1-week placebo run-in phase, and an 8-week flexible dosing phase. Males and females aged between 21 and 65 who were diagnosed with TRBDD while being mentally and physically capable of consenting to the study were considered. The study was powered for 70 patients (35 in each treatment arm) to complete 8 weeks of active medication with an anticipated 10% dropout rate based on experience with our patient population in the preceding five years. One treatment arm consisted of ESC in combination with CBX (
n = 26) while the other arm received ESC with PBO (
n = 21).
Screening visit 1 consisted of a physical exam, blood draws, and urinalysis to obtain CBC, CMP, thyroid function, lipid profile, hCG pregnancy test, and toxicology screen. Subjects were diagnosed with TRBDD through structured interviews using the Mini International Neuropsychiatric Interview (MINI) and the Maudsley Staging Scales. Depression severity and associated symptoms were quantified using Hamilton Rating Scales for Depression (HAMD) and Anxiety (HAMA), Clinical Global Impressions (CGI), and Columbia Suicide Severity Rating Scale (CSSRS). Psychiatric and family histories were obtained through interviewing and with focused questionnaires. After the 1-week placebo run-in phase, subjects were evaluated to effectively rule out placebo responders. Successful placebo non-responders were randomized in a 1:1 fixed assignment ration to receive either ESC + CBX or ESC + PBO. Our approach to stratification included two age groups (21–45 and 46–65) plus binary genders, and group assignment was based on a pharmacy-generated randomization code. The randomization code was generated by the study biostatistician and kept by the institutional pharmacist. Study medications were prepared by the pharmacist, sealed in envelopes, administered to subjects, and returned to the study coordinator after consumption to ensure compliance.
CBX was dosed at 200 mg twice daily, while ESC was started at 10 mg per day and later titrated up to 10 mg twice per day. However, several exceptions became necessary to optimize clinical response and minimize adverse side effects: in the ESC + CBX arm, 6 patients were dosed at 10 mg of ESC and 1 patient at 30 mg ESC; in the ESC + PBO arm, 3 patients were dosed at 10 mg ESC, and 2 patients were dosed at 30 mg and 40 mg ESC. Along with the study medication, patients were prescribed one or more of the following medications for mood stabilization as indicated: Quetiapine, Lamotrigine, Divalproex sodium, Buspirone, Topiramate, Ziprasidone, Oxcarbazepine, Gabapentin, Carbamazepine, Asenapine, Risperidone, Olanzapine, Aripiprazole, Zolpidem, and Lurasidone.
A minimum score of 18 on the HAMD-17 scale was required for enrollment. “Responders” to treatment were defined as those whose baseline HAMD-17 scored dropped by at least 50% by week 8 but was still above a score of 7. “Non-responders” were defined as subjects whose HAMD-17 scores dropped less than 50% by week 8. Remission was defined as a score of ≤7 on HAMD-17 at the treatment endpoint (week 8). For participants who dropped out of the study after week 6, their last observation was carried forward in the analysis.
2.4. Laboratory Measurements and Calculation of SII
Subjects returned for follow-up visits at weeks 1, 2, 4, and 8 for blood draws and medication management to assess safety and efficacy. Blood draws occurred consistently between 9 and 10 am to control for diurnal variations. For purposes of this secondary analysis, we utilized the complete blood count with differential (CBC w/diff) at two timepoints, baseline and week 8. The SII was calculated in the following manner:
2.5. Additional Blood Biomarkers
The following blood biomarkers were analyzed from two timepoints, baseline and week 8, to perform a correlational analysis with SII. Inflammation biomarkers included high-sensitivity C-reactive protein (CRP); the interleukins IL-1A, IL-2, IL-4, IL-6, IL-8, IL-10, IL-18; interferon gamma (IFN-γ); tumor necrosis factor alpha (TNF-α); and the chemokine monocyte chemoattractant protein 1 (MCP-1). The growth factors included epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF). The kynurenine pathway (KP) metabolites included tryptophan (TRP), kynurenine (KYN), Kynurenic acid (KYNA), 3-hydroxykynurenine (3HK), anthranilic acid (AA), xanthurenic acid (XA), picolinic acid (PIC), quinolinic acid (QUIN), and quinaldehyde (QUINA). Biologically pertinent KP metabolite ratios were also calculated, including KYN/TRP, KYNA/KYN, AA/KYNA, 3HK/KYNA, QUIN/PIC, and QUIN/KYNA.
Plasma samples were analyzed using the Zymutest High Sensitivity CRP enzyme-linked immunosorbent assay (ELISA) kit (Hyphen Biomed®, Neuville-sur-Oise, France). This is a highly sensitive “one step” sandwich ELISA technique specific for human CRP. Levels of cytokines and growth factors were measured using a Randox Cytokine and Growth Factors High-Sensitivity Array assay (Randox®, London, UK). This is a chemiluminescent immunoassay that operates on a sandwich principle similar to that used in ELISA. Procedures were followed according to the protocols for both assays. Kynurenine pathway metabolites were measured via Ultra Performance Liquid Chromatography/Mass Spectrometry (UPLC-MS), using a Waters Acquity UPLC connected to a Xevo TQ MS triple-quadrupole mass spectrometer, equipped with a Z-spray ESI ion source (Waters, Milford, MA, USA). Separation was carried out using a Kinetex XBC18, 2.6 μm, 2.1 × 150 mm column (Phenomenex, Torrance, CA, USA).
2.6. Clinical Outcome Variable
Depression severity was quantitated using the total score of the Hamilton Depression Rating Scale 17 Item (HAMD-17) administered at baseline and the treatment endpoint (week 8). The total HAMD-17 score was used as the primary clinical outcome (continuous variable). Secondary outcome variables were constructed (categorical, dichotomous), including treatment “response”, defined as a ≥50% reduction of the HAMD-17 total score between baseline and week 8, and treatment “remission”, defined as a HAMD-17 total score ≤7 at the treatment endpoint (week 8) regardless of baseline HAMD-17. For the purpose of this study, we only used the HAMD-17 total score, none of the other rating instruments.
2.7. Statistical Analysis
Statistical analysis was conducted using R-3.6.3. Associations with p-values < 0.05 were considered statistically significant, but p-values < 0.1 were also explored based on the exploratory nature of this study. BMI and biomarkers were natural-log-transformed to meet the assumption of normal distributions. We first performed a descriptive analysis of demographic, clinical, and biomarker variables comparing clinical subgroups (using t-tests, ANOVA, and chi-square). In order to screen for relevant covariates for later modelling, we then tabulated the univariate relationships of all variables with HAMD-17, SII, and individual cell counts using linear regressions.
Retrospective power analysis was conducted using an approximate correlation power calculation (arctangh transformation). In the retrospective power analysis, we calculated R = 0.377 based on parameters of N = 52, significance level of p = 0.05, and power = 0.80. The power calculation yielded R = 0.377, where R2 = 0.3772 = 0.142. Based on these findings, we would expect a biomarker term to explain 14.2% of the variance in the outcome (HAMD-17 week 8) with the above parameters.
In the subsequent modelling steps, dichotomous variables of sex, treatment arm, response, and remission were treated as dummy-coded variables with respective reference levels: male, placebo, non-responder, and non-remitter. In the first model, we contrasted the SII to HAMD-17 between treatment timepoints (baseline and week 8) using a robust linear mixed model with timepoint as the random variable. In the second model, we used multiple linear regression to describe post-treatment depressive severity (HAMD-17 at week 8) according to the SII, adjusting for demographics, treatment arm, and pre-treatment depressive severity (HAMD-17 at baseline). This model was finalized through the inclusion of significant interactions between the SII and neutrophils with each of the demographic variables, and this final model was depicted visually with interaction plots.
3. Results
3.1. Sample Characteristics and Group Comparisons (Table 1)
Our sample with available CBC data consisted of 52 TRBDD subjects and 32 HCs. Compared to HCs, the TRBDD sample had significantly fewer females (p = 0.016), elevated BMI (p < 0.001), and trending older age (p = 0.083). There were no significant group differences in baseline CBC-related biomarkers (neutrophils, monocytes, lymphocytes, or SII) when comparing HC to TRBDD subjects.
The TRBDD sample consisted of N = 23 in the placebo (ESC + PBO) arm and N = 29 in the treatment arm (ESC + CBX). On group comparison of TRBDD by treatment arm, ESC+ CBX was significantly younger compared to the placebo group (p = 0.026), but was similar in regard to sex and BMI. The baseline HAMD-17 was similar (p = 0.66) between arms, but the ESC + CBX arm had a significantly lower HAMD-17 score by week 8 (p = 0.007). This is congruent with significantly more remitters (p = 0.003) and trending toward more responders (p = 0.063) in the ESC + CBX arm by week 8. There were no significant differences in CBC-related biomarkers between treatment arms at either timepoint. There were no baseline differences in inflammatory markers between arms, but at week 8, CRP was lower (p = 0.004) and IL-1β trended lower (p = 0.093) in the ESC + CBX arm. Baseline VEGF trended higher (p = 0.057) and FGF trended lower (p = 0.074) in the ESC + CBX arm compared to the PBO arm at week 8. There were no differences in KP metabolites or ratios according to treatment arm at baseline, but at week 8, the ESC + CBX arm had a significantly higher KYNA/KYN ratio (p = 0.05) and lower AA/KYNA ratio (p = 0.038) compared to the PBO arm.
3.2. Univariate Relationships of Sample Characteristics with Baseline HAMD-17 (Table 2)
On univariate linear regression of patient characteristics by pre-treatment HAMD-17, there were no significant associations with demographic variables. Baseline HAMD-17 trended toward lower baseline levels of monocytes (p = 0.058) and higher MCP-1 at baseline (p = 0.087). Meanwhile, baseline HAMD-17 was significantly associated with lower IL-2 at baseline (p = 0.035), higher TNF-α at week 8 (p = 0.043), and lower QUINA at week 8 (p = 0.015).
3.3. Univariate Relationships of Sample Characteristics by Baseline SII (Table 3)
Baseline SII trended lower in female subjects (p = 0.087). Baseline SII was associated with higher platelet counts (p < 0.001), higher neutrophil counts (p < 0.001) at both timepoints, and lower lymphocyte counts at baseline only (p = 0.02). From the standpoint of inflammatory markers, baseline SII trended toward lower baseline IL-4 (p = 0.074) and lower baseline IFN-γ (p = 0.089); baseline SII was also associated with biomarkers at week 8, including higher IL-1B (p = 0.03) and higher CRP (p = 0.048). There was a significant positive association between baseline levels of SII and VEGF (p = 0.011). Meanwhile, baseline SII trended toward lower 3-HK at baseline (p = 0.082) and toward a higher baseline QUIN/KYNA ratio at baseline and week 8 (p = 0.06 and p = 0.09, respectively).
3.4. Modeling HAMD-17 by SII-to-Timepoint Relationship
The purpose of the first model was to assess whether the relationship between depressive severity and SII contrasts between baseline and week 8. To that end, we constructed a robust linear mixed effects model (
Table 4) with the total HAMD-17 score (continuous outcome variable) and SII being dependent on treatment timepoint (main dependent variables), adjusting for age, BMI and sex (dummy-coded categorical variable with ‘male’ as reference category), and BMI and treatment arm (dummy-coded dichotomous variable with reference level ‘ESC + PBO’). BMI was log-transformed to meet the assumption of normality. The SII-to-timepoint relationship was assessed using an interaction variable, SII*Timepoint (where Timepoint was a dummy-coded variable with baseline as the reference level), which was not significant
(p = 0.498).
3.5. Modelling HAMD18 (Week 8) by SII (Baseline)
The purpose of the second model (multiple linear regression,
Table 5) was to describe the association between the clinical outcome (HAMD-17 at week 8, continuous outcome variable) and the baseline biomarker (SII baseline, dependent variable of interest) in order to test whether the treatment outcome is modulated by the pre-treatment biomarker. This model was adjusted for age, BMI and sex (dummy-coded categorical variable with ‘male’ as reference category), and treatment arm (dummy-coded dichotomous variable with reference level ‘ESC + PBO’). We also included an adjustment for HAMD-17 (baseline), in order to control for variation in baseline depressive severity. There was no independent significant association (
p = 0.45) between the SII (baseline) and post-treatment depressive severity (HAMD-17, week 8).
3.6. Modelling HAMD-17 (Week 8) by SII (baseline), including Interaction Screen
We included an interaction screen with baseline SII (dependent variable of interest) in order to assess whether the effect of the SII (baseline) on HAMD-17 (week 8) is dependent on sex, age, BMI, and treatment arm. There was no direct covariance between the SII (baseline) and demographic variables (see
Table 3). Even though there were no independent effects of the SII (baseline) on HAMD-17 (week 8) in the prior model (
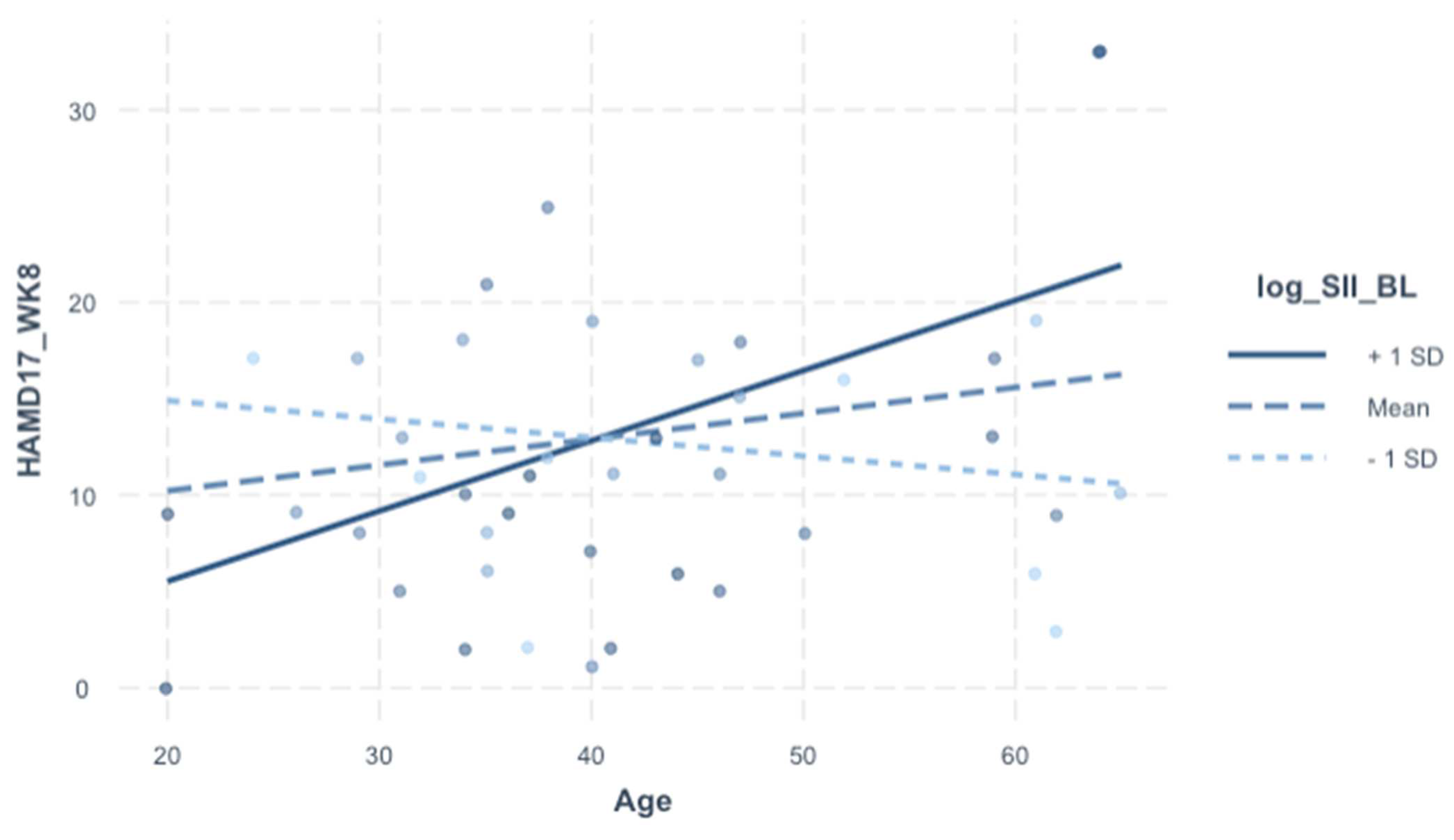
Table 5), the interaction screen was pursued because sex, age, and BMI are relevant for the SII and depression (both biologically and clinically). The interaction term was not significant for sex or BMI, but it was for age (β = 0.43, 95% CI [0.16–0.70],
p = 0.003). In the final model (
Table 6), the analytically pertinent variable is portrayed visually in
Figure 1; even the independent effects of the SII and age were statistically significant; they do not bear explanatory significance in relation to outcome in the context of this interaction model.
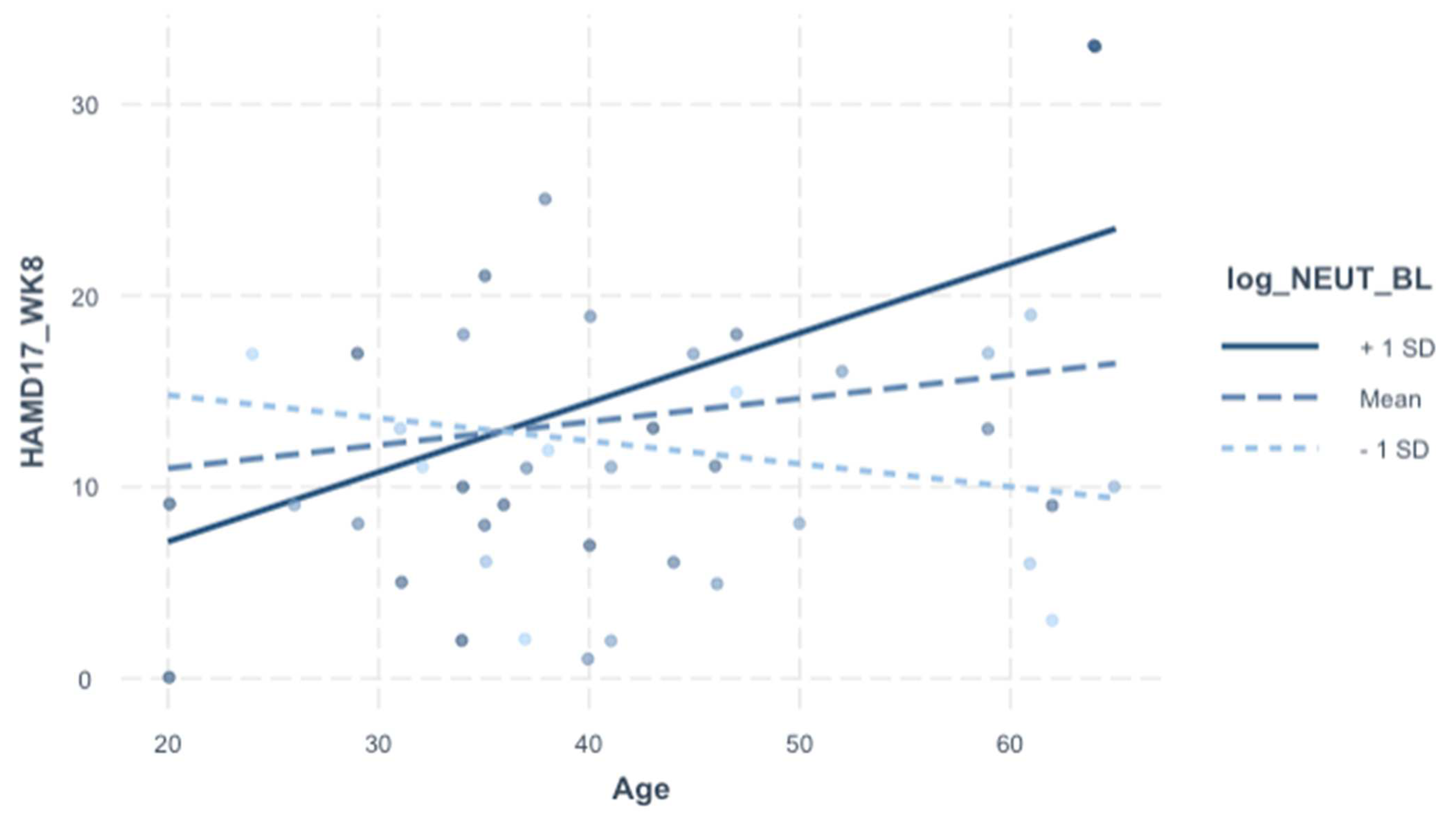
3.7. Relationships of HAMD-17 with Individual Cell Counts (Table 7 and Table 8)
For exploratory purposes, we included additional analysis into the individual cell counts integral to the SII (neutrophils, platelets, lymphocytes). The rationale was to delineate which cell type may be contributing to the significant SII-to-age interaction on HAMD-17 (Week 8) depicted in
Table 6. HAMD-17 (week 8) was significantly associated with age–neutrophils interaction specifically (but not with platelets or lymphocytes), and this relationship survived adjustment for demographics, baseline HAMD-17, and treatment arm (
p = 0.006) (
Table 7 and
Table 8,
Figure 2).
5. Conclusions, Strengths, and Limitations
To our knowledge, this is the first study of the SII in TRBDD. The central finding was an age-dependent predictive association with baseline SII and treatment outcomes. From a biological standpoint, this finding is compatible with the emerging notion that systemic inflammation is important for bipolar neuroprogression. Further, it invites exploration into mechanisms related to innate immunity that may contribute to an underlying vulnerability to treatment refractoriness in TRBDD. From a translational standpoint, the SII seems to bear prognostic value for TRBDD, with the advantages of being affordable and routinely accessible. Further studies are needed to better characterize the precise biological significance of the SII, including its relationships with other known immune-metabolic markers. One peculiarity worth noting is that the predictive value of baseline SII was not significantly dependent on treatment arm, even though the combination of ESC + CBX independently predicted treatment response compared to ESC + PBP. As such, given the design constraints of this secondary post hoc analysis, it is difficult to conclude whether baseline SII (amongst older patients with TRBDD) is a predictive marker for response to CBX augmentation or ESC, which was present in both arms. Larger follow-up studies are needed for further validation of these relationships. Taken together, these findings represent a novel, preliminary step to better clarify TRBDD mechanisms and the impact of systemic inflammation on treatment response.