Abstract
Atrial fibrillation is the most common type of cardiac arrhythmias in humans, mostly caused by hyper excitation of specific areas in the atrium resulting in dyssynchronous atrial contractions, leading to severe consequences such as heart failure and stroke. Current therapeutics aim to target this condition through both pharmacological and non-pharmacological approaches. To test and validate any of these treatments, an appropriate preclinical model must be carefully chosen to refine and optimise the therapy features to correctly reverse this condition. A broad range of preclinical models have been developed over the years, with specific features and advantages to closely mimic the pathophysiology of atrial fibrillation. In this review, currently available models are described, from traditional animal models and in vitro cell cultures to state-of-the-art organoids and organs-on-a-chip. The advantages, applications and limitations of each model are discussed, providing the information to select the appropriate model for each research application.
1. Introduction
Atrial fibrillation (Afib) is the most common type of cardiac arrhythmia in humans, characterised by high-frequency excitation of the atrium, which results in dyssynchronous atrial contraction and irregularities in ventricular excitation [1]. Afib is increasingly a widespread health problem as its prevalence is on the rise, with an estimated 33 million people worldwide diagnosed with Afib in 2020; this figure is expected to double by 2050 [2]. Afib patients have increased morbidity and mortality rates due to the severe consequences associated with thromboembolism and stroke [3]. Ischemic stroke and Afib commonly exhibit overlapping risk factors, including dyslipidemia, diabetes mellitus, and hypertension. Oral anticoagulants are widely prescribed for stroke prevention in patients with Afib. Nevertheless, it is noteworthy that these anticoagulants solely address the thromboembolic aspect and do not comprehensively target the entirety of factors associated with Afib pathology [4]. Moreover, COVID-19 infection is reported to be associated with more frequent occurrences of arrhythmias, predominantly Afib cases [5], making Afib a global concern that needs more efficient and effective treatment options.
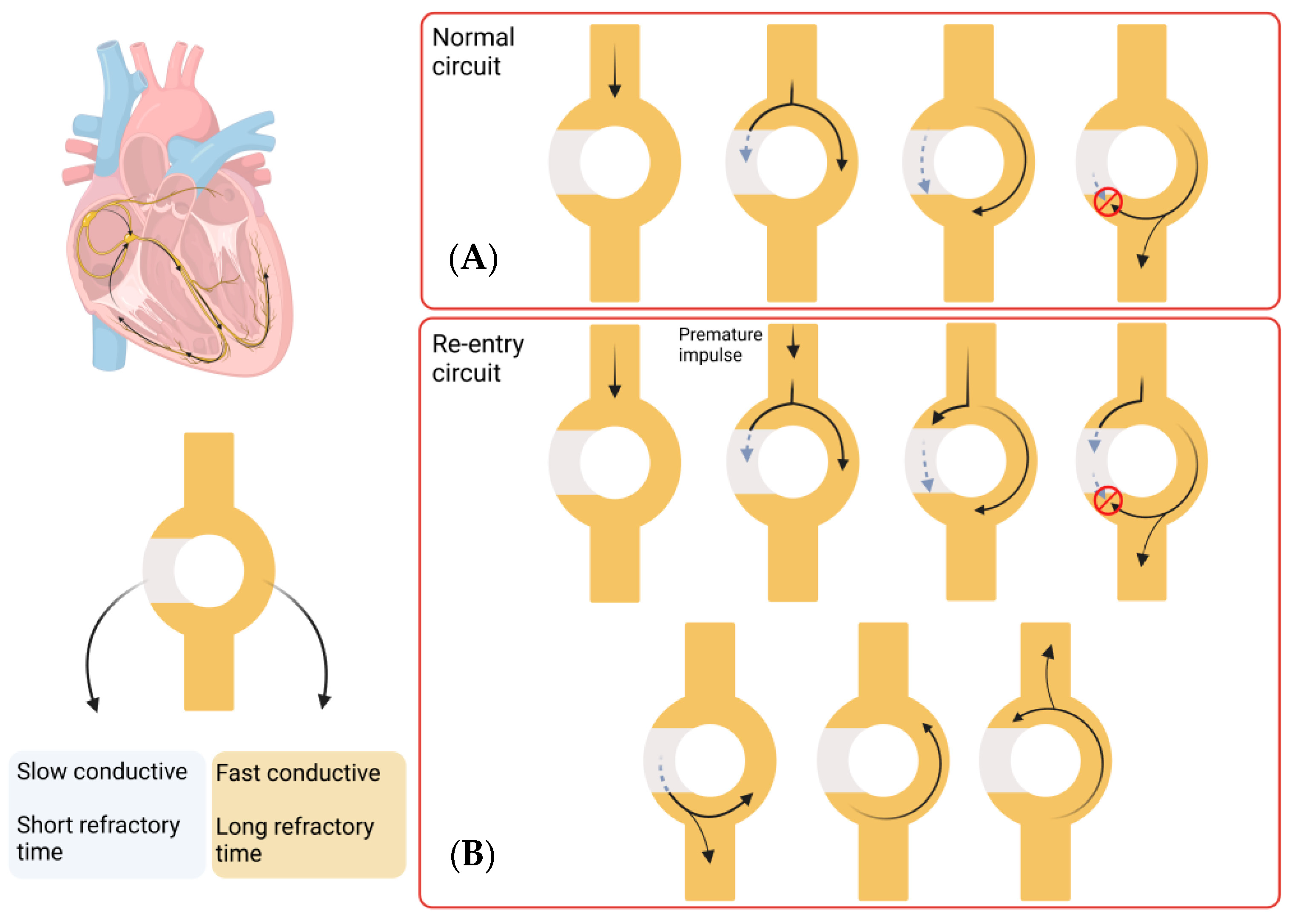
The pathophysiology underlying Afib involves a complex process that is not yet fully understood. Clinically, Afib is divided into paroxysmal, persistent, long-standing persistent, or permanent, depending on the duration of the symptoms. Paroxysmal Afib typically resolves within 7 days of onset, while persistent Afib is sustained for longer than 7 days. Long-standing persistent Afib lasts more than 12 months, and Afib is considered permanent when there has been a joint decision by the patient and clinician to cease further attempts to restore or maintain sinus rhythm [6]. A key component in the maintenance of the Afib arrhythmia state is the process of re-entry, which occurs when an impulse travels abnormally around a cardiac circuit repetitively. This is a triggered process that initiates an arrhythmia, which is commonly induced by an ectopic firing focus. The first reported focal ectopic firing loci were found in the pulmonary veins (PVs) in patients with paroxysmal Afib; ablation of these ectopic foci was shown to reduce Afib burden, demonstrating a role for PVs in Afib genesis [7]. Several subsequent studies have provided evidence for the role of PVs in Afib initiation [8,9,10]. These processes are defined as the group of molecular, cellular and interstitial changes that can manifest as changes in size, mass, geometry and function of the heart after injury [11]. Some of these changes include progressive atrial dilatation [12] or changes in ion channel activity, (particularly Na+ and K+ currents) [13]. Overall, this results in a vulnerable tissue substrate that facilitates the process of re-entry that maintains Afib. Re-entry itself is a process that occurs when a propagating impulse fails to fade after normal activation of the heart and persists, resulting in continuous re-excitation of the heart even after the refractory period has ended. Re-entry requires some type of stimulus that triggers a vulnerable circuit (tissue substrate) where the depolarising signal never encounters refractory tissue which results in the typical increase in heart rate of arrhythmic patients [14]. A schematic representation of this process is shown in Figure 1.
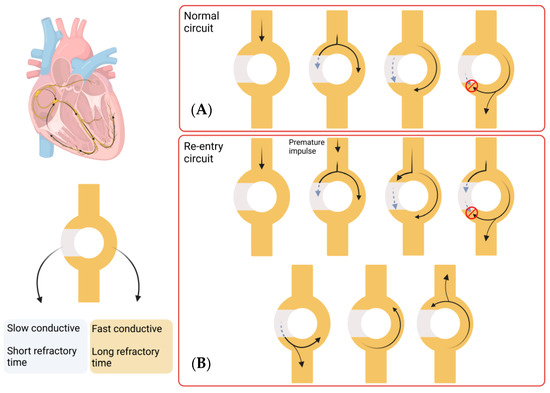
Figure 1.
Re-entry process in Afib. Most of the re-entry circuits happen above the level of the ventricles, but this process can take place in any area of the heart. This circuit consists of a single pathway that divides in two, one which shows slow conductivity and short refractory time and the other which shows fast conductivity and longer refractory time. (A) In a normal circuit, an impulse travels through the circuit and splits into both pathways. The fast conductive pathway propagates the impulse quicker, but the long refractory period makes it inaccessible for a long time (red circle indicates the pulse cannot propagate). The slow conductive pathway propagates the impulse slower, with a quicker recovery. When the impulse in the fast conductive pathway reaches the next pathway, it splits again and propagates the impulse, while the other component interacts with the slow conductive pathway impulse and annulates each other. In this way, the impulse is transmitted forward. (B) A re-entry circuit is created by a premature impulse, which would take the slow conductive pathway due to the short refractory time. This leads to an impulse that does not fade and persists in propagating impulses in both directions, leading to a continuous re-excitation of the heart. (Images created with BioRender.com (accessed on 21 January 2023)).
1.1. Current Therapeutic Options for Atrial Fibrillation
The main therapeutic goal in the management of Afib is the restoration and maintenance of a sinus rhythm. Anti-arrhythmic drugs (AADs) remain the most widely prescribed treatment for Afib patients; however, they are not always effective and show significant adverse effects, including some that for certain patients are pro-arrhythmic [15]. Anti-arrhythmic drugs typically act on ion channels, with the main goal of reducing the frequency and duration of episodes of arrhythmias. AADs are classified depending on the mechanism of action and specific effects on the heart. Class I AADs are sodium channel blockers, and they reduce the conduction velocity and are commonly used to treat atrial and ventricular arrhythmias. They are further subdivided into IA (such as quinidine or procainamide), IB (such as lidocaine or mexiletine) and IC (such as flecainide and propafenone). Class II AADs are beta-blockers, and they stop epinephrine and norepinephrine from binding to the beta-adrenergic receptors, decreasing conduction through the AV node. Some examples are propranolol and metoprolol. Class III AADs block potassium channels, repolarising the cells and prolonging the action potential duration. The most used are amiodarone, sotalol and dronedarone [16,17]. However, the positive effects of these drugs are accompanied by increases in mortality risk in long-term treatments and, on some occasions, exacerbation of the arrhythmia that they are meant to treat [15,18]. In this review, we will focus on non-pharmacological treatment approaches with a specific focus on the utility of in vitro models in supporting the development and enhancement of catheter-based ablation techniques.
1.2. Catheter Ablation
Catheter ablation aims to isolate, disrupt or possibly destroy abnormal foci responsible for Afib. The four myocardial sheaths of the left atrium arising from the pulmonary veins (PV) are identified as the primary trigger source in 82% to 90% of patients with paroxysmal atrial fibrillation. Therefore, PVs are the most common anatomical structures targeted by catheter ablation in the treatment of atrial fibrillation [3]. Some evidence also supports the idea of ablation targeting the ostium of the left atrial appendage (LAA) due to the prevalence of abnormal firing in patients with recurrent Afib [19]. Currently, for Afib, the treatment falls under a thermal or non-thermal ablation. However, recently, the non-thermal approach of pulsed-field ablation (PFA) has been gaining prominence [20]. The most common thermal method is in the form of radiofrequency ablation (RFA), followed by high-intensity focused ultrasound (HIFU) or cryoablation. Currently, catheter ablation is now considered the first-line treatment strategy for symptomatic paroxysmal atrial fibrillation [21].
Radiofrequency ablation uses heat to destroy the area of tissue triggering the Afib. Reported Afib treatment using RFA shows higher efficacy than AAD therapy, with reduced complications [22]. Despite increasing adoption in the clinic, the success rate with RFA varies depending on the patient and the type of Afib present. For the ideal candidate receiving ablation therapy, presenting for paroxysmal Afib, the effectiveness is between 60 and 80% [23]. However, for less-than-optimal patients, such as a patient with persistent Afib, the success rate lies between 50 and 70% [23].
High-intensity focused ultrasound is based on the mechanism of vibration that produces mechanical movement of particles within a medium, which is then converted to heat causing thermal tissue injury. HIFU creates wide and deep lesions (up to 11 mm in depth) and it is energy dose-dependent [24]. HIFU has been shown to have the ability to precisely target a defined location, for example, creating lesions in the cardiac tissue while ensuring that the epicardium and endocardium remain unaffected [25]. In comparison to cryotherapy or RFA, HIFU can easily penetrate soft tissues and can produce an ablation region without the need for direct physical contact. This feature makes HIFU advantageous in situations where a challenging location of the tissue impacts the stability and consistency required for tissue contact. Limitations to HIFU include reported permanent injuries to extracardiac tissue such as the wall of the oesophagus and the phrenic nerve leading to nerve palsy and dyspnoea [26].
Cold ablation or cryotherapy is characterised by the formation of intracellular and extracellular ice crystals, which results in apoptosis of the surrounding tissue. These crystals are produced by cryothermal energy that is produced by the injection of refrigerant through a fine tube catheter, freezing the tissue. The refrigerant vaporises at the tip of a cryoablation catheter and freezes the tissue [27]. While freezing, the catheter tip adheres to the affected tissue, which enables the application of stable energy delivery. In contrast to RFA, tissue lesions induced by cryoablation maintain the tissue structure, including fibrocytes and collagen [28] and present a lower risk of cardiac perforation or thrombogenicity compared to RFA [29]. However similarly to RF, cryo-based ablations can result in damages to the oesophagus [30].
The latest emerging therapy for Afib is pulsed-field ablation. The PFA modality is a non-thermal catheter-based ablation technology that uses high-voltage pulsed electrical fields to ablate tissues through a mechanism known as irreversible electroporation (IRE). This process is based on the application of high electric fields to a cell, leading to the formation of large pores in the cell membrane, ultimately resulting in cell death. IRE has been used previously in other medical areas such as oncology, and due to the reported increased tissue specificity compared to other approaches, PFA may present a safer catheter-ablation option compared to RFA [31] or cryoablation [32].
1.3. Limitations of Current Treatment Approaches
Current therapeutic options are expanding and improving to maximise efficiency and reduce adverse effects on the patient. However, the extensive number of potential targets, variables and parameters associated with the ideal catheter-based intervention creates a challenge in achieving maximally successful control of Afib. The current range of applied parameters may contribute to the variable successes reported in the clinic. To facilitate the development of an experimental-based set of parameters and to achieve successful treatment at minimal cost, it is essential to design an appropriate testing platform that allows us to refine parameters as well as predict and mitigate potential negative side effects. There is a need to strike a balance between the testing and optimisation of the treatments while avoiding the risk to the life and well-being of the patients. The development of appropriate models of Afib is essential not only as a disease model to better understand the mechanisms underlying Afib but also as a therapeutic screening platform to facilitate the testing and development of different treatment options. The absence of appropriate and well-defined models for Afib for in vitro and preclinical testing can be an obstacle to developing improved therapeutics. A good model should allow the testing of a broad spectrum of treatments, which would result in the selection of the best option to be included in future clinical trials. Without these models, only limited information can be obtained, and important side effects can be missed [33,34]. In recent years, several research models have been developed to mimic human cardiac atrial fibrillation, each of which can provide different information depending on their advantages and limitations. In this review, we report on the different models available for the study of Afib, highlighting their advantages and disadvantages, relevant publications/outcomes and most common uses.
2. Current Experimental Models of Atrial Fibrillation
When choosing an experimental model of Afib in the context of device ablations, it is important to consider the level of complexity that is required to answer the experimental question being asked. Animal models provide the highest level of complexity (most commonly dogs, pigs and horses), which provide insight into heart function, effects on surrounding tissues, other organ systems and behavioural responses to interventions. Ex vivo isolated heart studies offer the opportunity to analyse tissue mechanisms under controlled conditions which are very relevant for local short-term acute effects. Finally, the use of in vitro cellular models, both in single-cell monocultures of cardiomyocytes or in the creation of multi-layered and multi-cellular cardiac tissues provides insight into the cellular and molecular mechanisms involved in Afib processes and the effect of the potential treatments, allowing the specific study of changes in cell death and beating patterns. As the complexity of the model increases, typically, the opportunity for higher throughput testing decreases. Thus, the development and optimisation of treatments require a balanced approach and planning a pipeline of tests across multiple levels. Different in vivo animal models are currently available and show many similarities to human Afib studies in terms of benefits but also limitations in terms of costs and ethical considerations. While the data from animal studies have provided valuable information on Afib and its pathophysiology and recent research has successfully induced Afib in animal models [35,36], our understanding and the development of treatment options and clinical targets were revealed through several clinical studies in humans. For instance, Haïssaguerre et al. reported that Afib is initiated by focal areas near the pulmonary veins (PVs) [7]. Furthermore, they showed that RFA of the area surrounding the PVs, so-called pulmonary vein isolation (PVI), was sufficient to terminate Afib [37]. The focus of this review is ex vivo and in vitro models of Afib, as animal models have been extensively reviewed elsewhere [38].
2.1. Ex Vivo Models
Cardiac tissue isolated from animal hearts has been widely used for cardiac studies using a range of biochemical, electrophysiological, pharmacological and morphological approaches, particularly related to antiarrhythmic drug safety [39,40]. Availability of human cardiac tissue is, in general, limited to very small amounts of fresh and viable sections which are considered surgical waste [41,42]. However, these small samples do contribute to the study of the mechanisms and effects of Afib therapy [43,44,45]. Human-explanted tissues help to overcome some of the physiological differences seen in animal models. Nonetheless, the amount and complexity of the available tissue are usually insufficient, often lacking the multi-layered structures which make them closer to conventional in vitro models. These models have the great advantage that they avoid any interference from the autonomic nervous system and internal organ communications, which allows the examination of pure cardiac responses against different interventions. The use of ex vivo cardiac models to determine the ablated area produced by a certain ablation modality has the potential to allow for the testing of a wide array of experimental parameters [46,47,48]. Relevant mechanical and physical aspects of catheter ablation, such as contact angle or contact force, can be optimised for an optimal ablation outcome by ex vivo models as previously reported [49]. Similarly, the effect of temperature or the irrigation solution used in the RF ablation process can be easily tested and measured in excised porcine ventricles [50].
The use of isolated whole hearts was pioneered by Oscar Langendorff, who developed the isolated perfused mammalian heart in the year 1895 [51], and since then, it has been widely used and has aided our understanding of the fundamental physiology of the heart. This model has been used in physiological studies of Afib to better understand the mechanisms of this pathology in numerous animals including rabbits [52], sheep [53], guinea pigs [54] and mice [55]. The Langendorff setup enables the delivery of different compounds or the application of catheters. Recently, the use of actual ex vivo human hearts has become a reality and 83 human hearts declined for transplant were resuscitated in the Visible Heart® Laboratory (Minneapolis, MN, USA), showing pulsatile perfusion and evidence of electrocardiogram rhythm, highlighting the significant potential of perfused ex vivo systems previously used with animal hearts [56]. The Langendorff heart-perfused systems offer a powerful testing platform for catheter-based therapies, particularly epicardial devices. The access to a beating heart allows the physical positioning of the catheter to the desired part of the heart, as well as endocardial devices. The Langendorff heart allows complex processes such as the opening of the catheter inside the heart and the localisation of the electrodes to be tested, which is not as easily achieved in vivo. Ablation in perfused systems has been previously reported for ventricular fibrillation [57]; however, nothing has been reported for the atrium. This opens a new avenue of investigation for catheter ablation testing before in vivo studies, obtaining important information regarding ablation size and the effect on the conductive properties of the heart. This model has been reported to be used for monitoring HIFU-induced modifications of electrical conduction in cardiac tissue using real-time fluorescence, aiming to develop appropriate future clinical interventions [58].
Despite the advances and potential of the ex vivo models for Afib studies, these models are complex and challenging to establish and maintain. Typically, the isolated heart models derive from an animal source, which means they do not fully recreate the human condition. The isolated heart is studied in an environment and conditions that do not fully represent those found in the animal or human body, such as the composition of the blood that is commonly substituted with a surrogate buffer [59]. Several molecular processes become less effective after removing the heart from the blood supply, particularly ATP depletion [60].
2.2. In Vitro Models
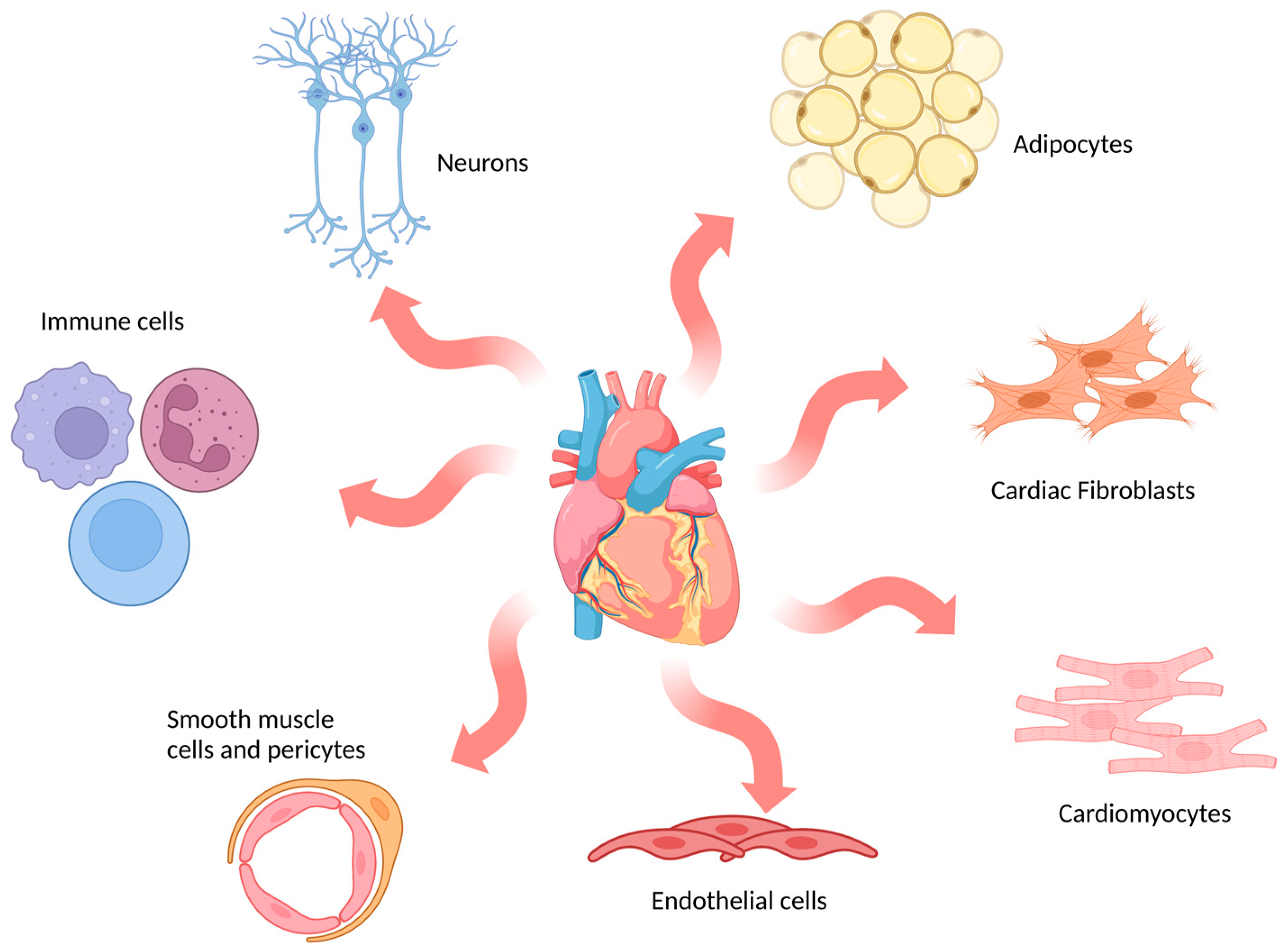
The adult mammalian heart is composed of multiple cell types (Figure 2), including cardiomyocytes, fibroblasts, endothelial cells, immune cells and mural cells (smooth muscle cells and pericytes), together with adipocytes and neuronal cells [61]. In humans, cardiomyocytes are the dominant cell type by volume (70–80% of the adult heart) and are specialised into atrial or ventricular myocytes which are responsible for the contractile forces of the heart [62]. However, they are not the most numerous cell type. Fibroblasts, which are essential for maintaining the structural, electrical and mechanical features of the heart, are the most abundant cell type in the adult heart [63]. In vitro models encompass a broad range of options that mimic cardiac physiology and pathology to different extents.
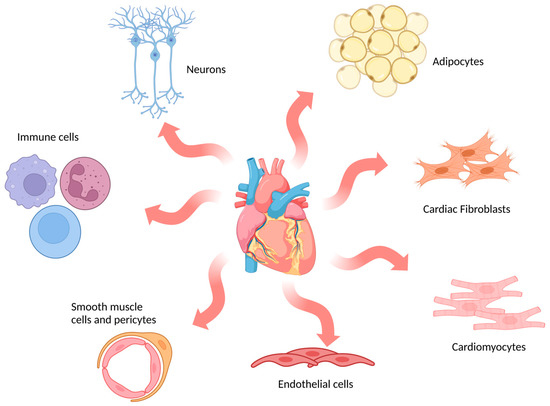
Figure 2.
Schematic representation of main cell types in the human heart. The most abundant cell types found in cardiac tissue are indicated in the image: cardiomyocytes, cardiac fibroblasts, endothelial cells, smooth muscle cells and pericytes, immune cells, neurons and adipocytes. (Image created with BioRender.com (accessed on 21 January 2023)).
Different cell sources are available for in vitro modelling based on the specific requirements of the study. Primary cardiac cells have been the most commonly used cells in the study of cardiac arrhythmias, typically in monolayer cultures. Primary adult cardiomyocytes with a mature ion channel population and sarcomeric structures present are ideal for electrical [64,65], contractile [66] and calcium dynamic studies [67,68,69], including the effects of ablation parameters on these properties of cardiomyocyte physiology. However, they are complex to maintain in culture and show limited capacity for experimentation, as they can only be maintained for a short period of time after isolation. The isolation process can also be challenging and contamination with other unwanted cell types is common [70]. To overcome these limitations, immortalised cell lines have been developed, normally obtained by introducing an oncogene that sustains active cell proliferation. HL-1 cells are an example of immortalised cell line established from an AT-1 subcutaneous tumour excised from an adult mouse [71] and have been used for the identification of specific proteins and regulatory pathways involved in Afib-related atrial remodelling such as calpain [72], endoplasmic reticulum stress-associated autophagy [73] and more general features of Afib [74]. The immature H9c2 cell line was originally derived from embryonic rat ventricular tissue [75]. These cells are widely used in hypertrophy studies related to heart failure [76,77]. The most recent immortalised cell line is AC16 cells, derived from primary human ventricular cardiomyocytes fused with SV40 transformed human skin fibroblasts [78]. This cell line has been used to study cardiac hypertrophy, oxidative stress, mitochondrial dysfunction and electroporation thresholds [79,80,81,82].
Over the last decade, the emergence of induced pluripotent stem cell (iPSC) technology has greatly advanced our understanding of patient-specific molecular mechanisms of disease and serves as a platform for the development of new therapeutics and drug screening [83]. This technology is based on the ability to reprogram disease-specific patient fibroblasts by forcing the expression of specific transcription factors (Oct4, Sox2, cMyc and Klf4), resulting in a pluripotent state [84]. Subsequently, these pluripotent cells are then differentiated into specific mature cells of interest [85]. This approach has the advantage of maintaining the patient’s complete genetic background and allows the impact of certain key mutations on pathophysiology to be studied. iPSCs have been differentiated into cardiomyocytes through a broad variety of protocols [86] and they have provided new insights into the molecular mechanisms of cardiac diseases [87]. The advantages and limitations of patient-derived cardiomyocytes have been extensively reviewed elsewhere [88].
In vivo tissues are not composed of only one cell type, so the inclusion of two or more cell types in the same system, known as a co-culture, has been shown to offer many advantages when simulating the native tissue [89]. Cardiac tissue has a heterogenous and diverse cell composition. Atrial tissues contain mostly cardiomyocytes and fibroblasts together with other less abundant cell types such as smooth muscle, endothelial and immune cells [61]. It has been reported that essential crosstalk through soluble factors between the cardiomyocytes and fibroblasts is vital for the correct functioning of the cardiac tissue but also can become pathogenic during injury, resulting in a general impairment in electric conduction [90,91]. The ability to include several cell types together in the same space makes the co-culture an attractive model to understand the complex interactions between these cell types and their involvement in Afib [92,93].
Regardless of the selected cell source, cells can be organised and distributed differently to create systems with varying levels of complexity. Models with higher complexity aim to closely resemble the physiological conditions of cardiac tissue but may also require more challenging setups and laborious work. In this review, these models are classified based on their dimensionality into 2D and 3D models.
2.2.1. Adherent Cell (2D) Models
Adherent cell, monolayers or 2D culture is the most common type of in vitro model system. The process is based on cell seeding over a substrate that facilitates growth and proliferation in a single-layer structure. These models are attractive because of their ease of use, i.e., cell growth, manipulation and imaging, which allows for an increased number of replicates and higher throughput. Two-dimensional cultures are well established and reported, with a broad number of studies allowing for results comparison between them. In conventional 2D cultures, the whole cell population has even contact with the nutrients and growth factors present in the medium, which results in homogenous growth and proliferation [94]. Even though the 2D cultures are simplified models, they allowed for discoveries such as the importance of inflammatory processes [95] and the identification of potential drugs such as pioglitazone [96]. Moreover, cell monolayers can be ablated, then the affected area can be assessed, showing the potential for preclinical testing of ablation devices for Afib therapy [82]. Recent developments in iPSC-derived monolayers of cardiomyocytes allowed for a detailed investigation of cell death, induced by PFA and the associated temperature changes [97].
2.2.2. 3D Cell Culture Models
Despite the obvious advantages, 2D systems have certain cell and tissue features that differ appreciably from an in vivo system. One of the most negative examples is the different morphology and lack of tissue organisation found in the native tissue. To overcome these limitations and create a smaller gap between 2D in vitro models and in vivo models, some improvements to the classic 2D system have been developed. Cells respond to geometrical and mechanical patterns present in their environment, and the use of cellular micropatterning provides an opportunity to overcome the lack of such patterns when using the standard tissue culture system [98]. Micropatterning is a technique which allows the position of cells in specific areas of a substrate, enabling the control of cell shape, position and culture architecture [99]. Several methods can be used for this aim, such as the use of polymers as a stamp of the desired microstructure (soft lithography) [100] or using UV through photomasks (photolithography) [101]. Some studies have taken advantage of this technique to create cardiac fibres in vitro to closely mimic the heart tissue environment [102,103]. Important features such as calcium handling, action potential firing and conductional velocities observed in micropatterned cultures makes them more like adult mouse myocardium than traditional monolayer cultures [104]. The use of iPSC together with micropatterning further recapitulates in vivo atrial conduction using a 1D spiral pattern [105], thus overcoming the slow conduction present in other iPSC models of Afib [106].
The use of cell monolayers on rigid plastic culture dishes does not allow the cardiac cells to perform physiological contractions, which is the main feature of cardiomyocytes in vivo [107]. In the last few decades, in vitro studies have been trying to overcome this limitation by generating 3D models that can closely represent the physiological behaviour of the cardiac tissue. However, the focus of these models in cardiac research has been primarily on regenerative medicine, specifically the cell replacement lost after cardiac infarction [108]. The number of 3D cell culture systems reported is increasing and the utility of the models is improving. The use of novel 3D in vitro techniques creates new insights into the physiological and pathological processes in heart tissue, making them attractive treatment screening platforms. To date, numerous 3D in vitro models have been developed with different levels of complexity, such as cellular hydrogels and engineered heart tissues (EHTs), organoids and organs-on-a-chip.
3D Hydrogels
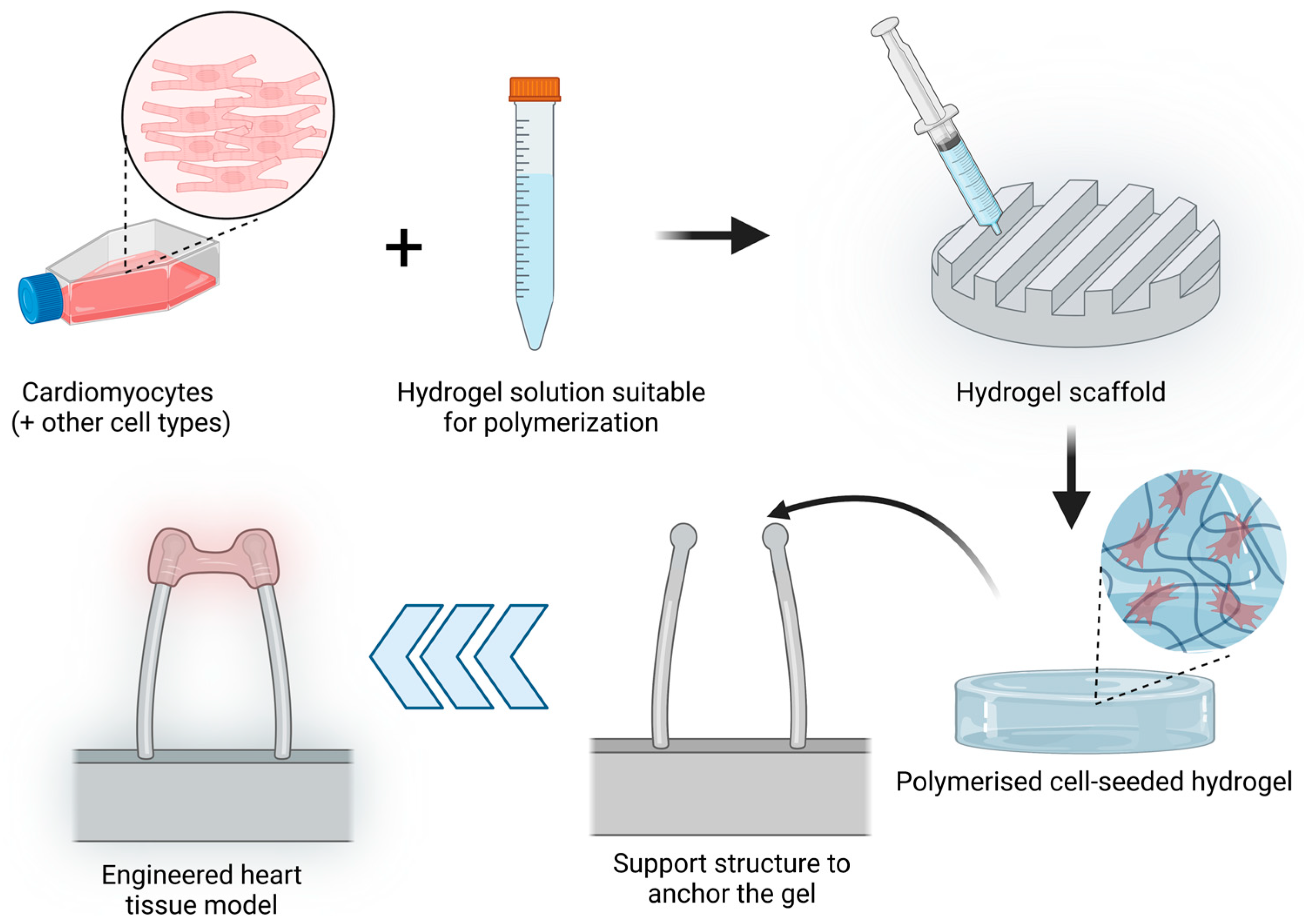
Hydrogels are crosslinked water-soluble polymers that allow the incorporation of cells embedded into the gel tridimensional structure. The porosity of the hydrogel allows molecules such as growth factors, nutrients or drug loading inside the hydrogel [109] and interact with the seeded cells. Initial 3D hydrogel culture studies were based on single compounds such as collagen type I scaffolds [110] but have been rapidly updated by the addition of several components of the extracellular matrix such as fibrinogen or basement-membrane matrix such as Matrigel [111]. Hydrogels have been widely used to develop EHTs, which are tridimensional muscle constructs made from isolated cardiomyocytes of different animals, human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs), first described in 1997 [110,112]. The elements needed to produce an EHT are cardiac cells, a liquid hydrogel that can be polymerised, a scaffold that will determine the overall EHT shape and a support structure to which the hydrogel will be anchored or attached. The simplified process is described in Figure 3. Over the years, different modifications have been introduced to the support structure, including the use of stretching devices to promote hypertrophy and improved contractile function [113] or two elastic silicone posts to allow auxotonic contractions of the EHT [111].
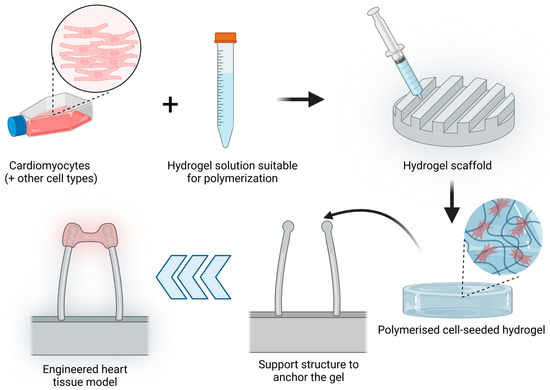
Figure 3.
EHT model production process. Cardiomyocytes alone or together with other cell types (such as fibroblasts) are resuspended in a hydrogel solution that can be polymerised. This mix is added to a scaffold with the desired shape and polymerised to obtain a cell-seeded hydrogel. This hydrogel is further placed in a support structure to anchor the gel. In the image, an example of a commonly used support structure is shown to allow the contraction of the cardiomyocyte-seeded hydrogel. (Image created with BioRender.com (accessed on 21 January 2023)).
EHTs can contract due to the structure of the hydrogel and the interaction between the cardiomyocytes, showing organised sarcomeres and defined beating patterns [114]. These processes, together with electrical stimulation, are essential for the further maturation of the hiPSC-Cardiomyocytes [115]. Recently, the inclusion of other cell types such as fibroblasts has helped create a more accurate representation of the cardiac tissue [116,117]. Furthermore, particularly atrial and ventricular EHTs, can be designed and have shown different features with similarities to those seen in vivo, allowing a highly specific model for the study of cardiac pathologies such as Afib [118]. While EHTs have a great potential for preclinical therapeutic models, as drug screening platforms [119], they are also showing promise as test platforms for ablation-related therapies (Figure 4). The complexity of these models can produce a more accurate result of a given ablation, incorporating a 3D structure as ex vivo models together with viable and healthy cells that can interact and communicate, more representative of the physiological state of the cardiac tissue. Moreover, they keep the relative ease of use and high reproducibility of an in vitro model, avoiding the use of animals for early stages of ablation parameter screening. However, these models are still developing.
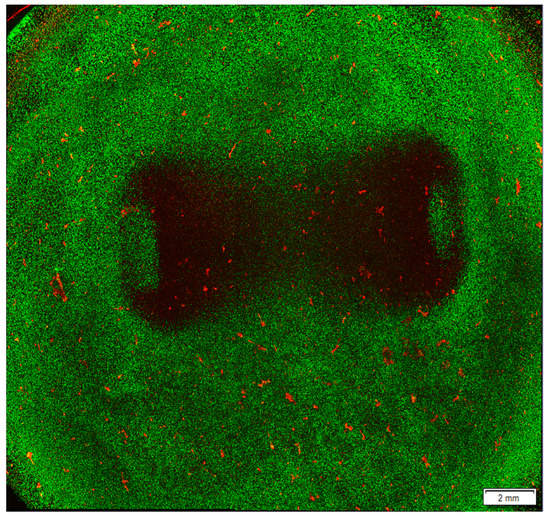
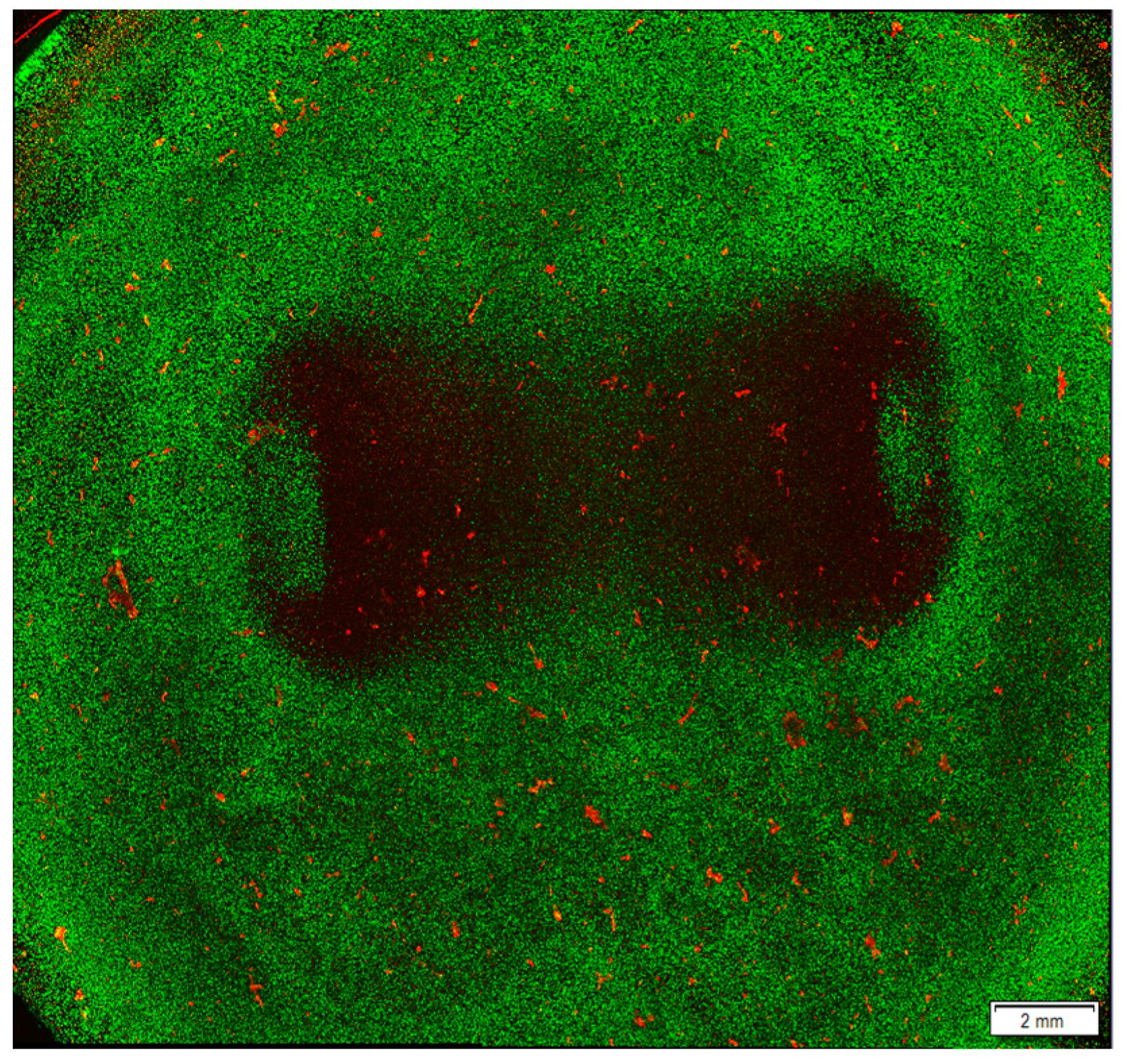
Figure 4.
Ablated area after ablation treatment in a hyaluronic acid hydrogel model seeded with cardiomyocytes. AC16 cardiomyocyte cell line was seeded in a hyaluronic acid hydrogel model and treated with 36,000 biphasic pulses of 2 µs duration and 1250 V of input voltage. After ablation, the cells were stained with Life/Dead staining, which labels live cells in green and dead cells in red. The image was obtained using a confocal microscope to visualise the lesion area. Results obtained by the authors of this study and more details on the method used are described in our previous work [79].
Hydrogels can be designed to be temperature-sensitive which would help to predict possible thermal damage risk of a given set of ablation parameters and set up before further animal and clinical studies [120,121]. EHTs based on 3D hydrogels, together with in silico models, can prove beneficial for predicting and planning therapeutic ablation interventions in Afib, as has been shown previously for cancer treatment models [122,123].
3D Organoids
Organoids are tridimensional, multicellular cultures which are capable of self-organisation into complex tissue-like and organ-like structures, supported by an extracellular matrix (ECM). The ability to create these structures is mostly restricted to stem cells, such as pluripotent embryonic stem cells (ESC) or hiPSCs, which can be further matured into the desired cell phenotype. Other cell types that can create organoids are the tissue-resident adult stem cells (ASCs), which are present in adult tissues and show the ability to self-renew and differentiate into other cell types while preserving their tissue specificity [124,125,126]. ASC-based organoids need to be supported by a cocktail of growth factors in the culture media involved in signalling control in vivo tissue conditions.
In recent years, several production methods have been developed, the most common of which is based on cell seeding over or embedded into a matrix such as Matrigel (an ECM protein mix), which provides an appropriate environment. This method allows the monitoring of processes such as cell adhesion, migration and chemotaxis [127]. The use of spinning reactors allows batch production of organoids with larger sizes. Cells are placed in a container which is constantly stirred to avoid cell attachment [128]. The hanging drop method has been used for decades, taking advantage of gravity to induce cell aggregation and assembly in a droplet of medium typically hanging from the lid of a culture dish. This approach was enhanced using hanging drop plates (HDPs), creating an array of spheroids [129]. Other methods employ non-adherent surfaces to cultivate the cells, facilitating them to form aggregates and eventually make spheroids, which is a simple method with high throughput and is more cost-effective than other methods [130,131]. Magnetic nanoparticles have also been employed, relying on the fact that they are taken up by the cultured cells, allowing them to float in the media and favouring its aggregation and production of an ECM [132]. Finally, the use of bioprinting for organoid formation has gained increasing interest in recent years [133]. This technique allows precise control of the shape and distribution of the ECM and organoids, enabling a better representation of the in vivo environment. Bioprinters’ function is based on additive manufacturing, which deposits the desired material layer by layer until reaching the final desired structure [134].
Human cardiac organoids have a huge potential as disease models for heart disorders. They have the capacity to produce spontaneous and induced electrical activity, showing higher conduction velocities than 2D cultures. Cardiac organoids can help study complex electrical arrhythmic processes such as re-entry, hereditary arrhythmias and personalised medicine using hiPSCs. Shinnawi et al. created a cardiac organoid model based on hiPSCs from patients with the arrhythmogenic syndrome, short QT syndrome. This model showed a similar development of re-entry arrhythmia as seen in patients with this syndrome. This study provides evidence of the ability of cardiac organoids to recapitulate disease phenotype and new insights into the mechanisms underlying this arrhythmia syndrome [135]. Organoid cultures show differences in gene expression patterns compared to the in vivo tissues they try to mimic [136]. Organoid cultures show a high heterogeneity of cell phenotypes, containing various clones in different proportions, making studying specific mutations difficult [137]. The lack of vascular and immune systems in organoid cultures limits the representation of the tissue microenvironment [138]; additionally, due to geometrical constraints, device-based intervention testing is limited. Moreover, the use of hiPSC creates ethical regulation challenges [139]. Despite all of these issues, there is significant potential for applications of organoids in the areas of disease modelling and drug screening.
3. Conclusions and Future Challenges
Current therapeutic options do not fully address the clinical need for Afib. One potential reason is the lack of appropriate preclinical models to sufficiently support a broad array of test parameters, and treatment options to be assessed and allowing safety testing. Some of the limitations of current models are based on inter-species physiological differences (in vivo models) or the lack of tissue complexity and organ interactions (ex vivo and in vitro models). Due to the complexity of factors involved in Afib, the differences between species and the regulatory processes involved in the use of animals and their organs, the choice of the best preclinical model is a challenge that can be key to the success of the treatment in a clinical setting. There is no perfect Afib model for both disease modelling and treatment screening. To ensure an appropriate model selection, it is crucial to consider the study’s requirements and objectives. A summary of the advantages and limitations of each model is shown in Table 1.

Table 1.
Classification of research models of Afib with the advantages and limitations of each system.
For instance, in PFA testing, a model’s tridimensionality and size are critical factors to determine the ablation zone, whereas these factors may not be as crucial when determining the cell response to an AAD. In vitro models have undergone a revolution in the last number of years, especially with the arrival of hiPSC technology and complex 3D culture systems. Improvements are required to obtain completely mature patient-derived cardiomyocytes, combined with the efforts in optimising 3D models and EHTs such that we can create a useful preclinical model for high-throughput testing of device-based innervations. Including multiple cell types and creating a similar environment to in vivo would provide the influence of the crosstalk between different cell types, which is essential in the heart’s functionality. In the future, these models could be combined with perfusion, and hiPSC-derived heart cells to create the idealised tridimensional in vitro patient-specific heart model that would allow the testing of therapies in advance, allowing clinicians to plan and optimise each patient’s best treatment modality and mitigate risks.
Author Contributions
Conceptualisation, J.M.B.-M. and L.R.Q.; writing—original draft preparation, J.M.B.-M.; writing—review and editing, J.M.B.-M., M.J.K., M.O., E.D. and L.R.Q.; project administration, M.O., E.D. and L.R.Q.; funding acquisition, M.O and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Disruptive Technologies Innovation Fund (DTIF), grant number DT20180031A/B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.C.M.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Morin, D.P.; Bernard, M.L.; Madias, C.; Rogers, P.A.; Thihalolipavan, S.; Estes, N.A.M. The state of the art: Atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin. Proc. 2016, 91, 1778–1810. [Google Scholar] [CrossRef] [PubMed]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Rotter, I. Ischemic Stroke Risk Factors in Patients with Atrial Fibrillation Treated with New Oral Anticoagulants. J. Clin. Med. 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Gawałko, M.; Kapłon-Cieślicka, A.; Hohl, M.; Dobrev, D.; Linz, D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int. J. Cardiol. Heart Vasc. 2020, 30, 100631. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Wu, T.J.; Liang, K.W.; Ting, C.T. Relation between the rapid focal activation in the pulmonary vein and the maintenance of paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2001, 24, 902–905. [Google Scholar] [CrossRef]
- Sanders, P.; Morton, J.B.; Davidson, N.C.; Spence, S.J.; Vohra, J.K.; Sparks, P.B.; Kalman, J.M. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation 2003, 108, 1461–1468. [Google Scholar] [CrossRef]
- Mahida, S.; Sacher, F.; Derval, N.; Berte, B.; Yamashita, S.; Hooks, D.; Denis, A.; Amraoui, S.; Hocini, M.; Haissaguerre, M.; et al. Science linking pulmonary veins and atrial fibrillation. Arrhythm. Electrophysiol. Rev. 2015, 4, 40–43. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac remodeling: Concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, A.J.; Abascal, V.M.; Sheehan, M.; Oertel, L.B.; Harrigan, P.; Hughes, R.A.; Weyman, A.E. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990, 82, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Veenhuyzen, G.D.; Simpson, C.S.; Hoshiar, A. Atrial fibrillation. CMAJ 2004, 171, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Valembois, L.; Bergmann, J.-F.; Belmin, J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2015, 3, CD005049. [Google Scholar] [CrossRef]
- Zimetbaum, P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 2012, 125, 381–389. [Google Scholar] [CrossRef]
- EM, V.W. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J. Clin. Pharmacol. 1984, 24, 129–147. [Google Scholar]
- Roden, D.M. Risks and benefits of antiarrhythmic therapy. N. Engl. J. Med. 1994, 331, 785–791. [Google Scholar]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef]
- Bi, S.; Jia, F.; Lv, C.; He, Q.; Xu, X.; Xue, Z.; Su, S. Preclinical study of biphasic asymmetric pulsed field ablation. Front. Cardiovasc. Med. 2022, 9, 859480. [Google Scholar] [CrossRef]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; Crijns, H.J.; Damiano, R.J., Jr.; Davies, D.W.; DiMarco, J.; et al. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm 2012, 9, 632–696.e21. [Google Scholar] [PubMed]
- Calkins, H.; Reynolds, M.R.; Spector, P.; Sondhi, M.; Xu, Y.; Martin, A.; Williams, C.J.; Sledge, I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: Two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2009, 2, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Haegeli, L.M.; Calkins, H. Catheter ablation of atrial fibrillation: An update. Eur. Heart J. 2014, 35, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- He, D.S.; Zimmer, J.E.; Hynynen, K.; Marcus, F.I.; Caruso, A.C.; Lampe, L.F.; Aguirre, M.L. Application of ultrasound energy for intracardiac ablation of arrhythmias. Eur. Heart J. 1995, 16, 961–966. [Google Scholar] [CrossRef]
- Engel, D.J.; Muratore, R.; Hirata, K.; Otsuka, R.; Fujikura, K.; Sugioka, K.; Marboe, C.; Lizzi, F.L.; Homma, S. Myocardial lesion formation using high-intensity focused ultrasound. J. Am. Soc. Echocardiogr. 2006, 19, 932–937. [Google Scholar] [CrossRef]
- Schmidt, B.; Antz, M.; Ernst, S.; Ouyang, F.; Falk, P.; Chun, J.K.R.; Kuck, K.-H. Pulmonary vein isolation by high-intensity focused ultrasound: First-in-man study with a steerable balloon catheter. Heart Rhythm 2007, 4, 575–584. [Google Scholar] [CrossRef]
- Jin, E.S.; Wang, P.J. Cryoballoon ablation for atrial fibrillation: A comprehensive review and practice guide. Korean Circ. J. 2018, 48, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, D.K. Mechanisms of tissue destruction following cryosurgery. Ann. R. Coll. Surg. Engl. 1984, 66, 313–318. [Google Scholar] [PubMed]
- Kaszala, K.; Ellenbogen, K.A. Biophysics of the second-generation cryoballoon: Cryobiology of the big freeze. Circ. Arrhythm. Electrophysiol. 2015, 8, 15–17. [Google Scholar] [CrossRef]
- Nair, K.K.M.; Danon, A.; Valaparambil, A.; Koruth, J.S.; Singh, S.M. Atrioesophageal fistula: A review. J. Atr. Fibrillation 2015, 8, 1331. [Google Scholar]
- Nuccitelli, R. Application of pulsed electric fields to cancer therapy. Bioelectricity 2018, 1, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wittkampf, F.H.M.; van Es, R.; Neven, K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin. Electrophysiol. 2018, 4, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Guichard, J.-B.; Dobrev, D.; Nattel, S. Translational challenges in atrial fibrillation. Circ. Res. 2018, 122, 752–773. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Algalarrondo, V.; Voigt, N.; Melka, J.; Wehrens, X.H.T.; Dobrev, D.; Nattel, S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: A critical analysis. Cardiovasc. Res. 2016, 109, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Halațiu, V.B.; Perian, M.; Balan, A.I.; Scridon, A. Transesophageal atrial burst pacing for atrial fibrillation induction in rats. J. Vis. Exp. 2022, 180, e63567. [Google Scholar] [CrossRef]
- Scridon, A.; Perian, M.; Grigoraş, T.; Halaţiu, V.; Vântu, A.; Balan, A.; Cosma, I.; Barmou, A.; Finascu, B.; Moldovan, D.; et al. Spontaneous atrial fibrillation after long-term transesophageal atrial burst pacing in rats. Technical and procedural approach to a new in vivo atrial fibrillation model. Rev. Romana Med. Lab. 2018, 26, 105–111. [Google Scholar] [CrossRef]
- Tayebjee, M.H.; Hunter, R.J.; Baker, V.; Creta, A.; Duncan, E.; Sporton, S.; Earley, M.J.; Schilling, R.J. Pulmonary vein isolation with radiofrequency ablation followed by cryotherapy: A novel strategy to improve clinical outcomes following catheter ablation of paroxysmal. Europace 2011, 13, 1250–1255. [Google Scholar] [CrossRef]
- Schüttler, D.; Bapat, A.; Kääb, S.; Lee, K.; Tomsits, P.; Clauss, S.; Hucker, W.J. Animal models of atrial fibrillation. Circ. Res. 2020, 127, 91–110. [Google Scholar] [CrossRef]
- Bussek, A.; Schmidt, M.; Bauriedl, J.; Ravens, U.; Wettwer, E.; Lohmann, H. Cardiac tissue slices with prolonged survival for in vitro drug safety screening. J. Pharmacol. Toxicol. Methods 2012, 66, 145–151. [Google Scholar] [CrossRef]
- Rosen, M. Isolated tissue models and proarrhythmia. Eur. Heart J. Suppl. 2001, 3, K64–K69. [Google Scholar] [CrossRef]
- Boukens, B.J.; Sulkin, M.S.; Gloschat, C.R.; Ng, F.S.; Vigmond, E.J.; Efimov, I.R. Transmural APD gradient synchronizes repolarization in the human left ventricular wall. Cardiovasc. Res. 2015, 108, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jost, N.; Virág, L.; Comtois, P.; Ordög, B.; Szuts, V.; Seprényi, G.; Bitay, M.; Kohajda, Z.; Koncz, I.; Nagy, N.; et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J. Physiol. 2013, 591, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Gramley, F.; Lorenzen, J.; Jedamzik, B.; Gatter, K.; Koellensperger, E.; Munzel, T.; Pezzella, F. Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc. Pathol. 2010, 19, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yi, X.; Li, X.; Jiang, X. Fibroblast growth factor-21 is positively associated with atrial fibrosis in atrial fibrillation patients with rheumatic heart disease. Int. J. Clin. Exp. Pathol. 2015, 8, 14901–14908. [Google Scholar]
- Csepe, T.A.; Hansen, B.J.; Fedorov, V.V. Atrial fibrillation driver mechanisms: Insight from the isolated human heart. Trends Cardiovasc. Med. 2017, 27, 1–11. [Google Scholar] [CrossRef][Green Version]
- Calzolari, V.; De Mattia, L.; Basso, F.; Crosato, M.; Scalon, A.; Squasi, P.A.M.; Del Favero, S.; Cernetti, C. Ablation catheter orientation: In vitro effects on lesion size and in vivo analysis during PVI for atrial fibrillation. Pacing Clin. Electrophysiol. 2020, 43, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.D.; Ravi, V.; Rhodes, P.; Du-Fay-de-Lavallaz, J.M.; Winterfield, J.; Allen-Proctor, M.; Wasserlauf, J.; Krishnan, K.; Trohman, R.; Sharma, P.S.; et al. Use of infrared thermography to delineate temperature gradients and critical isotherms during catheter ablation with normal and half normal saline: Implications for safety and efficacy. J. Cardiovasc. Electrophysiol. 2021, 32, 2035–2044. [Google Scholar] [CrossRef]
- Masnok, K.; Watanabe, N. Catheter contact area strongly correlates with lesion area in radiofrequency cardiac ablation: An ex vivo porcine heart study. J. Interv. Card. Electrophysiol. 2022, 63, 561–572. [Google Scholar] [CrossRef]
- Huang, S.-T.; Dong, J.-Z.; Du, X.; Wu, J.-H.; Yu, R.-H.; Long, D.-Y.; Ning, M.; Sang, C.-H.; Jiang, C.-X.; Bai, R.; et al. Relationship Between Ablation Lesion Size Estimated by Ablation Index and Different Ablation Settings-an Ex Vivo Porcine Heart Study. J. Cardiovasc. Transl. Res. 2020, 13, 965–969. [Google Scholar] [CrossRef]
- Park, S.; Hwang, J.; Park, J.E.; Ahn, Y.C. Application of ultrasound thermal imaging for monitoring laser ablation in ex vivo cardiac tissue. Lasers Surg. Med. 2020, 52, 218–227. [Google Scholar] [CrossRef]
- Langendorff, O. Untersuchungen am überlebenden Säugethierherzen. Pflügers Arch. 1895, 61, 291–332. [Google Scholar] [CrossRef]
- Ninio, D.M.; Murphy, K.J.; Howe, P.R.; Saint, D.A. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J. Cardiovasc. Electrophysiol. 2005, 16, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, F. Cholinergic atrial fibrillation: IK,ACh gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc. Res. 2003, 59, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, J.E.; Diness, J.G.; Sheykhzade, M.; Grunnet, M.; Jespersen, T. Synergistic antiarrhythmic effect of combining inhibition of Ca2+-activated K+ (SK) channels and voltage-gated Na+ channels in an isolated heart model of atrial fibrillation. Heart Rhythm 2015, 12, 409–418. [Google Scholar] [CrossRef]
- Liao, C.; Akazawa, H.; Tamagawa, M.; Ito, K.; Yasuda, N.; Kudo, Y.; Yamamoto, R.; Ozasa, Y.; Fujimoto, M.; Wang, P.; et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J. Clin. Investig. 2010, 120, 242–253. [Google Scholar] [CrossRef]
- Chen, J.; Mandapati, R.; Berenfeld, O.; Skanes, A.C.; Gray, R.A.; Jalife, J. Dynamics of wavelets and their role in atrial fibrillation in the isolated sheep heart. Cardiovasc. Res. 2000, 48, 220–232. [Google Scholar] [CrossRef]
- Pak, H.-N.; Oh, Y.-S.; Liu, Y.-B.; Wu, T.-J.; Karagueuzian, H.S.; Lin, S.-F.; Chen, P.-S. Catheter ablation of ventricular fibrillation in rabbit ventricles treated with beta-blockers. Circulation 2003, 108, 3149–3156. [Google Scholar] [CrossRef]
- Deng, C.X.; Qu, F.; Nikolski, V.P.; Zhou, Y.; Efimov, I.R. Fluorescence imaging for real-time monitoring of high-intensity focused ultrasound cardiac ablation. Ann. Biomed. Eng. 2005, 33, 1352–1359. [Google Scholar] [CrossRef]
- Skrzypiec-Spring, M.; Grotthus, B.; Szelag, A.; Schulz, R. Isolated heart perfusion according to Langendorff—Still viable in the new millennium. J. Pharmacol. Toxicol. Methods 2007, 55, 113–126. [Google Scholar] [CrossRef]
- Doring, H.J.; Dehnert, H. The Isolated Perfused Heart according to Langendorff; BVM-Biomesstechnik Verlag: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes structure, function and associated pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Doppler, S.A.; Carvalho, C.; Lahm, H.; Deutsch, M.-A.; Dreßen, M.; Puluca, N.; Lange, R.; Krane, M. Cardiac fibroblasts: More than mechanical support. J. Thorac. Dis. 2017, 9, S36–S51. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.A.; Creazzo, T.; Hartzell, H.C. A time-dependent and voltage-sensitive K+ current in single cells from frog atrium. J. Gen. Physiol. 1986, 88, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Jahnel, U.; Nawrath, H.; Carmeliet, E.; Vereecke, J. Depolarization-induced influx of sodium in response to phenylephrine in rat atrial heart muscle. J. Physiol. 1991, 432, 621–637. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Lee, H.; Bray, M.-A.; Geisse, N.A.; Huang, Y.-T.; Adams, W.J.; Sheehy, S.P.; Parker, K.K. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 2012, 181, 2030–2037. [Google Scholar] [CrossRef]
- Vites, A.M.; Pappano, A.J. Distinct modes of inhibition by ruthenium red and ryanodine of calcium-induced calcium release in avian atrium. J. Pharmacol. Exp. Ther. 1994, 268, 1476–1484. [Google Scholar]
- Cheung, J.Y.; Tillotson, D.L.; Yelamarty, R.V.; Scaduto, R.C. Cytosolic free calcium concentration in individual cardiac myocytes in primary culture. Am. J. Physiol. 1989, 256, C1120–C1130. [Google Scholar] [CrossRef]
- Sowell, B.; Fast, V.G. Ionic mechanism of shock-induced arrhythmias: Role of intracellular calcium. Heart Rhythm 2012, 9, 96–104. [Google Scholar] [CrossRef][Green Version]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Lanson, N.A.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef]
- Ke, L.; Qi, X.Y.; Dijkhuis, A.-J.; Chartier, D.; Nattel, S.; Henning, R.H.; Kampinga, H.H.; Brundel, B.J.J.M. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J. Mol. Cell. Cardiol. 2008, 45, 685–693. [Google Scholar] [CrossRef]
- Wiersma, M.; Meijering, R.A.M.; Qi, X.-Y.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B.J.J.M. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. J. Am. Heart Assoc. 2017, 6, e006458. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Deng, C.-Y.; Wu, S.-L.; Xiao, D.-Z.; Yu, X.-Y.; Kuang, S.-J.; Lin, Q.-X.; Shan, Z.-X. Involvement of Src in L-type Ca2+ channel depression induced by macrophage migration inhibitory factor in atrial myocytes. J. Mol. Cell. Cardiol. 2009, 47, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Kimes, B.W.; Brandt, B.L. Properties of a clonal muscle cell line from rat heart. Exp. Cell Res. 1976, 98, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chueh, P.J.; Tseng, C.-T.; Liu, K.-Y.; Tsai, H.-Y.; Kuo, W.-W.; Chou, M.-Y.; Yang, J.-J. ZAK re-programs atrial natriuretic factor expression and induces hypertrophic growth in H9c2 cardiomyoblast cells. Biochem. Biophys. Res. Commun. 2004, 324, 973–980. [Google Scholar] [CrossRef]
- Vindis, C.; D’Angelo, R.; Mucher, E.; Nègre-Salvayre, A.; Parini, A.; Mialet-Perez, J. Essential role of TRPC1 channels in cardiomyoblasts hypertrophy mediated by 5-HT2A serotonin receptors. Biochem. Biophys. Res. Commun. 2010, 391, 979–983. [Google Scholar] [CrossRef]
- Davidson, M.M.; Nesti, C.; Palenzuela, L.; Walker, W.F.; Hernandez, E.; Protas, L.; Hirano, M.; Isaac, N.D. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 39, 133–147. [Google Scholar] [CrossRef]
- Dutta, D.; Xu, J.; Kim, J.-S.; Dunn, W.A.; Leeuwenburgh, C. Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 2013, 9, 328–344. [Google Scholar] [CrossRef]
- Cui, L.; Guo, J.; Zhang, Q.; Yin, J.; Li, J.; Zhou, W.; Zhang, T.; Yuan, H.; Zhao, J.; Zhang, L.; et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol. Lett. 2017, 275, 28–38. [Google Scholar] [CrossRef]
- Wen, J.; Shen, J.; Zhou, Y.; Zhao, X.; Dai, Z.; Jin, Y. Pyrroloquinoline quinone attenuates isoproterenol hydrochloride-induced cardiac hypertrophy in AC16 cells by inhibiting the NF-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 873–885. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; O’Halloran, T.; Clarke, C.; Donaghey, K.; Dunne, E.; O’Halloran, M.; Quinlan, L.R. Electroporation parameters for human cardiomyocyte ablation in vitro. J. Cardiovasc. Dev. Dis. 2022, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Banerjee, I. Recent advances in the applications of iPSC technology. Curr. Opin. Biotechnol. 2019, 60, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G. Stem cells. Diseases in a dish take off. Science 2010, 330, 1172–1173. [Google Scholar] [CrossRef]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar]
- Funakoshi, S.; Yoshida, Y. Recent progress of iPSC technology in cardiac diseases. Arch. Toxicol. 2021, 95, 3633–3650. [Google Scholar]
- Knollmann, B.C. Induced pluripotent stem cell-derived cardiomyocytes: Boutique science or valuable arrhythmia model? Circ. Res. 2013, 112, 969–976; discussion 976. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Ono, K.; Hata, S.; Suzuki, T.; Kumamoto, H.; Sasano, H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J. Steroid Biochem. Mol. Biol. 2012, 131, 68–75. [Google Scholar] [CrossRef]
- Cartledge, J.E.; Kane, C.; Dias, P.; Tesfom, M.; Clarke, L.; Mckee, B.; Al Ayoubi, S.; Chester, A.; Yacoub, M.H.; Camelliti, P.; et al. Functional crosstalk between cardiac fibroblasts and adult cardiomyocytes by soluble mediators. Cardiovasc. Res. 2015, 105, 260–270. [Google Scholar] [CrossRef]
- Thompson, S.A.; Copeland, C.R.; Reich, D.H.; Tung, L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation 2011, 123, 2083–2093. [Google Scholar] [CrossRef]
- Rahmutula, D.; Marcus, G.M.; Wilson, E.E.; Ding, C.-H.; Xiao, Y.; Paquet, A.C.; Barbeau, R.; Barczak, A.J.; Erle, D.J.; Olgin, J.E. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-β1. Cardiovasc. Res. 2013, 99, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Rohr, S. Arrhythmogenic implications of fibroblast-myocyte interactions. Circ. Arrhythm. Electrophysiol. 2012, 5, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Sirish, P.; Li, N.; Timofeyev, V.; Zhang, X.-D.; Wang, L.; Yang, J.; Lee, K.S.S.; Bettaieb, A.; Ma, S.M.; Lee, J.H.; et al. Molecular mechanisms and new treatment paradigm for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e003721. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, X.; Wang, Q.-X.; Guo, M.; Liu, F.; Song, Z.-P.; Zhang, D.-D. Beneficial effects of pioglitazone on atrial structural and electrical remodeling in vitro cellular models. J. Mol. Cell. Cardiol. 2013, 65, 1–8. [Google Scholar] [CrossRef]
- Casciola, M.; Feaster, T.K.; Caiola, M.J.; Keck, D.; Blinova, K. Human in vitro assay for irreversible electroporation cardiac ablation. Front. Physiol. 2022, 13, 1064168. [Google Scholar] [CrossRef]
- Théry, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Bélisle, J.M.; Kunik, D.; Costantino, S. Rapid multicomponent optical protein patterning. Lab Chip 2009, 9, 3580–3585. [Google Scholar] [CrossRef]
- Badie, N.; Bursac, N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 2009, 96, 3873–3885. [Google Scholar] [CrossRef]
- McDevitt, T.C.; Angello, J.C.; Whitney, M.L.; Reinecke, H.; Hauschka, S.D.; Murry, C.E.; Stayton, P.S. In Vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J. Biomed. Mater. Res. 2002, 60, 472–479. [Google Scholar] [CrossRef]
- Thomas, S.P.; Bircher-Lehmann, L.; Thomas, S.A.; Zhuang, J.; Saffitz, J.E.; Kléber, A.G. Synthetic strands of neonatal mouse cardiac myocytes: Structural and electrophysiological properties. Circ. Res. 2000, 87, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Biendarra-Tiegs, S.M.; Yechikov, S.; Shergill, B.; Brumback, B.; Takahashi, K.; Shirure, V.S.; Gonzalez, R.E.; Houshmand, L.; Zhong, D.; Weng, K.-C.; et al. An iPS-derived in vitro model of human atrial conduction. Physiol. Rep. 2022, 10, e15407. [Google Scholar] [CrossRef] [PubMed]
- Laksman, Z.; Wauchop, M.; Lin, E.; Protze, S.; Lee, J.; Yang, W.; Izaddoustdar, F.; Shafaattalab, S.; Gepstein, L.; Tibbits, G.F.; et al. Modeling Atrial Fibrillation using Human Embryonic Stem Cell-Derived Atrial Tissue. Sci. Rep. 2017, 7, 5268. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Yasuda, S.; Katoh, M.; Yamada, K.P.; Yamashita, H.; Saeki, Y.; Sunagawa, K.; Nagai, R.; Hisada, T.; Sugiura, S. Single cell mechanics of rat cardiomyocytes under isometric, unloaded, and physiologically loaded conditions. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H196–H202. [Google Scholar] [CrossRef] [PubMed]
- Häneke, T.; Sahara, M. Progress in bioengineering strategies for heart regenerative medicine. Int. J. Mol. Sci. 2022, 23, 3482. [Google Scholar] [CrossRef]
- Mishra, B.; Upadhyay, M.; Adena, S.R. Hydrogels: An introduction to a controlled drug delivery device, synthesis and application in drug delivery and tissue engineering. Austin. J. Biomed. Eng. 2017, 4, 1037. [Google Scholar]
- Eschenhagen, T.; Fink, C.; Remmers, U.; Scholz, H.; Wattchow, J.; Weil, J.; Zimmermann, W.; Dohmen, H.H.; Schäfer, H.; Bishopric, N.; et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart muscle model system. FASEB J. 1997, 11, 683–694. [Google Scholar] [CrossRef]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwoerer, A.P.; Uebeler, J.; Eschenhagen, T. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Schneiderbanger, K.; Schubert, P.; Didié, M.; Münzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef]
- Fink, C.; Ergün, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000, 14, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human engineered heart tissue: Analysis of contractile force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.; Jackman, C.P.; Li, Y.; Bursac, N. Developmental stage-dependent effects of cardiac fibroblasts on function of stem cell-derived engineered cardiac tissues. Sci. Rep. 2017, 7, 42290. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Eder, A.; Vollert, I.; Hansen, A.; Eschenhagen, T. Human engineered heart tissue as a model system for drug testing. Adv. Drug Deliv. Rev. 2016, 96, 214–224. [Google Scholar] [CrossRef]
- Maruyama, H.; Yokota, Y.; Hosono, K.; Arai, F. Hydrogel Heart Model with Temperature Memory Properties for Surgical Simulation. Sensors 2019, 19, 1102. [Google Scholar] [CrossRef]
- Yokota, Y.; Maruyama, H.; Arai, F. Hydrogel Heart Model Having Temperature Indicating Function. In Proceedings of the 2018 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 9–12 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–2. [Google Scholar]
- Arena, C.B.; Szot, C.S.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. A three-dimensional in vitro tumor platform for modeling therapeutic irreversible electroporation. Biophys. J. 2012, 103, 2033–2042. [Google Scholar] [CrossRef]
- Wasson, E.M.; Alinezhadbalalami, N.; Brock, R.M.; Allen, I.C.; Verbridge, S.S.; Davalos, R.V. Understanding the role of calcium-mediated cell death in high-frequency irreversible electroporation. Bioelectrochemistry 2020, 131, 107369. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.A.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Kumar, A.; Starly, B. Large scale industrialized cell expansion: Producing the critical raw material for biofabrication processes. Biofabrication 2015, 7, 044103. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef]
- Yuhas, J.M.; Li, A.P.; Martinez, A.O.; Ladman, A.J. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977, 37, 3639–3643. [Google Scholar]
- Tseng, H.; Gage, J.A.; Raphael, R.M.; Moore, R.H.; Killian, T.C.; Grande-Allen, K.J.; Souza, G.R. Assembly of a three-dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng. Part C Methods 2013, 19, 665–675. [Google Scholar] [CrossRef]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D bioprinting of organoids. Bio-Des. Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Shinnawi, R.; Shaheen, N.; Huber, I.; Shiti, A.; Arbel, G.; Gepstein, A.; Ballan, N.; Setter, N.; Tijsen, A.J.; Borggrefe, M.; et al. Modeling Reentry in the Short QT Syndrome With Human-Induced Pluripotent Stem Cell-Derived Cardiac Cell Sheets. J. Am. Coll. Cardiol. 2019, 73, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N. Organoids. Nat. Methods 2018, 15, 23. [Google Scholar] [CrossRef]
- Munsie, M.; Hyun, I.; Sugarman, J. Ethical issues in human organoid and gastruloid research. Development 2017, 144, 942–945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).