Human Exome Sequencing and Prospects for Predictive Medicine: Analysis of International Data and Own Experience
Abstract
1. Introduction
2. Human Monogenic Diseases: Population Genetics Research
2.1. Human Monogenic Diseases
2.2. Population Genetic Researches for Monogenic Diseases
2.3. WES Application to Identify New Variants in the Genomes of Patients
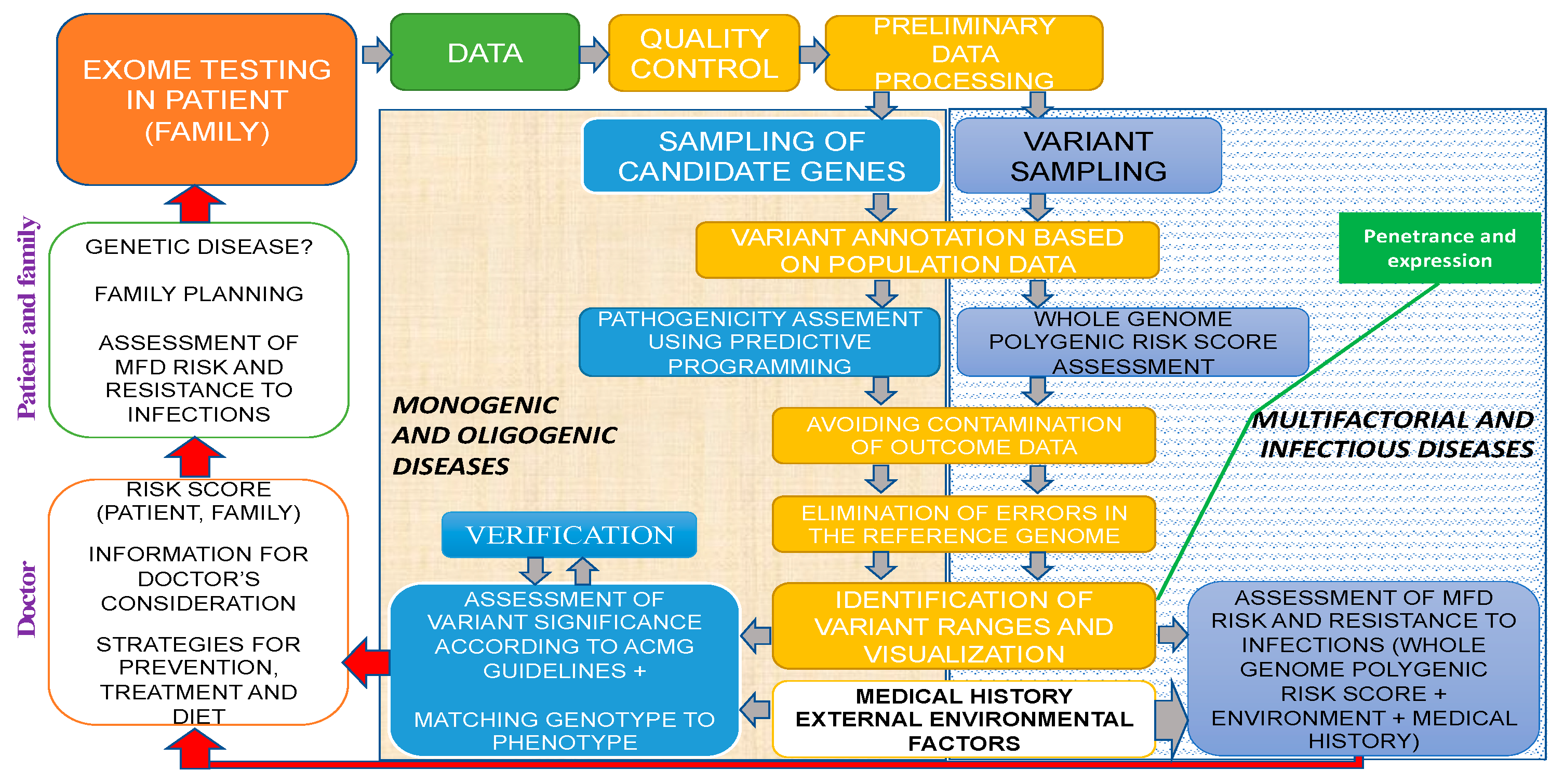
2.4. General Strategy and Algorithm of WES Implementation in Human Genetic Pathology Diagnostics
3. New-Generation Sequencing, Phenotypic Screening, Oligogenic and Multifactorial Diseases
3.1. Oligogenic Etiology of Cardiomyopathies
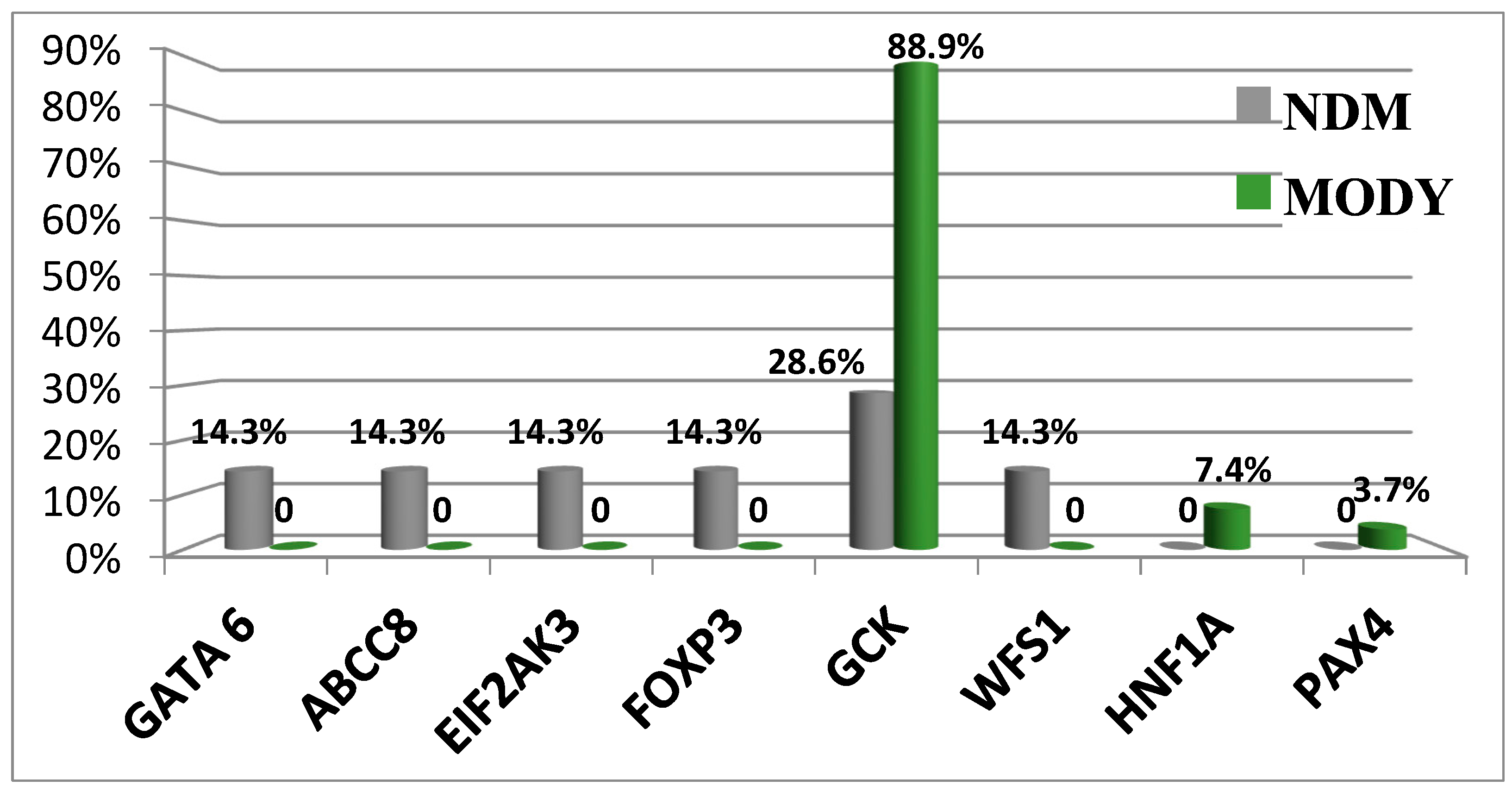
3.2. Monogenic Diabetes Mellitus
3.3. Multifactorial Diseases (MFDs): Type 2 Diabetes Mellitus
3.4. Prospects of Comprehensive Individualized Screening for MFD Polygenic Factors
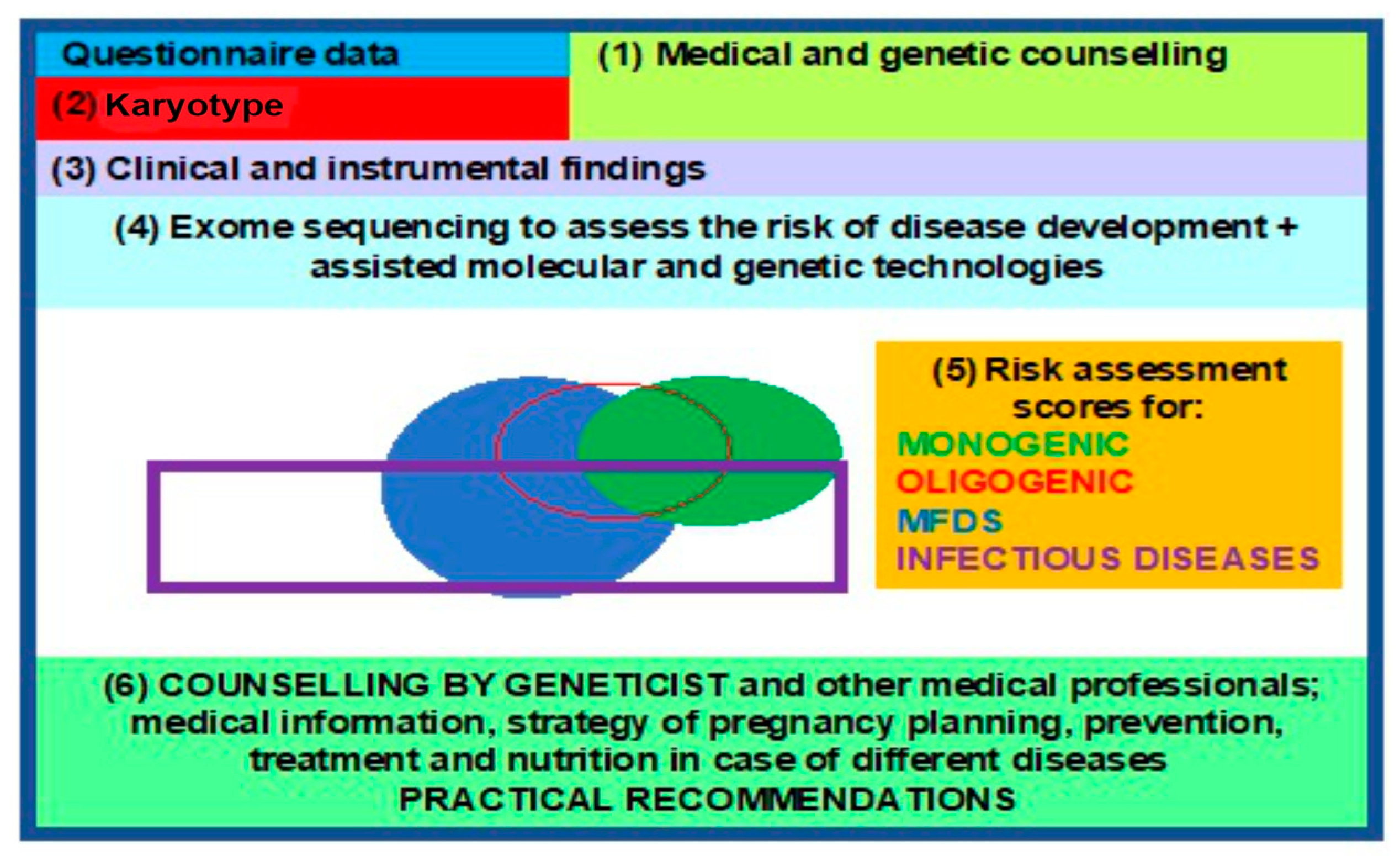
3.5. Clinical Genetic Passport (CGP)
- -
- Penetrance (the percentage of carriers of the corresponding genotype that exhibit the trait);
- -
- Expressivity (varying manifestation of the trait in individuals with the same genotype) [98].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baranov, V.S.; Baranova, E.V.; Aseev, M.V. Evolution of Predictive Medicine; Baranov, V.S., Ed.; Eko-Vektor Publisher: St. Petersburg, Russia, 2021; 359p. [Google Scholar]
- Frazer, K.A.; Ballinger, D.G.; Cox, D.R.; Hinds, D.A.; Stuve, L.L.; Gibbs, R.A.; Belmont, J.W.; Boudreau, A.; Hardenbol, P.; Leal, S.M.; et al. The International Hapmap Consortium. A second-generation human haplotype map of over 3.1 million SNPs. Nature 2007, 449, 851–861. [Google Scholar] [CrossRef]
- Stankov, K.; Benc, D.; Draskovic, D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics 2013, 132, 1112–1122. [Google Scholar] [CrossRef]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef]
- Ng, S.B.; Turner, E.H.; Robertson, P.D.; Flygare, S.D.; Bigham, A.W.; Lee, C.; Shaffer, T.; Wong, M.; Bhattacharjee, A.; Eichler, E.E.; et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature 2009, 461, 272–276. [Google Scholar] [CrossRef]
- Rabbani, B.; Mahdieh, N.; Hosomichi, K.; Nakaoka, H.; Inoue, I. Next-generation sequencing: Impact of exome sequencing in characterizing Mendelian disorders. J. Hum. Genet. 2012, 57, 621–632. [Google Scholar] [CrossRef]
- Goloshchapov, O.V.; Bakin, E.A.; Kucher, M.A.; Stanevich, O.V.; Suvorova, M.A.; Gostev, V.V.; Glotov, O.S.; Eismont, Y.A.; Polev, D.E.; Lobenskaya, A.Y.; et al. Bacteroides fragilis is a potential marker of effective microbiota transplantation in acute graft-versus-host disease treatment. Cell. Ther. Transplant. 2020, 9, 47–59. [Google Scholar] [CrossRef]
- Suwinski, P.; Ong, C.K.; Ling, M.H.T.; Poh, Y.M.; Khan, A.M.; Ong, H.S. Advancing Personalized Medicine through the Application of Whole Exome Sequencing and Big Data Analytics. Front. Genet. 2019, 10, 49. [Google Scholar] [CrossRef]
- Belkadi, A.; Bolze, A.; Itan, Y.; Cobat, A.; Vincent, Q.B.; Antipenko, A.; Shang, L.; Boisson, B.; Casanova, J.-L.; Abel, L. Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proc. Natl. Acad. Sci. USA 2015, 112, 5473–5478. [Google Scholar] [CrossRef]
- Alfares, A.; Aloraini, T.; Subaie, L.A.; Alissa, A.; Qudsi, A.A.; Alahmad, A.; Mutairi, F.A.; Alswaid, A.; Alothaim, A.; Eyaid, W.; et al. Whole-genome sequencing offers additional but limited clinical utility compared with reanalysis of whole-exome sequencing. Genet. Med. 2018, 20, 1328–1333. [Google Scholar] [CrossRef]
- Barbitoff, Y.A.; Polev, D.E.; Glotov, A.S.; Serebryakova, E.A.; Shcherbakova, I.V.; Kiselev, A.M.; Kostareva, A.A.; Glotov, O.S.; Predeus, A.V. Systematic dissection of biases in whole-exome and whole-genome sequencing reveals major determinants of coding sequence coverage. Sci. Rep. 2020, 10, 2057. [Google Scholar] [CrossRef]
- AllSeq. WGS vs. WES. Available online: http://allseq.com/kb/wgsvswes (accessed on 6 January 2023).
- Glotov, O.S.; Serebryakova, E.A.; Turkunova, M.E.; Efimova, O.A.; Glotov, A.S.; Barbitoff, Y.A.; Nasykhova, Y.A.; Predeus, A.V.; Polev, D.E.; Fedyakov, M.A.; et al. Whole-exome sequencing for monogenic diabetes in Russian children reveals wide spectrum of genetic variants in MODY-related and unrelated genes. Mol. Med. Rep. 2019, 20, 4905–4914. [Google Scholar]
- Barbitoff, Y.A.; Serebryakova, E.A.; Nasykhova, Y.A.; Predeus, A.V.; Polev, D.E.; Shuvalova, A.R.; Vasiliev, E.V.; Urazov, S.P.; Sarana, A.M.; Scherbak, S.G.; et al. Identification of Novel Candidate Markers of Type 2 Diabetes and Obesity in Russia by Exome Sequencing with a Limited Sample Size. Genes 2018, 9, 415. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Li, G.; Lu, H. Identification of potential markers for type 2 diabetes mellitus via bioinformatics analysis. Mol. Med. Rep. 2020, 22, 1868–1882. [Google Scholar] [CrossRef]
- Alur, V.; Raju, V.; Vastrad, B.; Vastrad, C.; Kavatagimath, S.; Kotturshetti, S. Bioinformatics Analysis of Next Generation Sequencing Data Identifies Molecular Biomarkers Associated with Type 2 Diabetes Mellitus. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231155635. [Google Scholar] [CrossRef]
- Retterer, K.; Juusola, J.; Cho, M.T.; Vitazka, P.; Millan, F.; Gibellini, F.; Vertino-Bell, A.; Smaoui, N.; Neidich, J.; Monaghan, K.G.; et al. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016, 18, 696–704. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, C.; Li, K.; Hu, X.; Gao, H.; Zeng, J.; Guo, R.; Liu, J.; Guo, J.; Li, Z.; et al. Clinical Application of Whole Exome Sequencing for Monogenic Disorders in PICU of China. Front. Genet. 2021, 12, 677699. [Google Scholar] [CrossRef]
- Hegde, M.; Santani, A.; Mao, R.; Ferreira-Gonzalez, A.; Weck, K.E.; Voelkerding, K.V. Development and Validation of Clinical Whole-Exome and Whole-Genome Sequencing for Detection of Germline Variants in Inherited Disease. Arch. Pathol. Lab. Med. 2017, 141, 798–805. [Google Scholar] [CrossRef]
- Chung, C.C.Y.; Hue, S.P.Y.; Ng, N.Y.T.; Doong, P.H.L.; Chu, A.T.W.; Chung, B.H.Y. Meta-analysis of the diagnostic and clinical utility of exome and genome sequencing in pediatric and adult patients with rare diseases across diverse populations. Genet. Med. 2023, 25, 100896. [Google Scholar] [CrossRef]
- Khusnutdinova, E.K.; Fatkhlislamova, R.I.; Khidiiatova, I.M.; Viktorova, T.V.; Grinchuk, O.V. Restriction-deletion polymorphism of V-region of mitochondrial DNA in populations of peoples of Volga-Ural region. Genetics 1997, 33, 996–1000. [Google Scholar]
- Stepanov, V.A. Ethnogenomics of the Population of Northern Eurasia; Pechatnaya Manufaktura: Tomsk, Russia, 2002; 244p. [Google Scholar]
- Baranov, V.S. Genome Paths: A Way to Personalized and Predictive Medicine. Acta Naturae 2009, 1, 70–80. [Google Scholar] [CrossRef][Green Version]
- Glotov, O.S.; Glotov, A.S.; Tarasenko, O.A.; Ivashchenko, T.E.; Baranov, V.S. Study of functionally significant polymorphism of ACE, AGTR1, ENOS, MTHFR, MTRR and APOE genes in population of North-West region of Russia. Ecol. Genet. 2004, 2, 32–35. [Google Scholar] [CrossRef]
- Hofmann, A.L.; Behr, J.; Singer, J.; Kuipers, J.; Beisel, C.; Schraml, P.; Moch, H.; Beerenwinkel, N. Detailed simulation of cancer exome sequencing data reveals differences and common limitations of variant callers. BMC Bioinform. 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shashikant, C.S.; Jensen, M.; Altman, N.S.; Girirajan, S. Novel metrics to measure coverage in whole exome sequencing datasets reveal local and global non-uniformity. Sci. Rep. 2017, 7, 885. [Google Scholar] [CrossRef]
- OMIM Gene Map Statistics. Available online: https://www.omim.org/statistics/geneMap (accessed on 6 January 2023).
- Zhao, Y.; Fang, L.T.; Shen, T.w.; Choudhari, S.; Talsania, K.; Chen, X.; Shetty, J.; Kriga, Y.; Tran, B.; Zhu, B.; et al. Whole genome and exome sequencing reference datasets from a multi-center and cross-platform benchmark study. Sci. Data 2021, 8, 296. [Google Scholar] [CrossRef]
- Janicki, E.; De Rademaeker, M.; Meunier, C.; Boeckx, N.; Blaumeiser, B.; Janssens, K. Implementation of Exome Sequencing in Prenatal Diagnostics: Chances and Challenges. Diagnostics 2023, 13, 860. [Google Scholar] [CrossRef]
- Szustakowski, J.D.; Balasubramanian, S.; Kvikstad, E.; Khalid, S.; Bronson, P.G.; Sasson, A.; Wong, E.; Liu, D.; Wade Davis, J.; Haefliger, C.; et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 2021, 53, 942–948. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, X.; Zhang, P.; Yang, L.; Lu, Y.; Xiao, F.; Wu, B.; Wang, H.; Zhou, W.; Dong, X. Systematic estimation of cystic fibrosis prevalence in Chinese and genetic spectrum comparison to Caucasians. Orphanet J. Rare Dis. 2022, 17, 129. [Google Scholar] [CrossRef]
- Barbitoff, Y.A.; Skitchenko, R.K.; Poleshchuk, O.I.; Shikov, A.E.; Serebryakova, E.A.; Nasykhova, Y.A.; Polev, D.E.; Shuvalova, A.R.; Shcherbakova, I.V.; Fedyakov, M.A.; et al. Whole exome sequencing provides insights into monogenic disease prevalence in Northwest Russia. Mol. Genet. Genom. Med. 2019, 7, e964. [Google Scholar] [CrossRef]
- Sheremet, N.L.; Zhorzholadze, N.V.; Ronzina, I.A.; Grushke, I.G.; Kurbatov, S.A.; Chukhrova, A.L.; Loginova, A.N.; Shcherbakova, P.O.; Tanas, A.S.; Polyakov, A.V.; et al. Molecular genetic diagnosis of Stargardt disease. Vestn. Oftalmol. 2017, 133, 4–11. [Google Scholar] [CrossRef]
- Al-Khuzaei, S. An Overview of the Genetics of ABCA4 Retinopathies, an Evolving Story. Genes 2021, 12, 1241. [Google Scholar] [CrossRef]
- Abramov, D.D.; Kadochnikova, V.V.; Yakimova, E.G.; Belousova, M.V.; Maerle, A.V.; Sergeev, I.V.; Ragimov, A.A.; Donnikov, A.E.; Trofimov, D.Y. High carrier frequency of CFTR gene mutations associated with cystic fibrosis, and PAH gene mutations associated with phenylketonuria in Russian population. Bull. Russ. State Med. Univ. 2015, 4, 32–35. [Google Scholar]
- Guo, J.; Garratt, A.; Hill, A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J. Cyst. Fibros. 2022, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Duga, S.; Peyvandi, F. Recessively inherited coagulation disorders. Blood 2004, 104, 1243–1253. [Google Scholar] [CrossRef]
- Hillert, A. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Collet, C. High genetic carrier frequency of Wilson’s disease in France: Discrepancies with clinical prevalence. BMC Med. Genet. 2018, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.T.; FFACMG. Classic Galactosemia and Clinical Variant Galactosemia Synonyms: Galactose-1-Phosphate Uridylyltranserase Deficiency, GALT Deficiency; Initial Posting: 4 February 2000, Last Update: 11 March 2021; National Library of Medicine: Bethesda, MD, USA, 2021.
- Lazarin, G.A.; Haque, I.S.; Nazareth, S.; Iori, K.; Patterson, A.S.; Jacobson, J.L.; Marshall, J.R.; Seltzer, W.K.; Patrizio, P.; Evans, E.A.; et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: Results from an ethnically diverse clinical sample of 23,453 individuals. Genet. Med. 2013, 15, 178–186. [Google Scholar] [CrossRef]
- Abramov, D.D.; Belousova, M.V.; Kadochnikova, V.V.; Ragimov, A.A.; Trofimov, D.Y. Carrier frequency of GJB2 and GALT mutations associated with sensorineural hearing loss and galactosemia in the Russian population. Bull. Russ. State Med. Univ. 2017, 6, 20–23. [Google Scholar] [CrossRef][Green Version]
- Trujillano, D.; Bertoli-Avella, A.M.; Kumar Kandaswamy, K.; Weiss, M.E.; Köster, J.; Marais, A.; Paknia, O.; Schröder, R.; Garcia-Aznar, J.M.; Werber, M.; et al. Clinical exome sequencing: Results from 2819 samples reflecting 1000 families. Eur. J. Hum. Genet. 2017, 25, 176–182. [Google Scholar] [CrossRef]
- Trinh, J.; Kandaswamy, K.K.; Werber, M.; Weiss, M.E.R.; Oprea, G.; Kishore, S.; Lohmann, K.; Rolfs, A. Novel pathogenic variants and multiple molecular diagnoses in neurodevelopmental disorders. J. Neurodev. Disord. 2019, 11, 11. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, Z.; Yi, S.; Wei, H.; Zhou, X.Z.; Su, J. Clinical application of whole-exome sequencing: A retrospective, singlecenter study. Exp. Ther. Med. 2021, 22, 753. [Google Scholar] [CrossRef]
- Miroshnikova, V.V.; Romanova, O.V.; Ivanova, O.N.; Fedyakov, M.A.; Panteleeva, A.A.; Barbitoff, Y.A.; Muzalevskaya, M.V.; Urazgildeeva, S.A.; Gurevich, V.S.; Urazov, S.P.; et al. Identification of novel variants in the LDLR gene in Russian patients with familial hypercholesterolemia using targeted sequencing. Biomed. Rep. 2021, 14, 15. [Google Scholar] [CrossRef]
- EAS Familial Hypercholesterolaemia Studies Collaboration; Vallejo-Vaz, A.J.; Marco, M.D.; Stevens, C.A.T.; Akram, A.; Freiberger, T.; Hovingh, G.K.; Kastelein, J.J.P.; Mata, P.; Raal, F.J.; et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries-The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 2018, 277, 234–255. [Google Scholar] [CrossRef]
- Barbitoff, Y.A.; Bezdvornykh, I.V.; Polev, D.E.; Serebryakova, E.A.; Glotov, A.S.; Glotov, O.S.; Predeus, A.V. Catching hidden variation: Systematic correction of reference minor alleles in clinical variant calling. Genet. Med. 2018, 20, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, M.D.; Giacobbe, C.; Fortunato, G. Familial hypercholesterolemia: A complex genetic disease with variable phenotypes. Eur. J. Med. Genet. 2020, 63, 103831. [Google Scholar] [CrossRef]
- Alaverdian, D.A.; Fedyakov, M.; Polennikova, E.; Ivashchenko, T.; Shcherbak, S.; Urasov, S.; Tsay, V.; Glotov, O.S. X-linked and autosomal dominant forms of the ichthyosis in coinheritance. Drug Metab. Pers. Ther. 2019, 34, 20190008. [Google Scholar] [CrossRef] [PubMed]
- Koshevaya, Y.S.; Kusakin, A.V.; Buchinskaia, N.V.; Pechnikova, V.V.; Serebryakova, E.A.; Koroteev, A.L.; Glotov, A.S.; Glotov, O.S. Description of first registered case of the Lopes-Maciel-Rodan syndrome in Russia. Int. J. Mol. Sci. 2022, 23, 12437. [Google Scholar] [CrossRef]
- Turkunova, M.E.; Barbitoff, Y.A.; Serebryakova, E.A.; Polev, D.E.; Berseneva, O.S.; Bashnina, E.B.; Baranov, V.S.; Glotov, O.S.; Glotov, A.S. Molecular Genetics and Pathogenesis of the Floating Harbor Syndrome: Case Report of Long-Term Growth Hormone Treatment and a Literature Review. Front. Genet. 2022, 13, 846101. [Google Scholar] [CrossRef]
- Scholz, T.; Blohm, M.E.; Kortüm, F.; Bierhals, T.; Lessel, D.; van der Ven, A.T.; Lisfeld, J.; Herget, T.; Kloth, K.; Singer, D.; et al. Whole-Exome Sequencing in Critically Ill Neonates and Infants: Diagnostic Yield and Predictability of Monogenic Diagnosis. Neonatology 2021, 118, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Balashova, M.S.; Tuluzanovskaya, I.G.; Glotov, O.S.; Glotov, A.S.; Barbitoff, Y.A.; Fedyakov, M.A.; Alaverdian, D.A.; Ivashchenko, T.E.; Romanova, O.V.; Sarana, A.M.; et al. The spectrum of pathogenic variants of the ATP7B gene in Wilson disease in the Russian Federation. J. Trace Elem. Med. Biol. 2020, 59, 126420. [Google Scholar] [CrossRef]
- Dillon, O.J.; Lunke, S.; Stark, Z.; Yeung, A.; Thorne, N.; Gaff, C.; White, S.M.; Tan, T.Y. Exome sequencing has higher diagnostic yield compared to simulated disease-specific panels in children with suspected monogenic disorders. Eur. J. Hum. Genet. 2018, 26, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.G.; Leach, N.T.; Pekarek, D.; Prasad, P.; Rose, N.C.; ACMG Professional Practice and Guidelines Committee. The use of fetal exome sequencing in prenatal diagnosis: A points to consider document of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2020, 22, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, S.; Aggarwal, V.; Giordano, J.L.; Stosic, M.; Wou, K.; Bier, L.; Spiegel, E.; Brennan, K.; Stong, N.; Jobanputra, V.; et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet 2019, 393, 758–767. [Google Scholar] [CrossRef]
- Lord, J.; McMullan, D.J.; Eberhardt, R.Y.; Rinck, G.; Hamilton, S.J.; Quinlan-Jones, E.; Prigmore, E.; Keelagher, R.; Best, S.K.; Carey, G.K.; et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study. Lancet 2019, 393, 747–757. [Google Scholar] [CrossRef]
- Best, S.; Wou, K.; Vora, N.; van der Veyver, I.B.; Wapner, R.; Chitty, L.S. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat. Diagn. 2018, 38, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fedyakov, M.A. Anauksetic Dysplasia: Clinic, Molecular Genetic Diagnosis and Treatment. Molecular Biological Technologies in Medical Practice; Maslennikov, A.B., Ed.; Akademizdat: Novosibirsk, Russia, 2021; Volume 32, pp. 81–92. [Google Scholar]
- Heng Mak, T.S.; Lee, Y.-K.; Tang, C.S.; Hai, J.J.S.H.; Ran, X.; Sham, P.-C.; Tse, H.-F. Coverage and diagnostic yield of Whole Exome Sequencing for the Evaluation of Cases with Dilated and Hypertrophic Cardiomyopathy. Sci. Rep. 2018, 8, 10846. [Google Scholar]
- Kousi, M.; Katsanis, N. Genetic modifiers and oligogenic inheritance. Cold Spring Harb. Perspect. Med. 2015, 5, a017145. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H. Genetics of Cardiomyopathy: Clinical and Mechanistic Implications for Heart Failure. Korean Circ. J. 2021, 51, 797836. [Google Scholar] [CrossRef]
- Glotov, A.S.; Kazakov, S.V.; Zhukova, E.A.; Alexandrov, A.V.; Glotov, O.S.; Pakin, V.S.; Danilova, M.M.; Poliakova, I.V.; Niyazova, S.S.; Chakova, N.N.; et al. Targeted next-generation sequencing (NGS) of nine candidate genes with custom AmpliSeq in patients and a cardiomyopathy risk group. Clin. Chim. Acta 2015, 446, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Komissarova, S.M.; Chakova, N.; Niyazova, S.S.; Kazakov, S.V.; Zhukova, E.A.; Aleksandrov, A.V.; Glotov, O.S.; Glotov, A.S. The specifics of hypertrophic cardiomyopathy clinical presentation in patients with various mutations of sarcomere genes. Russ. J. Cardiol. 2016, 1, 20–25. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Kelly, M.A.; Rehm, H.L.; Lakdawala, N.K.; Funke, B.H. Inherited cardiomyopathies: Molecular genetics and clinical genetic testing in the postgenomic era. J. Mol. Diagn. 2013, 15, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, A.; Nevanperä, N.; Remes, J.; Rahkonen, F.; Järvelin, M.-R.; Laitinen, J. Stress-related eating, obesity and associated behavioural traits in adolescents: A prospective population-based cohort study. BMC Public Health 2014, 14, 321. [Google Scholar] [CrossRef]
- Hattersley, A.T.; Greeley, S.A.W.; Polak, M.; Rubio-Cabezas, O.; Njølstad, P.R.; Mlynarski, W.; Castano, L.; Carlsson, A.; Raile, K.; Chi, D.V.; et al. ISPAD clinical practice consensus guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, F.; D’Annunzio, G. Genetic causes and treatment of neonatal diabetes and early childhood diabetes. Pest Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhao, Z.; Hu, Q.; Li, Y.; Zhao, W.; Li, C.; Xu, Y.; Rong, R.; Zhang, J.; Zhang, Z.; et al. Identification of Maturity-Onset Diabetes of the Young Caused by Mutation in FOXM1 via Whole-Exome Sequencing in Northern China. Front. Endocrinol. 2020, 11, 534362. [Google Scholar] [CrossRef]
- Bonnefond, A.; Philippe, J.; Durand, E.; Dechaume, A.; Huyvaert, M.; Montagne, L.; Marre, M.; Balkau, B.; Fajardy, I.; Vambergue, A.; et al. Whole-Exome Sequencing and High Throughput Genotyping Identified KCNJ11 as the Thirteenth MODY Gene. PLoS ONE 2012, 7, e37423. [Google Scholar] [CrossRef]
- Lemelman, M.B.; Letourneau, L.; Greeley, S. Neonatal diabetes mellitus: An update on diagnosis and management. Clin. Perinatol. 2018, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Greeley, S.A.; Naylor, R.N.; Philipson, L.H.; Bell, G.I. Neonatal diabetes: An expanding list of genes allows for improved diagnosis and treatment. Curr. Diab. Rep. 2011, 11, 519–532. [Google Scholar] [CrossRef]
- 100,000 Genomes Project. Available online: https://www.genomicsengland.co.uk (accessed on 6 January 2023).
- Petersen, I. Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology. Int. J. Mol. Sci. 2021, 22, 9418. [Google Scholar] [CrossRef]
- The NHGRI-EBI GWAS Catalog. Available online: https://www.ebi.ac.uk/gwas (accessed on 6 January 2023).
- Genetic Data-UK Biobank. Available online: https://www.ukbiobank.ac.uk/scientists-3/uk-biobank-axiom-array (accessed on 6 January 2023).
- Reich, D.E.; Lander, E.S. On the allelic spectrum of human disease. Trends Genet. 2001, 17, 502–510. [Google Scholar] [CrossRef]
- Baranov, V.S.; Glotov, A.S.; Glotov, O.S.; Ivaschenko, T.E.; Aseev, M.V.; Shved, N.Y.; Kozlovskaya, M.A.; Moskalenko, M.V.; Demin, G.S.; Bespalova, O.N.; et al. Genetic Passport the Basis of Individual and Predictive Medicine; Baranov, V.S., Ed.; SPb: ‘N-L’, Ltd.: Moscow, Russia, 2009; 527p. [Google Scholar]
- Wang, X.; Strizich, G.; Hu, Y.; Wang, T.; Kaplan, R.C.; Qi, Q. Genetic markers of type 2 diabetes: Progress in genome-wide association studies and clinical application for risk prediction. J. Diabetes 2016, 8, 24–35. [Google Scholar] [CrossRef]
- Scott, R.A.; Scott, L.J.; Mägi, R.; Marullo, L.; Gaulton, K.J.; Kaakinen, M.; Pervjakova, N.; Pers, T.H.; Johnson, A.D.; Eicher, J.D.; et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2018, 66, 2888–2902. [Google Scholar] [CrossRef]
- Loh, M.; Zhang, W.; Ng, H.K.; Schmid, K.; Lamri, A.; Tong, L.; Ahmad, M.; Lee, J.J.; Ng, M.C.Y.; Petty, L.E.; et al. Identification of genetic effects underlying type 2 diabetes in South Asian and European populations. Commun. Biol. 2022, 5, 329. [Google Scholar] [CrossRef]
- Zabetian, C.P.; Romero, R.; Robertson, D.; Sharma, S.; Padbury, J.F.; Kuivaniemi, H.; Kim, K.-S.; Kim, C.-H.; Köhnke, M.D.; Kranzler, H.R.; et al. A revised allele frequency estimate and haplotype analysis of the DBH deficiency mutation IVS1+2T->C in African- and European-Americans. Am. J. Med. Genet. Part A 2003, 123, 190–192. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Flajollet, S.; Poras, I.; Carosella, E.D.; Moreau, P. RREB-1 is a transcriptional repressor of HLA-G. J. Immunol. 2009, 183, 6948–6959. [Google Scholar] [CrossRef]
- Gibson, G. Rare and common variants: Twenty arguments. Nat. Rev. Genet. 2012, 18, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Collins, F.S.; McKusick, V.A. Implication of Human Genome Project for Medical Science. JAMA 2001, 285, 540–544. [Google Scholar] [CrossRef]
- Peltonen, L.; McKusick, V.A. Genomics and medicine. Dissecting human disease in the postgenomic era. Science 2001, 91, 1224–1229. [Google Scholar]
- Pendina, A.A.; Shilenkova, Y.V.; Talantova, O.E.; Efimova, O.A.; Chiryaeva, O.G.; Malysheva, O.V.; Dudkina, V.S.; Petrova, L.I.; Serebryakova, E.A.; Shabanova, E.S.; et al. Reproductive History of a Woman with 8p and 18p Genetic Imbalance and Minor Phenotypic Abnormalities. Front. Genet. 2019, 10, 1164. [Google Scholar] [CrossRef]
- Saifitdinova, A.F.; Glotov, O.S.; Poliakova, I.V.; Bichevaya, N.K.; Loginova, J.A.; Kuznetsova, R.A.; Leonteva, O.A.; Nevskaia, E.E.; Pavlova, O.A.; Puppo, I.L.; et al. Mosaicism in preimplantation human embryos. Integr. Physiol. 2020, 1, 225–230. [Google Scholar] [CrossRef]
- Shcherbak, S.G.; Anisenkova, A.Y.; Mosenko, S.V.; Glotov, O.S.; Chernov, A.N.; Apalko, S.V.; Urazov, S.P.; Garbuzov, E.Y.; Khobotnikov, D.N.; Klitsenko, O.A.; et al. Basic Predictive Risk Factors for Cytokine Storms in COVID-19 Patients. Front. Immunol. 2021, 12, 745515. [Google Scholar] [CrossRef]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of Protein-Coding Genetic Variation in 60,706 Humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Ryzhkova, O.P.; Kardymon, O.L.; Prohorchuk, E.B.; Konovalov, F.A.; Maslennikov, A.B.; Stepanov, V.A.; Afanasyev, A.A.; Zaklyazminskaya, E.V.; Kostareva, A.A.; Pavlov, A.E.; et al. Guidelines for interpretation of human DNA sequence data obtained by massively parallel sequencing (MPS) (revision 2018, version 2). Med. Genet. 2019, 18, 3–23. [Google Scholar]
- Inge-Vechtomov, S.G. Genetics with the Basics of Selection; Publishing House H-J: St. Petersburg, Russia, 2010; 720p. [Google Scholar]
- Tulzunovskaya, I.G.; Zhuchenko, N.A.; Balashova, M.; Filimonov, M.I.; Rosina, T.P.; Glotov, O.S.; Asanov, A.Y. Wilson-Conovalov disease: Intrafamilial clinical polymorphism. Pediatria J. GN SPERANSKY 2017, 96, 215–216. [Google Scholar]
- Abul-Husn, N.S.; Manickam, K.; Jones, L.K.; Wright, E.A.; Hartzel, D.N.; Gonzaga-Jauregui, C.; O’Dushlaine, C.; Leader, J.B.; Kirchner, H.L.; Lindbuchler, D.M.; et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016, 354, aaf7000. [Google Scholar] [CrossRef] [PubMed]
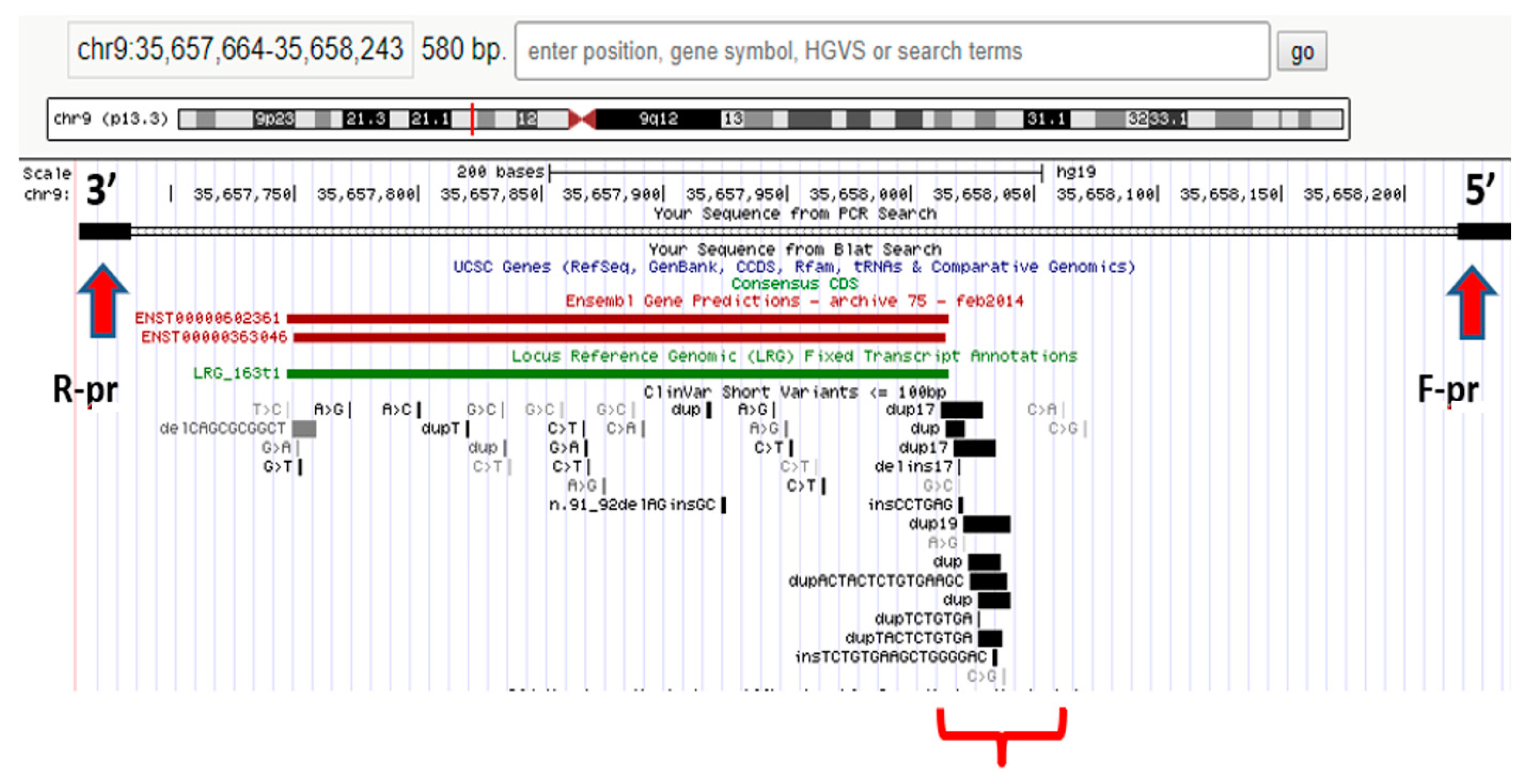
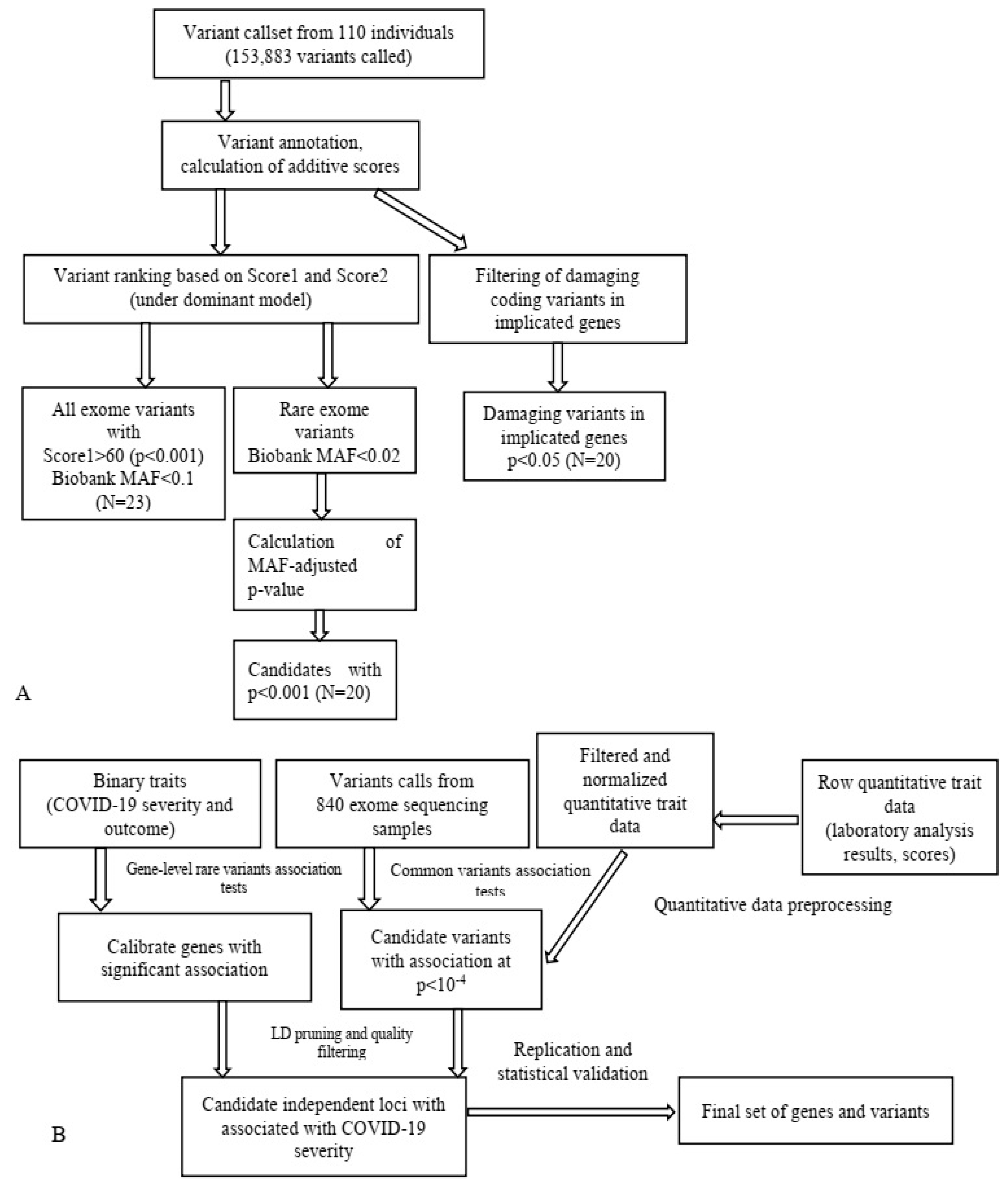
| NGS Technology | Study Aims | Working Principle | Data Size, GB | Research and Clinical Applications |
|---|---|---|---|---|
| WGS | Identification of genetic mutations and SNPs in coding and noncoding genome regions | DNA extraction, fragmentation, sequencing, data analysis, and identification of relevant variants | 90–120 | Research, diagnosis, and treatment |
| WES | Identification of variants in protein coding loci and genes (exome) | DNA extraction, fragmentation, target gene identification, sequencing, data analysis, and variant annotation | 6–12 | Clinical diagnosis, disease-causing genes |
| TS | Screening for DNA variants affecting numerous genes | DNA extraction, fragmentation, target gene sequence capture, sequencing, data analysis, and variant annotation | 0.5–3 | Treatment |
| RNA-seq | Identification of gene expression, new protein isoforms, detection of merging genes, SNPs, insertions, deletions, and small noncoding RNAs | RNA and cDNA extraction, fragmentation, sequencing, data analysis, and variant annotation | 3–6 | Research and therapy, biomarker discovery, and drug resistance |
| ChIP-seq | DNA fragmentation, binding beaded antibodies to target proteins, DNA purification, sequencing, and identification of gene variants | 1–2 | The influence of transcription factors on phenotype-affecting mechanisms and diseases |
| Disease/Condition | Gene | Allele Count | Carrier Frequency (Lower/Upper CI) | Disease Frequency (Lower/Upper CI) | Known Frequency | References |
|---|---|---|---|---|---|---|
| Retinal dystrophy, Stargardt disease | ABCA4 | 13 (23) | 0.0350 (0.0206/0.0589) | 3.1 × 10−4 (1.1 × 10−4/8.8 × 10−4) | 1 in 10,000 1 in 8000 | [33] [34] |
| Cystic fibrosis | CFTR | 11 (19) | 0.0296 (0.0167/0.0522) | 2.2 × 10−4 (6.9 × 10−5/6.9 × 10−4) | 1 in 10,000 1 in 3000–16,000 | Reported carrier frequency of 0.032 [35] [36] |
| Phenylketonuria | PAH | 11 (18) | 0.0296 (0.0167/0.0522) | 2.2 × 10−4 (6.9 × 10−5/6.9 × 10−4) | 1 in 10,000 1 in 4500 [Italy]–1 in 125,000 [Japan] | Reported carrier frequency of 0.029 [37] [38] |
| Wilson disease | ATP7B | 4 (6) | 0.0108 (0.0042/0.0274) | 2.9 × 10−5 (4.3 × 10−6/1.9 × 10−4) | 1 in 30,000 1 in 30,000 | Similar global incidence reported [39,40] |
| Galactosemia | GALT | 4 (5) | 0.0108 (0.0042/0.0274) | 2.9 × 10−5 (4.3 × 10−6/1.9 × 10−4) | 1 in 20,000 1 in 48,000 | Reported carrier frequency of 0.006 [35,41] |
| Gene | Patient ID | Exon/Intron | Variant | Allele Frequency in GnomAD | Allele Frequency in [49] | Variant Pathogenicity Classification by ACMG |
|---|---|---|---|---|---|---|
| LDLR | G31 | 4 | c.316_328delCCCAAGACGTGCT p.(Lis107Argfs*95) | Not found | Not found | P (PVS1 PS1 PM1 PM2 PP3) |
| LDLR | G29 | 4 | c.325T>G p.(Cys109Gly) | Not found | Not found | LP (PS1 PM1 PM2 PM5 PP3) |
| LDLR | G36 | 4 | c.401G>C (p.Cys134Ser) | Not found | Not found | LP (PS1 PM1 PM2 PM5 PP3) |
| LDLR | 1 | 4 | c.433_434insG p(Val145Glyfs*35) | Not found | Not found | P (PVS1 PM2 PP3) |
| LDLR | G18 | 4 | c.616A>C (p.Ser206Arg) | Not found | Not found | Uncertain significance (PM2 PP1 PP3) |
| LDLR | G21 | IVS6 | c.940+1_c.940+4 delGTGA (g.18154_18157delGTGA) | Not found | Not found | P (PVS1 PM1 PM2 PP3) |
| LDLR | 32 | 8 | c.1186G>C p.(Gly396Arg) | Not found | Not found | P (PVS1 PM1 PM2 PM5 PP3) |
| LDLR | G26 | IVS8 | c.1186+1G>T (g.22279G>T) | Not found | Not found | P (PVS1 PM2 PP3) |
| LDLR | G17 | 11 | c.1684_1691delTGGCCCAA p.(Pro563Hisfs*14) | Not found | Not found | P (PVS1 PM1 PM2 PP3) |
| Nosology | Efficiency of Diagnostics Prior to NGS, % | Efficiency of Diagnostics after WES, % | Efficiency of Diagnostics with Novel Variants Considered, % | Reference |
|---|---|---|---|---|
| Cystic fibrosis | 45–55 (1 mutation) 58 (35 mutations) | 67–80 | - | Unpublished |
| WD | Up to 75 (4 mutations) Up to 86 (12 mutations) | Up to 96 | 97 | [55] |
| MODY | 15–35 | 40–50 | 55 | [13] |
| Genetically heterogeneous condition | 28% | 65% | [56] | |
| Neurometabolic disorder | 24% | 35% | [56] | |
| Single anomalyy of the fetuses | 6% | [57] | ||
| Two and more anomalies of the fetuses | 35% | [57] | ||
| Anomalies of the fetuses | 10.3–18.9% | [58] | ||
| Anomalies of the fetuses | 8.5–15.4% | [59] | ||
| Anomalies of the fetuses | 6.2–80% | [60] |
| Gene | Nucleotide Change | Diseased/Risk/Healthy, % | Risk | Risk2 | p-Value | Polyphen 2 | SIFT | Clinical Verification |
|---|---|---|---|---|---|---|---|---|
| MYBPC3 | c.977G>A (NM_000256.3) | 5/4/0 | 19 | −99 | 0.41 | Benign | Damaging | [68] |
| MYBPC3 | c.2678G>T (NM_000256.3) | 5/17/0 | 16 | −96 | - | Probably damaging | Damaging | - |
| CASQ2 | c.1014+12delG (NM_001232.3) | 13/4/0 | 49 | −249 | 8.62 × 10−5 | - | - | - |
| TNNT2 | c.97+151delC (NM_000364.3) | 0/0/10 | −100 | 20 | 1.80 × 10−5 | - | - | - |
| TNNT2 | c.223+92G>C (NM_000364.3) | 0/0/29 | −300 | 60 | 1.902 × 10−7 | - | - | - |
| TNNT2 | c.223+93C>G (NM_000364.3) | 0/0/33 | −350 | 70 | 2.535 × 10−4 | - | - | - |
| Disease | Discovery GWAS, Case/Control | Prevalence in the Validation Dataset | Prevalence in the Testing Dataset | No. of SNPs in GPS | Tuning Parameter | AUC (95% CI) in the Validation Dataset | AUC (95% CI) in the Testing Dataset |
|---|---|---|---|---|---|---|---|
| CAD | 60,801/123,504 | 3963/120,280 (3.4%) | 8676/288,978 (3.0%) | 6,630,150 | LDPred (ρ = 0.001) | 0.81 (0.80–0.81) | 0.81 (0.81–0.81) |
| Atrial fibrillation | 17,931/115,142 | 2024/120,280 (1.7%) | 4576/288,978 (1.6%) | 6,730,541 | LDPred (ρ = 0.003) | 0.77 (0.76–0.78) | 0.77 (0.76–0.77) |
| T2D | 26,676/132,532 | 2785/120,280 (2.4%) | 5853/288,978 (2.0%) | 6,917,436 | LDPred (ρ = 0.01) | 0.72 (0.72–0.73) | 0.73 (0.72–0.73) |
| Inflammatory bowel disease | 12,882/21,770 | 1360/120,280 (1.1%) | 3102/288,978 (1.1%) | 6,907,112 | LDPred (ρ = 0.1) | 0.63 (0.62–0.65) | 0.63 (0.62–0.64) |
| Breast cancer | 122,977/105,974 | 2576/63,347 (4.1%) | 6586/1,576,895 (4.2%) | 5218 | Pruning and thresholding (r/2 < 0.2; p < 5 × 10−4 | 0.68 (0.67–0.69) | 0.69 (0.68–0.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glotov, O.S.; Chernov, A.N.; Glotov, A.S. Human Exome Sequencing and Prospects for Predictive Medicine: Analysis of International Data and Own Experience. J. Pers. Med. 2023, 13, 1236. https://doi.org/10.3390/jpm13081236
Glotov OS, Chernov AN, Glotov AS. Human Exome Sequencing and Prospects for Predictive Medicine: Analysis of International Data and Own Experience. Journal of Personalized Medicine. 2023; 13(8):1236. https://doi.org/10.3390/jpm13081236
Chicago/Turabian StyleGlotov, Oleg S., Alexander N. Chernov, and Andrey S. Glotov. 2023. "Human Exome Sequencing and Prospects for Predictive Medicine: Analysis of International Data and Own Experience" Journal of Personalized Medicine 13, no. 8: 1236. https://doi.org/10.3390/jpm13081236
APA StyleGlotov, O. S., Chernov, A. N., & Glotov, A. S. (2023). Human Exome Sequencing and Prospects for Predictive Medicine: Analysis of International Data and Own Experience. Journal of Personalized Medicine, 13(8), 1236. https://doi.org/10.3390/jpm13081236






