GERD as a Complication of Laparoscopic Sleeve Gastrectomy for the Treatment of Obesity: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
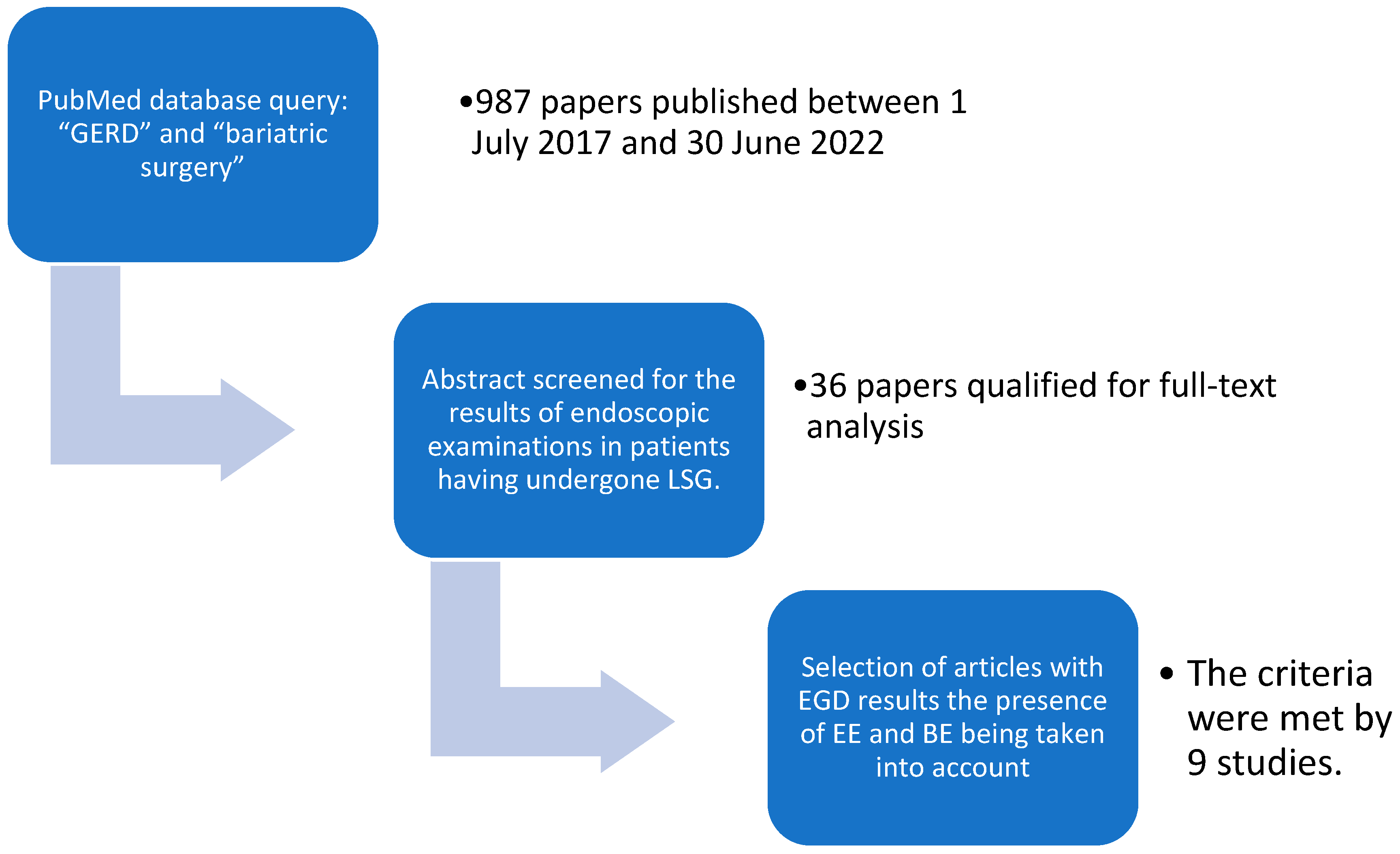
2. Materials and Methods
3. Results
3.1. Meta Analysis
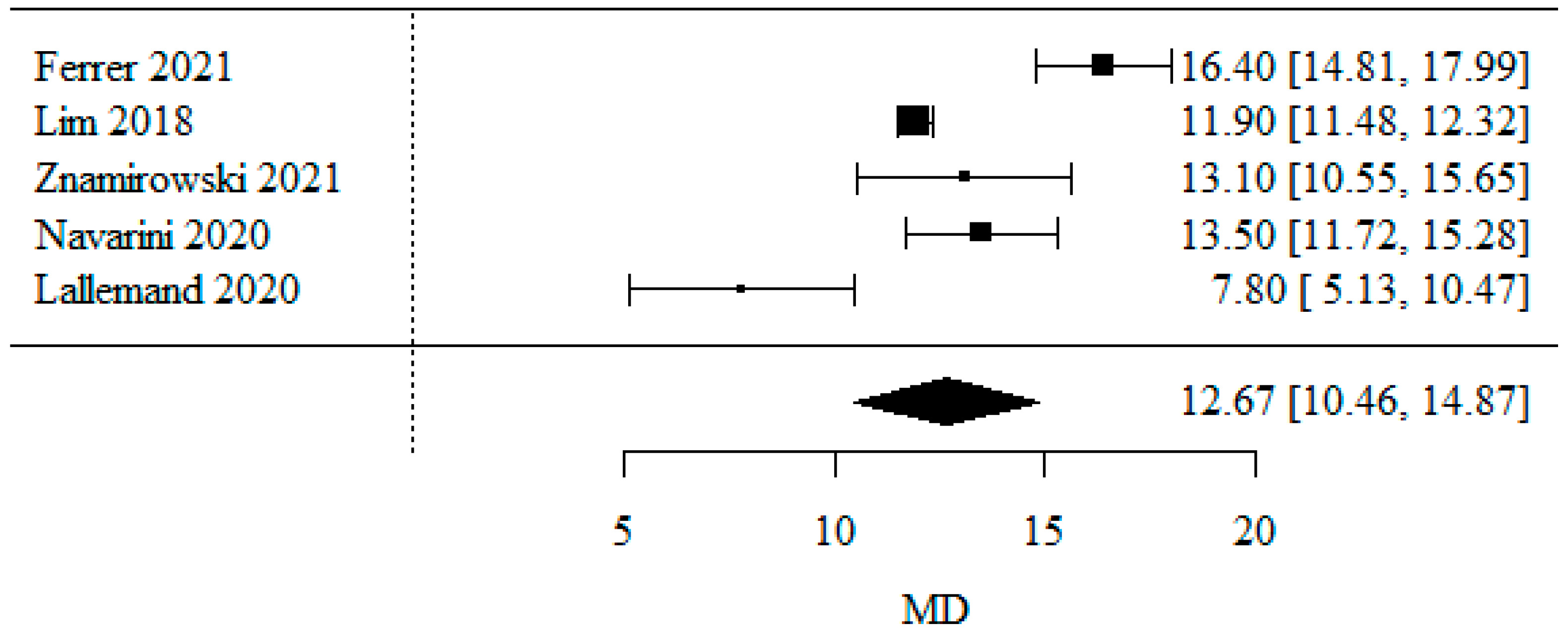
3.1.1. Body Mass Index
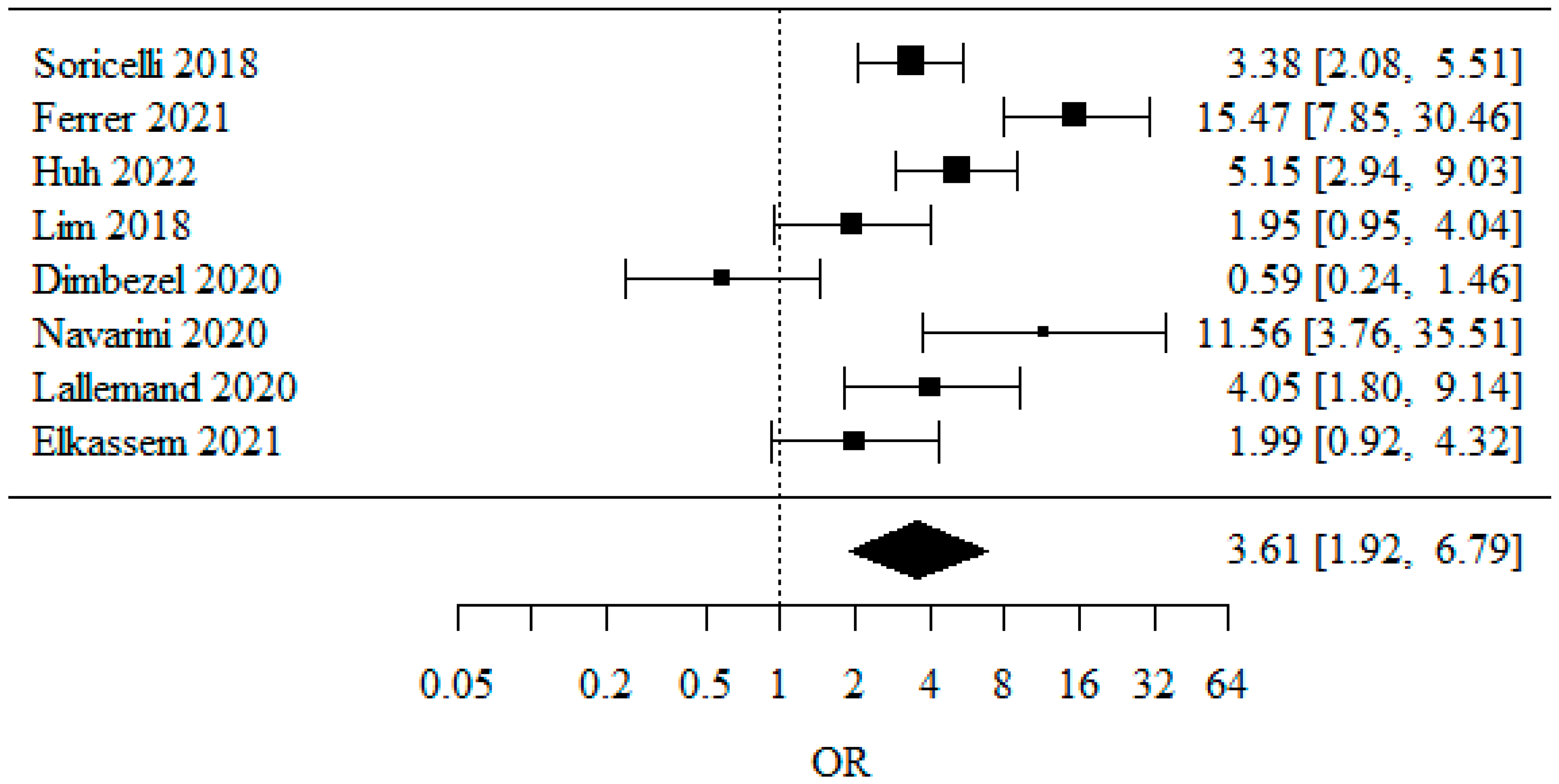
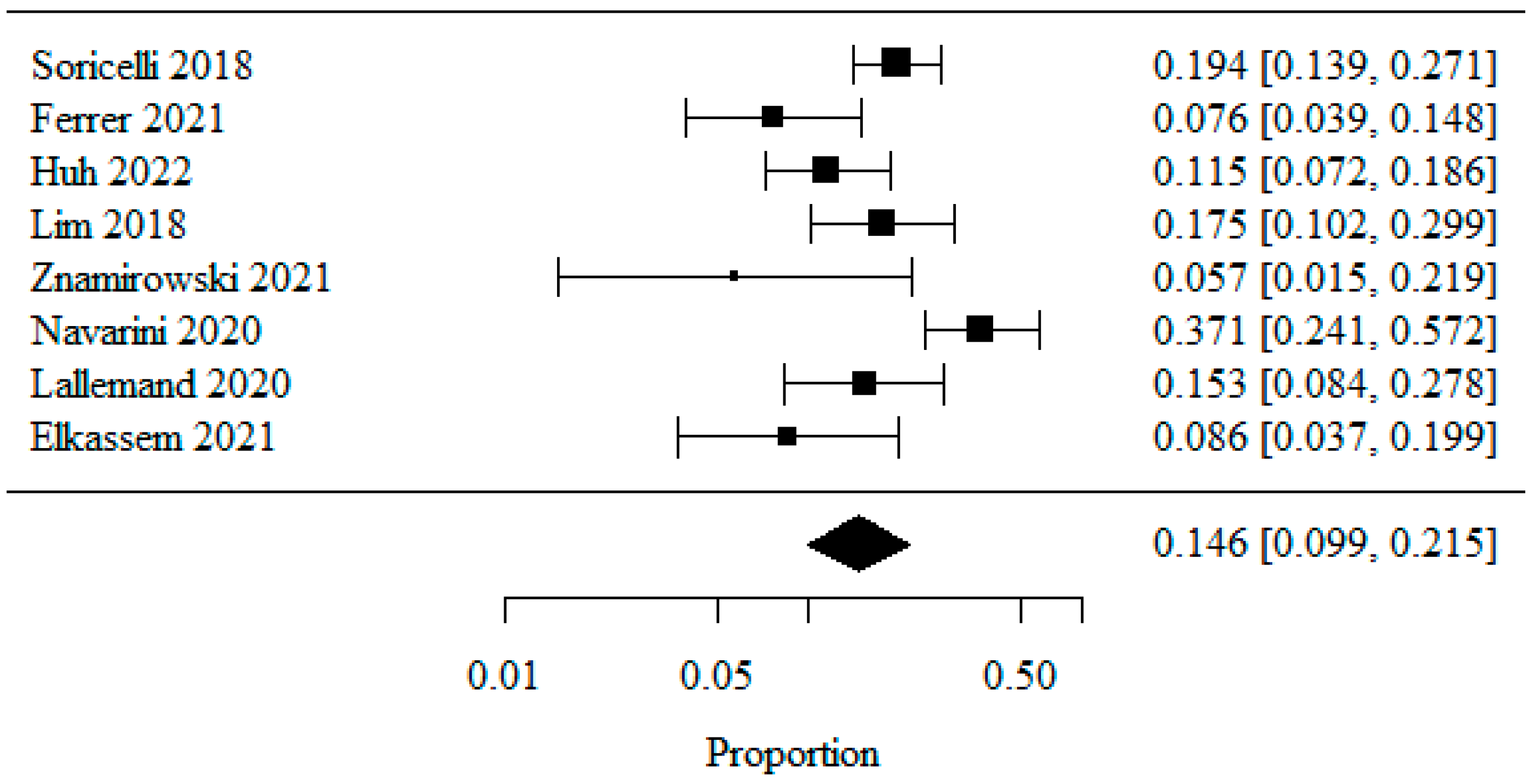
3.1.2. Overall GERD (Gastro-Esophageal Reflux Disease)
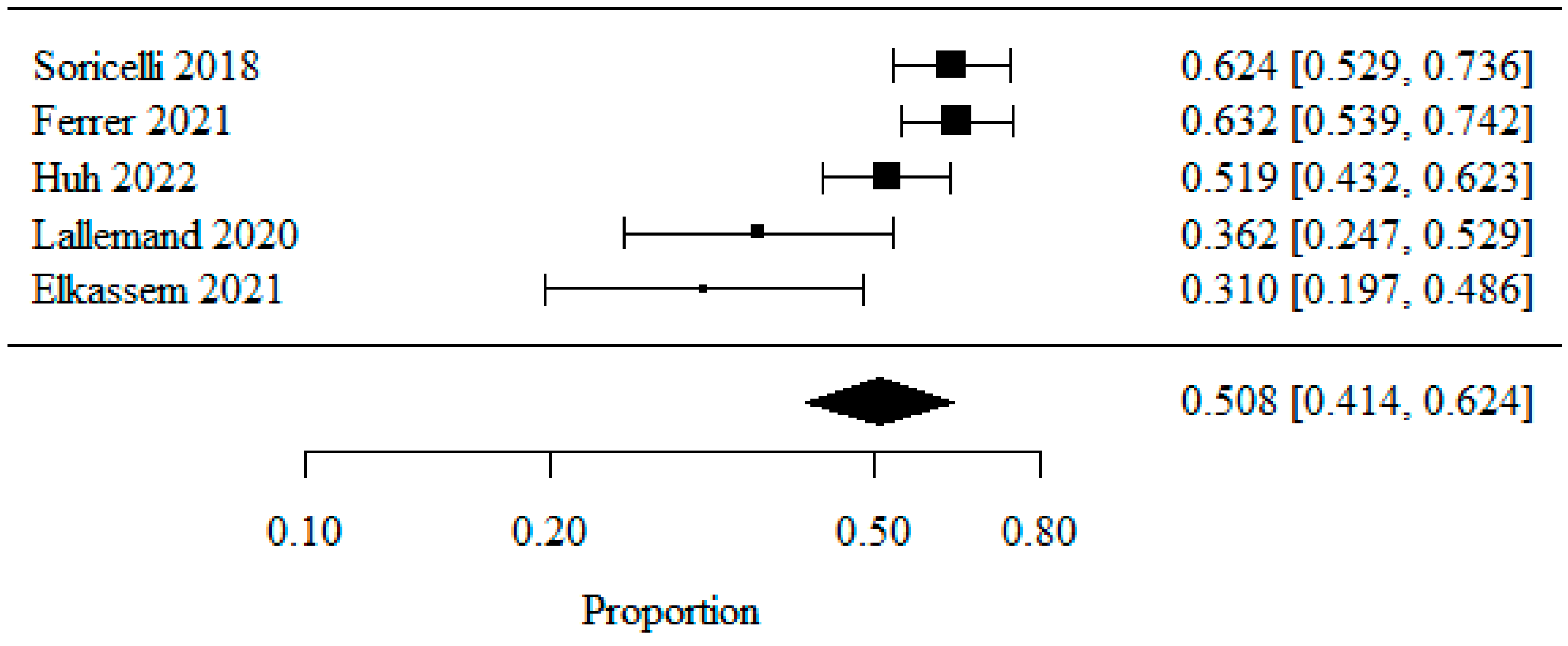
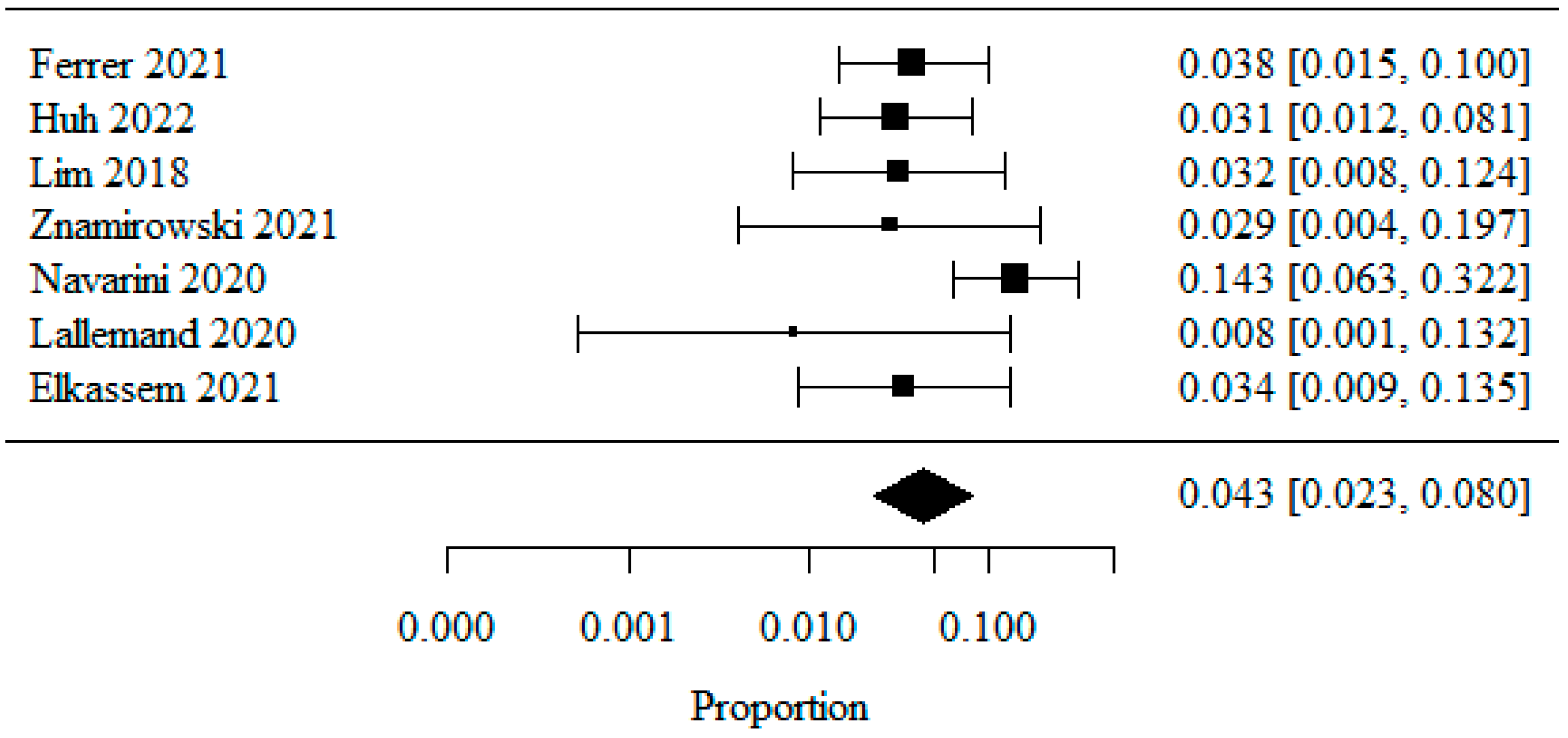
3.1.3. De Novo GERD
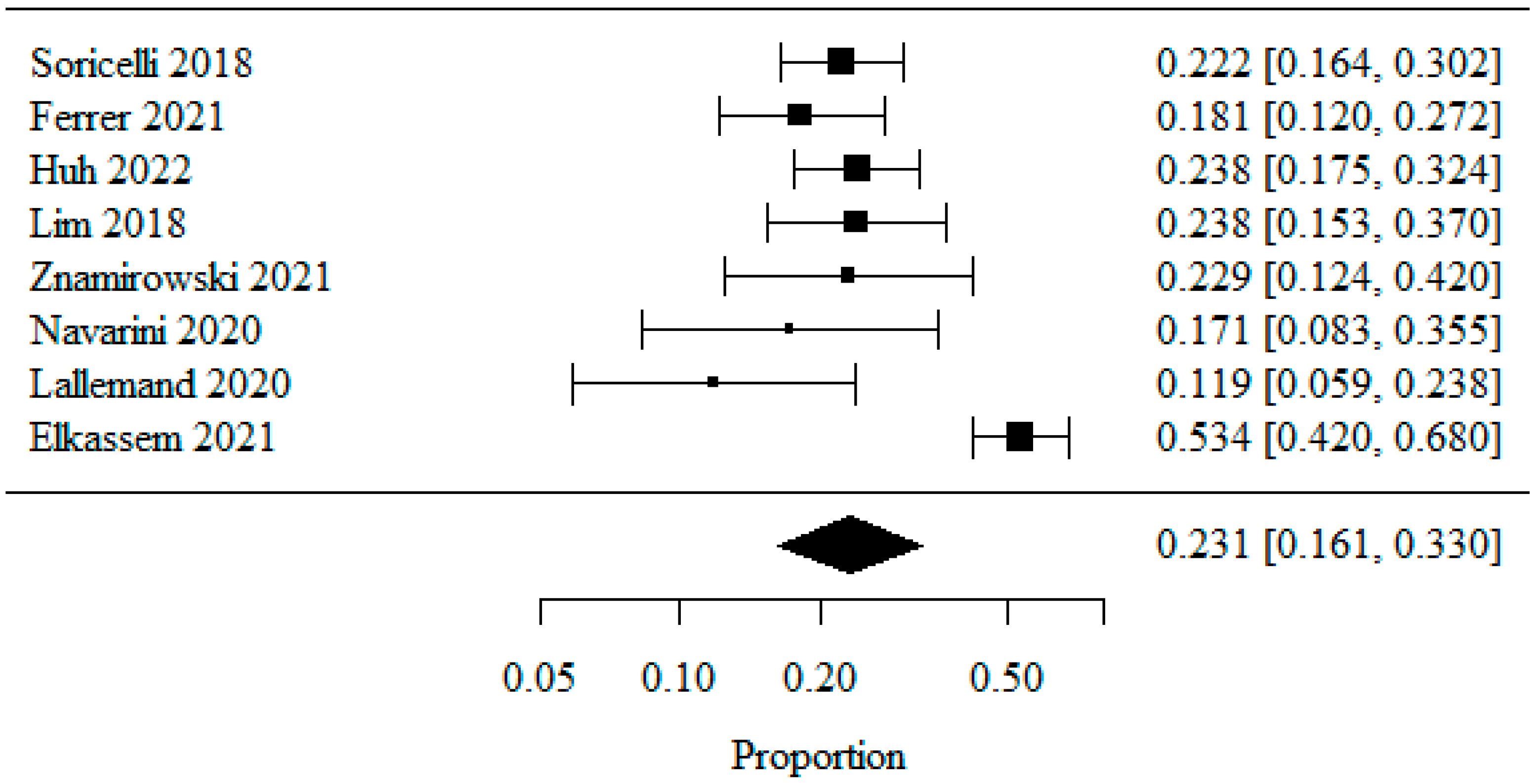
3.1.4. Reflux Esophagitis—Los Angeles Classification: LA Class A
3.1.5. Reflux Esophagitis—Los Angeles Classification: LA Class B
3.1.6. Reflux Esophagitis—Los Angeles Classification: LA Class C
3.1.7. Reflux Esophagitis—Los Angeles Classification: LA Class D
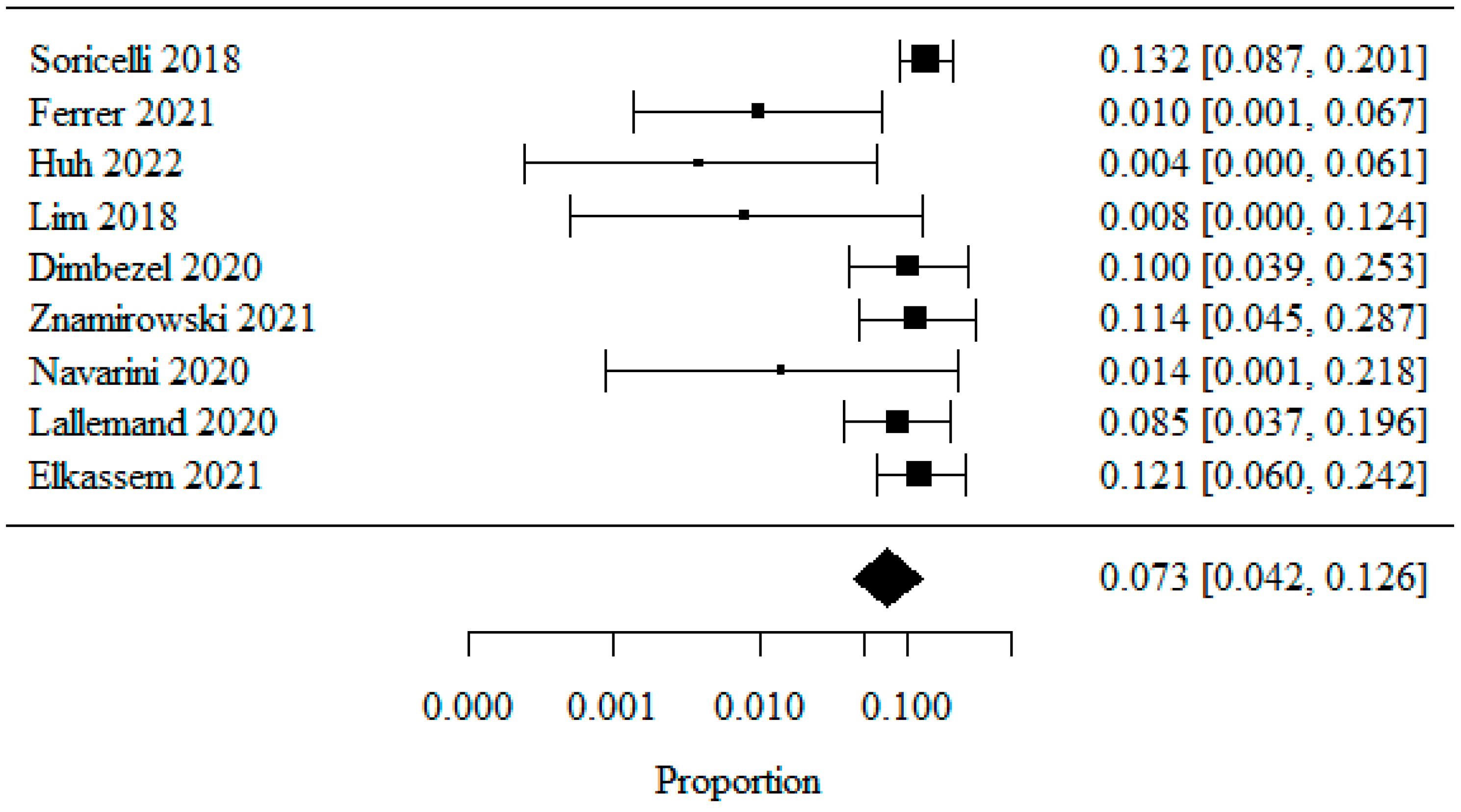
3.1.8. Barrett’s Esophagus
3.1.9. Other Limitations
3.2. Systematic Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 31 October 2022).
- Thompson, W.G.; Cook, D.A.; Clark, M.M.; Bardia, A.; Levine, J.A. Treatment of obesity. Mayo Clin. Proc. 2007, 82, 93–101. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [PubMed]
- Schneider, B.E.; Mun, E.C. Surgical management of morbid obesity. Diabetes Care 2005, 28, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Budzyński, A.; Major, P.; Głuszek, S.; Kaseja, K.; Koszustki, T.; Lęsniak, S.; Lewandowski, T.; Lipka, M.; Lisik, W.; Makarewicz, W.; et al. Polskie rekomendacje w zakresie chirurgii bariatrycznej i metabolicznej. Med. Prakt. Chir. 2016, 6, 3–25. [Google Scholar]
- Głuszek, S.; Bociek, A.; Suliga, E.; Matykiewicz, J.; Kołomańska, M.; Bryk, P.; Znamirowski, P.; Nawacki, Ł.; Głuszek-Osuch, M.; Wawrzycka, I.; et al. The Effect of Bariatric Surgery on Weight Loss and Metabolic Changes in Adults with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5342. [Google Scholar] [CrossRef]
- Walędziak, M.; Różańska-Walędziak, A.; Kowalewski, P.K.; Janik, M.R.; Brągoszewski, J.; Paśnik, K.; Bednarczyk, G.; Wallner, G.; Matłok, M. Present trends in bariatric surgery in Poland. Videosurgery Miniinv. 2019, 14, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Frezza, E.E.; Reddy, S.; Gee, L.L.; Wachtel, M.S. Complications after sleeve gastrectomy for morbid obesity. Obes. Surg. 2009, 19, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Braghetto, I.; Lanzarini, E.; Korn, O.; Valladares, H.; Molina, J.C.; Henriquez, A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes. Surg. 2010, 20, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidis, G.; Lazoura, O.; Sioka, E.; Tzovaras, G.; Antoniou, A.; Vassiou, K.; Zacharoulis, D. Anatomy and complications following laparoscopic sleeve gastrectomy: Radiological evaluation and imaging pitfalls. Obes. Surg. 2011, 21, 473–478. [Google Scholar] [CrossRef]
- Jones, R.; Galmiche, J.P. Review: What do we mean by GERD?—Definition and diagnosis. Aliment. Pharmacol. Ther. 2005, 22 (Suppl. S1), 2–10. [Google Scholar]
- American Gastroenterological Association. GERD Care pathway. Gastroenterology 2016, 150, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Rudolph, C.D.; Di Lorenzo, C.; Hassall, E.; Liptak, G.; Mazur, L.; Sondheimer, J.; Staiano, A.; Thomson, M.; Veereman-Wauters, G.; et al. Pediatric gastroesophageal reflux clinical practice guidelines: Joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 2009, 49, 498–547. [Google Scholar] [PubMed]
- Ronkainen, J.; Aro, P.; Storskrubb, T.; Johansson, S.E.; Lind, T.; Bolling-Sternevald, E.; Graffner, H.; Vieth, M.; Stolte, M.; Engstrand, L.; et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: A Kalixanda study report. Scand. J. Gastroenterol. 2005, 40, 275–285. [Google Scholar]
- Clermont, M.; Falk, G.W. Clinical Guidelines Update on the Diagnosis and Management of Barrett’s Esophagus. Dig. Dis. Sci. 2018, 63, 2122–2128. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef]
- Bujanda, D.E.; Hachem, C. Barrett’s Esophagus. Mo. Med. 2018, 115, 211–213. [Google Scholar]
- Soricelli, E.; Casella, G.; Baglio, G.; Maselli, R.; Ernesti, I.; Genco, A. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2018, 14, 751–756. [Google Scholar] [CrossRef]
- Ferrer, J.V.; Acosta, A.; García-Alementa, E.M.; García, A.T.; Del Castillo, D.; Espelta, M.V.; Del Val, I.D.; Lacorzana, J.O.; González-Argente, F.X.; Pagan, A.; et al. High rate of de novo esophagitis 5 years after sleeve gastrectomy: A prospective multicenter study in Spain. Surg. Obes. Relat. Dis. 2022, 18, 546–554. [Google Scholar]
- Huh, Y.-J.; Park, J.S.; Lee, S.; Han, S.-M. Impacts of sleeve gastrectomy on gastroesophageal reflux disease in severely obese Korean patients. Asian J. Surg. 2023, 46, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Lee, P.C.; Lim, E.; Tan, J.; Chan, W.H.; Tan, H.C.; Ganguly, S.; Tham, K.W.; Eng, A. Correlation Between Symptomatic Gastro-Esophageal Reflux Disease (GERD) and Erosive Esophagitis (EE) Post-vertical Sleeve Gastrectomy (VSG). Obes. Surg. 2019, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dimbezel, V.; Nedelcu, A.; Danan, M.; Carandina, S.; Collet, D.; Gronnier, C.; Nedelcu, M. Endoscopic Findings 5 Years Following Sleeve Gastrectomy. Obes. Surg. 2020, 30, 3847–3851. [Google Scholar] [CrossRef]
- Znamirowski, P.; Bryk, P.; Lewitowicz, P.; Kozieł, D.; Głuszek, S. GERD-A Burning Problem after Sleeve Gastrectomy? Int. J. Environ. Res. Public Health 2021, 18, 10829. [Google Scholar] [CrossRef]
- Navarini, D.; Madalosso, C.A.S.; Tognon, A.P.; Fornari, F.; Barão, F.R.; Gurski, R.R. Predictive Factors of Gastroesophageal Reflux Disease in Bariatric Surgery: A Controlled Trial Comparing Sleeve Gastrectomy with Gastric Bypass. Obes. Surg. 2020, 30, 1360–1367. [Google Scholar] [PubMed]
- Lallemand, L.; Duchalais, E.; Musquer, N.; Jacobi, D.; Coron, E.; des Varannes, S.B.; Mirallié, E.; Blanchard, C. Does Sleeve Gastrectomy Increase the Risk of Barret’s Esophagus? Obes. Surg. 2021, 31, 101–110. [Google Scholar] [CrossRef]
- Elkassem, S. Gastroesophageal Reflux Disease, Esophagitis, and Barrett’s Esophagus 3 to 4 Years Post Sleeve Gastrectomy. Obes. Surg. 2021, 31, 5148–5155. [Google Scholar] [CrossRef]
- Braghetto, I.; Csendes, A. Prevalence of Barrett’s Esophagus in Bariatric Patients Undergoing Sleeve Gastrectomy. Obes. Surg. 2016, 26, 710–714. [Google Scholar] [CrossRef]
- Migaczewski, M.; Czerwińska, A.; Rubinkiewicz, M.; Zarzycki, P.; Pisarska, M.; Rymarowicz, J.; Pędziwiatr, M.; Major, P. The prevalence of, and risk factors for, Barrett’s oesophagus after sleeve gastrectomy. Videosurgery Miniinv. 2021, 16, 710–714. [Google Scholar] [CrossRef]
- Felinska, E.; Billeter, A.; Nickel, F.; Contin, P.; Berlth, F.; Chand, B.; Grimminger, P.; Mikami, D.; Schoppmann, S.F.; Müller-Stich, B. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann. N. Y. Acad. Sci. 2020, 1482, 26–35. [Google Scholar] [CrossRef]
| Procedure | Number of Treatments | Percentage |
|---|---|---|
| Laparoscopic sleeve gastrectomy (LSG) | 1032 | 64.6% |
| Laparoscopic Roux-en-Y gastric bypass (LRYGB) | 291 | 18.2% |
| One anastomosis gastric bypass (OAGB) | 132 | 8.3% |
| Laparoscopic adjustable gastric banding (LAGB) | 117 | 7.3% |
| Class | Feature |
|---|---|
| Los Angeles A | Single erosion ≤ 5 mm in length |
| Los Angeles B | ≥1 erosion >5 mm in length, not extending over the entire distance between 2 adjacent esophageal folds |
| Los Angeles C | ≥1 erosion extending over the entire distance between ≥2 adjacent esophageal folds and covering ≤75% of the circumference |
| Los Angeles D | Mucosal loss covering ≥75% of the esophageal circumference |
| Reference | Country | Publication Year | Study Group Size (n) | Median BMI before Treatment (kg/m2) | Median BMI at Follow-Up (kg/m2) | Study Duration, Median (Months after Treatment) |
|---|---|---|---|---|---|---|
| [20] | Italy | 2018 | 144 | 46.2 ± 7.2 | N/A (−71.4% bw) | 66 |
| [21] | Spain | 2021 | 105 | 46.2 ± 6.6 | 29.8 ± 5.08 | 62 |
| [22] | South Korea | 2022 | 130 | 37.5 ± 4.7 | N/A | N/A |
| [23] | Singapore | 2018 | 63 | 42.1 ± 6.6 | 30.2 ± 1.18 | 13 |
| [24] | France | 2020 | 40 | 40.0 ± 1.89 | N/A | 62.4 |
| [25] | Poland | 2021 | 35 | 45.5 ± 5.48 | 32.4 ± 5.41 | 26.4 |
| [26] | Brazil | 2020 | 35 | 40.3 ± 4.0 | 26.8 ± 3.6 | N/A (12) |
| [27] | France | 2020 | 59 | 45.2 ± 8.1 | 37.4 ± 6.6 | N/A (60) |
| [28] | Canada | 2021 | 58 | 49.7 | 37.5 | N/A (36–48) |
| Overall | 669 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Znamirowski, P.; Kołomańska, M.; Mazurkiewicz, R.; Tymchyshyn, O.; Nawacki, Ł. GERD as a Complication of Laparoscopic Sleeve Gastrectomy for the Treatment of Obesity: A Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 1243. https://doi.org/10.3390/jpm13081243
Znamirowski P, Kołomańska M, Mazurkiewicz R, Tymchyshyn O, Nawacki Ł. GERD as a Complication of Laparoscopic Sleeve Gastrectomy for the Treatment of Obesity: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2023; 13(8):1243. https://doi.org/10.3390/jpm13081243
Chicago/Turabian StyleZnamirowski, Przemysław, Magdalena Kołomańska, Robert Mazurkiewicz, Oksana Tymchyshyn, and Łukasz Nawacki. 2023. "GERD as a Complication of Laparoscopic Sleeve Gastrectomy for the Treatment of Obesity: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 13, no. 8: 1243. https://doi.org/10.3390/jpm13081243
APA StyleZnamirowski, P., Kołomańska, M., Mazurkiewicz, R., Tymchyshyn, O., & Nawacki, Ł. (2023). GERD as a Complication of Laparoscopic Sleeve Gastrectomy for the Treatment of Obesity: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 13(8), 1243. https://doi.org/10.3390/jpm13081243