Beta-Lactam Antibiotics Can Be Measured in the Exhaled Breath Condensate in Mechanically Ventilated Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Preparation
2.2. Antibiotic Infusion
2.3. Bronchoalveolar Lavage
2.4. Plasma Sampling
2.5. HMEF Processing
2.6. Analysis of Beta-Lactam Antibiotic Concentrations
2.7. Statistical Analysis
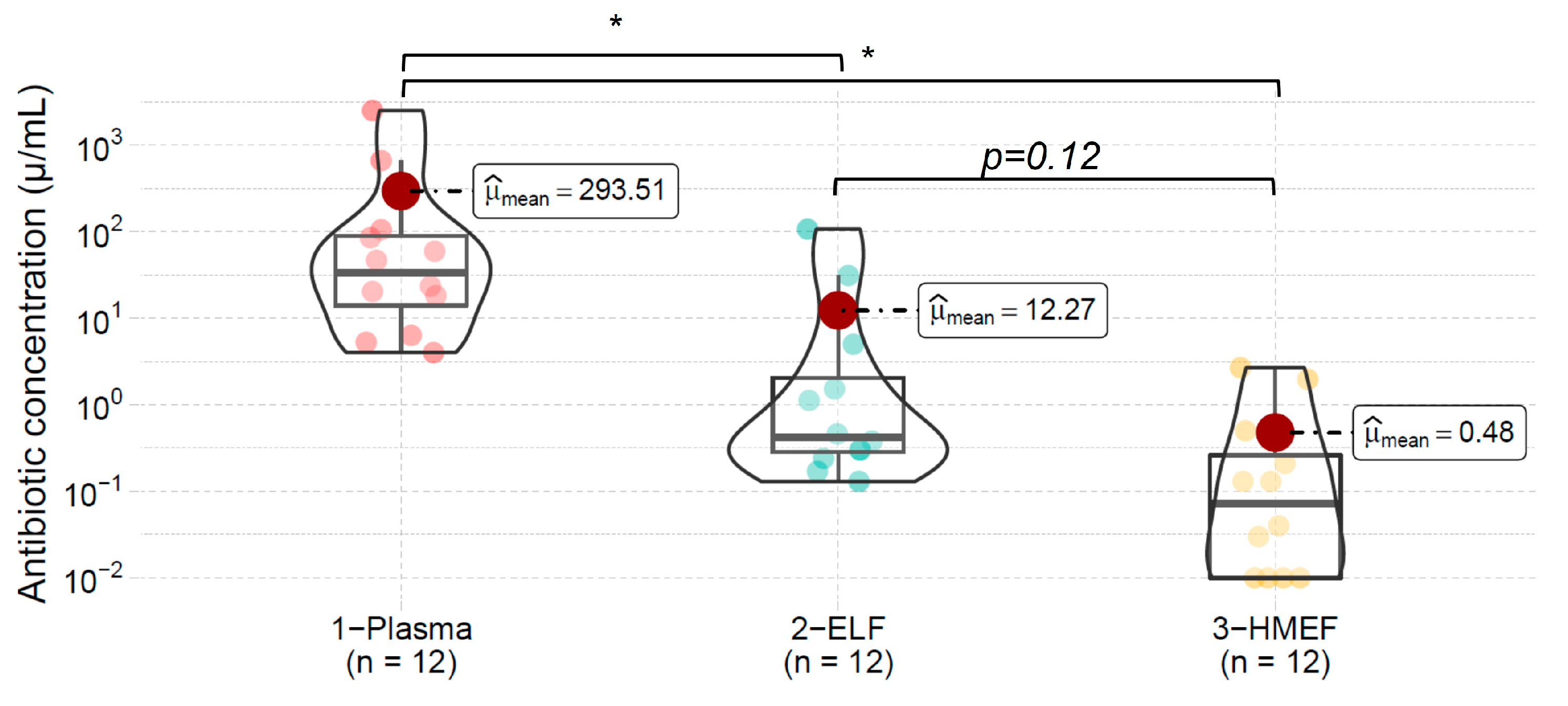
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Prim. 2021, 7, 25. [Google Scholar] [CrossRef]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- Meehan, T.P.; Fine, M.J.; Krumholz, H.M.; Scinto, J.D.; Galusha, D.H.; Mockalis, J.T.; Weber, G.F.; Petrillo, M.K.; Houck, P.M.; Fine, J.M. Quality of Care, Process, and Outcomes in Elderly Patients With Pneumonia. JAMA 1997, 278, 2080–2084. [Google Scholar] [CrossRef]
- Onufrak, N.J.; Forrest, A.; Gonzalez, D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016, 38, 1930–1947. [Google Scholar] [CrossRef] [Green Version]
- Hutschala, D.; Kinstner, C.; Skhirtladze, K.; Mayer-Helm, B.X.; Zeitlinger, M.; Wisser, W.; Müller, M.; Tschernko, E. The impact of perioperative atelectasis on antibiotic penetration into lung tissue: An in vivo microdialysis study. Intensive Care Med. 2008, 34, 1827–1834. [Google Scholar] [CrossRef]
- Baldwin, D.R. The penetration of novel intravenous cephalosporins into the lung. J. Chemother. 1996, 8 (Suppl. S2), 71–82. [Google Scholar] [PubMed]
- Riccobene, T.A.; Pushkin, R.; Jandourek, A.; Knebel, W.; Khariton, T. Penetration of Ceftaroline into the Epithelial Lining Fluid of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2016, 60, 5849–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, R.; Anantham, D. Role of bronchoalveolar lavage in the management of immunocompromised patients with pulmonary infiltrates. Ann. Transl. Med. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Caci, G.; Minervini, F.; Fechner, C.; Roos, J.E.; Obermann, E.C.; Azzola, A. Bronchoalveolar lavage-detected SARS-CoV-2 infection in presence of serial negative nasopharyngeal swabs: A case report. Ann. Transl. Med. 2021, 9, 583. [Google Scholar] [CrossRef]
- van der Zee, P.; van Walree, I.; Fijen, J.W.; van Houte, A.J.; van Velzen-Blad, H.; Rijkers, G.; Gommers, D.; Endeman, H. Cytokines and Chemokines Are Detectable in Swivel-Derived Exhaled Breath Condensate (SEBC): A Pilot Study in Mechanically Ventilated Patients. Dis. Markers 2020, 2020, 2696317. [Google Scholar] [CrossRef] [Green Version]
- McRae, K.; De Perrot, M.; Fischer, S.; Waddell, T.K.; Liu, M.; Keshavjee, S. Detection of IL-10 in the exhaled breath condensate, plasma and tissue during ischemia-reperfusion injury in experimental lung transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2001, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.B.; Shaver, C.M.; Kerchberger, V.E.; Russell, D.W.; Grove, B.S.; Warren, M.A.; Wickersham, N.E.; Ware, L.B.; McDonald, W.H.; Bastarache, J.A. Novel Method for Noninvasive Sampling of the Distal Airspace in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Herregodts, J.; Van Vooren, S.; Deschuyteneer, E.; Dhaese SA, M.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Measuring antibiotics in exhaled air in critically ill, non-ventilated patients: A feasibility and proof of concept study. J. Crit. Care 2019, 51, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, V.; Escalona, J.A.; Pavez, N.; Rivas, E.; Soto, D.; Severino, N.; Bachmann, M.C.; Bruhn, A.; Bugedo, G.; Retamal, J. Cuantificación de la concentración antibiótica en el agua pulmonar condensada en filtros humidificadores y en el lavado bronquioalveolar de pacientes conectados a ventilación mecánica. In Proceedings of the XXXVII Congreso Chileno de Medicina Intensiva, Iquique, Chile, 12–15 November 2019; Volume 54. [Google Scholar]
- Rodvold, K.A.; George, J.M.; Yoo, L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: Focus on antibacterial agents. Clin. Pharmacokinet. 2011, 50, 637–664. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D. Humidification of respired gases during mechanical ventilation: Mechanical considerations. Respir. Care Clin. North Am. 2006, 12, 253–261. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Dulhunty, J.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Araos, J.; Alegria, L.; Garcia, P.; Cruces, P.; Soto, D.; Erranz, B.; Amthauer, M.; Salomon, T.; Medina, T.; Rodriguez, F.; et al. Near-Apneic Ventilation Decreases Lung Injury and Fibroproliferation in an Acute Respiratory Distress Syndrome Model with Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2019, 199, 603–612. [Google Scholar] [CrossRef]
- Andresen, M.; Araos, J.; Wong, K.Y.; Leung, Y.C.; So, L.Y.; Wong, W.T.; Cabrera, S.; Silva, C.; Alegria, L.; Bruhn, A.; et al. Evaluation of Meropenem Pharmacokinetics in an Experimental Acute Respiratory Distress Syndrome (ARDS) Model during Extracorporeal Membrane Oxygenation (ECMO) by Using a PenP β-Lactamase Biosensor. Sensors 2018, 18, 1424. [Google Scholar] [CrossRef] [Green Version]
- Andresen, M.; Wong, K.Y.; Leung, Y.C.; Wong, W.T.; Chan, P.H.; Andresen-Vasquez, M.; Alegria, L.; Silva, C.; Tapia, P.; Downey, P.; et al. Method Based on the β-Lactamase PenPC Fluorescent Labeled for β-Lactam Antibiotic Quantification in Human Plasma. BioMed Res. Int. 2016, 2016, 4307987. [Google Scholar] [CrossRef] [Green Version]
- Beal, S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Dunne, A.; Kimko, H.; Nandy, P.; Vermeulen, A. Impact of low percentage of data below the quantification limit on parameter estimates of pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2011, 38, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Jansen, R.S.; Rosing, H.; Thijssen, B.; Beijnen, J.H.; Schellens, J.H.; Huitema, A.D. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol. Res. Perspect. 2015, 3, e00131. [Google Scholar] [CrossRef] [PubMed]
- Byon, W.; Fletcher, C.V.; Brundage, R.C. Impact of censoring data below an arbitrary quantification limit on structural model misspecification. J. Pharmacokinet. Pharmacodyn. 2008, 35, 101–116. [Google Scholar] [CrossRef]
- Acero Fernández, D.; Ferri Iglesias, M.J.; López Nuñez, C.; Louvrie Freire, R.; Aldeguer Manté, X. Considerar negativa una carga viral expresada como menor del límite inferior de cuantificación puede inducir a error en el diagnóstico y manejo terapéutico de la hepatitis C [To consider negative viral loads below the limit of quantification can lead to errors in the diagnosis and treatment of hepatitis C virus infection]. Gastroenterol. Y Hepatol. 2013, 36, 443–449. [Google Scholar] [CrossRef]
- Campanella, A.; De Summa, S.; Tommasi, S. Exhaled breath condensate biomarkers for lung cancer. J. Breath Res. 2019, 13, 044002. [Google Scholar] [CrossRef]
- Ghelli, F.; Panizzolo, M.; Garzaro, G.; Squillacioti, G.; Bellisario, V.; Colombi, N.; Bergamaschi, E.; Guseva Canu, I.; Bono, R. Inflammatory Biomarkers in Exhaled Breath Condensate: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9820. [Google Scholar] [CrossRef]
- Połomska, J.; Bar, K.; Sozańska, B. Exhaled Breath Condensate-A Non-Invasive Approach for Diagnostic Methods in Asthma. J. Clin. Med. 2021, 10, 2697. [Google Scholar] [CrossRef]
- Maniscalco, M.; Fuschillo, S.; Paris, D.; Cutignano, A.; Sanduzzi, A.; Motta, A. Clinical metabolomics of exhaled breath condensate in chronic respiratory diseases. Adv. Clin. Chem. 2019, 88, 121–149. [Google Scholar] [CrossRef]
- Desai, A.; Tankasala, D.; Ng, G.P.; Thakkar, P.; Hoilett, O.S.; Mather, K.J.; Linnes, J.C. Selective Collection of Exhaled Breath Condensate for Noninvasive Screening of Breath Glucose. J. Diabetes Sci. Technol. 2023, 2023, 19322968231179728. [Google Scholar] [CrossRef]
- Smyth, R.J.; Toomey, S.M.; Sartori, A.; O’Hanrahan, E.; Cuffe, S.D.; Breathnach, O.S.; Morgan, R.K.; Hennessy, B.T. Brief Report on the Detection of the EGFR T790M Mutation in Exhaled Breath Condensate from Lung Cancer Patients. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1213–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomba, L.; Paris, D.; Tramice, A.; Ambrosino, P.; Maniscalco, M.; Motta, A. Detection of Cells in Exhaled Breath Condensate Holds Potential for Pathophysiological Insights in Pulmonary Diseases. Am. J. Respir. Cell Mol. Biol. 2023, 69, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, E.; Khoubnasabjafari, M.; Hosseini, M.B.; Jouyban, A. Copper nanocluster-based sensor for determination of vancomycin in exhaled breath condensate: A synchronous fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2021, 196, 113906. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Fathi-Azarbayjani, A.; Rahimpour, E.; Jouyban-Gharamaleki, V.; Kim, H.Y.; Alffenaar, J.; Chan, H.K.; Jouyban, A. Concentration profile of tobramycin in exhaled breath condensate after inhalation of a single dose: A pilot study. J. Drug Deliv. Sci. Technol. 2021, 62, 102394. [Google Scholar] [CrossRef]
- Ates, H.C.; Mohsenin, H.; Wenzel, C.; Glatz, R.T.; Wagner, H.J.; Bruch, R.; Hoefflin, N.; Spassov, S.; Streicher, L.; Lozano-Zahonero, S.; et al. Biosensor-Enabled Multiplexed On-Site Therapeutic Drug Monitoring of Antibiotics. Adv. Mater. 2022, 34, e2104555. [Google Scholar] [CrossRef]
- Abhilash, B.; Tripathi, C.D.; Gogia, A.R.; Meshram, G.G.; Kumar, M.; Suraj, B. Variability in plasma concentration of cefotaxime in critically ill patients in an Intensive Care Unit of India and its pharmacodynamic outcome: A nonrandomized, prospective, open-label, analytical study. J. Pharmacol. Pharmacother. 2016, 7, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Paal, M.; Scharf, C.; Denninger, A.K.; Ilia, L.; Kloft, C.; Kneidinger, N.; Liebchen, U.; Michel, S.; Schneider, C.; Schröpf, S.; et al. Target Site Pharmacokinetics of Meropenem: Measurement in Human Explanted Lung Tissue by Bronchoalveolar Lavage, Microdialysis, and Homogenized Lung Tissue. Antimicrob. Agents Chemother. 2021, 65, e0156421. [Google Scholar] [CrossRef]
- Kiem, S.; Schentag, J.J. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 2008, 52, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Wellman, T.J.; de Prost, N.; Tucci, M.; Winkler, T.; Baron, R.M.; Filipczak, P.; Raby, B.; Chu, J.H.; Harris, R.S.; Musch, G.; et al. Lung Metabolic Activation as an Early Biomarker of Acute Respiratory Distress Syndrome and Local Gene Expression Heterogeneity. Anesthesiology 2016, 125, 992–1004. [Google Scholar] [CrossRef] [Green Version]
- Sá, R.C.; Henderson, A.C.; Simonson, T.; Arai, T.J.; Wagner, H.; Theilmann, R.J.; Wagner, P.D.; Prisk, G.K.; Hopkins, S.R. Measurement of the distribution of ventilation-perfusion ratios in the human lung with proton MRI: Comparison with the multiple inert-gas elimination technique. J. Appl. Physiol. 2017, 123, 136–146. [Google Scholar] [CrossRef] [Green Version]
- de Prost, N.; Tucci, M.R.; Melo, M.F. Assessment of lung inflammation with 18F-FDG PET during acute lung injury. Am. J. Roentgenol. 2010, 195, 292–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Bräse, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Preckel, B.; Bolten, J. Pharmacology of modern volatile anaesthetics. Best Pract. Research. Clin. Anaesthesiol. 2005, 19, 331–348. [Google Scholar] [CrossRef]
- Grossherr, M.; Hengstenberg, A.; Meier, T.; Dibbelt, L.; Igl, B.W.; Ziegler, A.; Schmucker, P.; Gehring, H. Propofol concentration in exhaled air and arterial plasma in mechanically ventilated patients undergoing cardiac surgery. Br. J. Anaesth. 2009, 102, 608–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
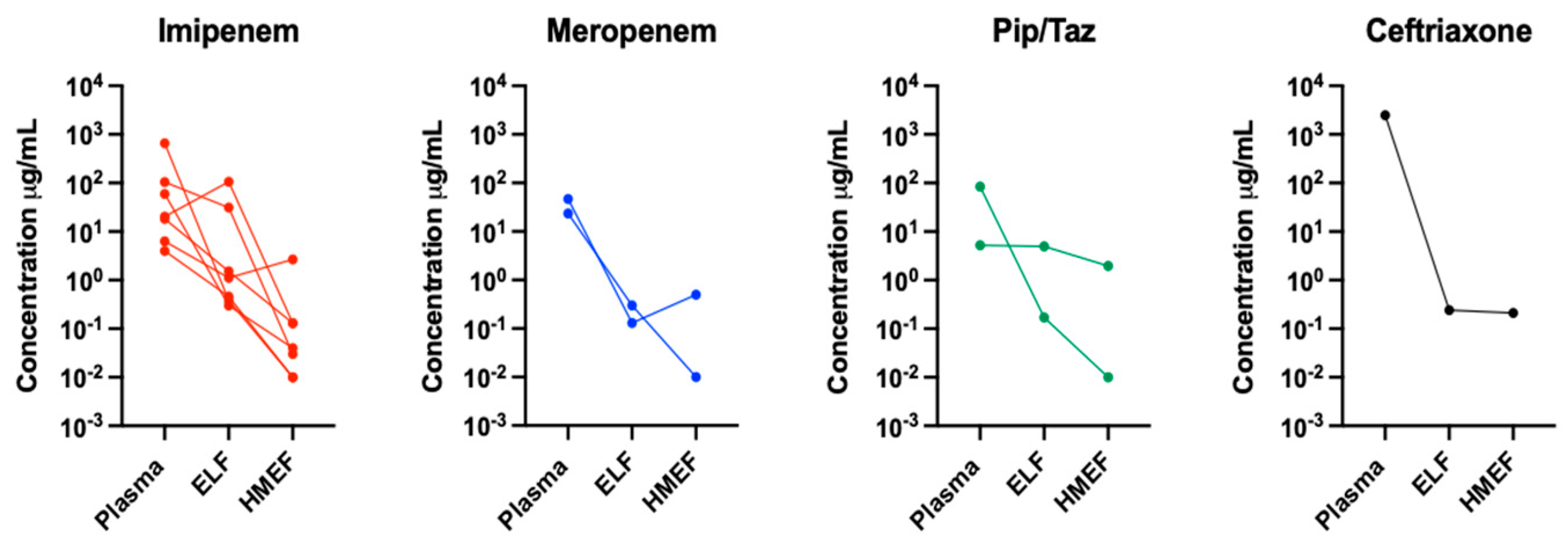
| Patient | BMI | PaO2/FIO2 | Antibiotic | Dose (Hours) | Diagnosis | eGFR (mL/min) | Albumin (gr/dL) | HMEF Time (Hours) | SOFA Score | Outcome | Antibiotic Concentration in the Three Compartments Examined. (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | ELF | HMEF | |||||||||||
| 1 | 21 | 272 | Imipenem | 500 mg c/8 | Pneumonia | 69 | 2.8 | 15 | 9 | Alive | 104.93 | 31.07 | 0.03 |
| 2 | 22.6 | 216 | Imipenem | 500 mg c/6 | Pneumonia | 90 | 2.9 | 7 | 6 | Dead | 20.28 | 106.56 | 0.13 |
| 3 | 30.7 | 172 | Imipenem | 1 gr per day | Pneumonia | 59 | 2.6 | 6 | 6 | Alive | 6.32 | 1.12 | 2.68 |
| 4 | 39.8 | 176 | Pip/Tazo | 4.5 gr c/8 | Pneumonia | 151 | 4.2 | 7 | 10 | Dead | 84.90 | 0.17 | 0.01 |
| 5 | 16.8 | 285 | Pip/Tazo | 4.5 gr c/8 | Pneumonia | 40 | 2.2 | 8 | 15 | Dead | 5.26 | 4.99 | 1.96 |
| 6 | 17.3 | 202 | Imipenem | 500 mg c/12 | Pneumonia | 17 | 2.7 | 11 | 8 | Alive | 4.01 | 0.46 | 0.01 |
| 7 | 29.1 | 63 | Imipenem | 1 gr c/12 | ARDS | 8 | 2.4 | 12 | 15 | Alive | 659.51 | 0.38 | 0.01 |
| 8 | 27.3 | 242 | Meropenem | 2 gr c/8 | Pulmonary sepsis | 125 | 2.2 | 6 | 6 | Alive | 46.52 | 0.13 | 0.51 |
| 9 | 24 | 162 | Meropenem | 2 gr c/8 | Pneumonia | 126 | 2.0 | 6 | 7 | Dead | 23.42 | 0.30 | 0.01 |
| 10 | 16.8 | 200 | Imipenem | 500 mg c/6 | Pulmonary sepsis | 110 | 2.3 | 19 | 8 | Alive | 18.18 | 1.52 | 0.13 |
| 11 | 26 | 141 | Imipenem | 500 mg c/6 | ARDS | 100 | 3.3 | 19 | 10 | Dead | 59.22 | 0.30 | 0.04 |
| 12 | 30.9 | 250 | Ceftriaxone | 2 gr per day | ARDS | 11 | 2.0 | 12 | 12 | Alive | 2489.6 | 0.24 | 0.21 |
| Mean | 25.1 | 198 | 75.5 | 2.6 | 10.7 | 9 | 293.51 | 12.27 | 0.48 | ||||
| SD | 6.9 | 62 | 49 | 0.63 | 5.1 | 3.2 | 715 | 31 | 0.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona, J.; Soto, D.; Oviedo, V.; Rivas, E.; Severino, N.; Kattan, E.; Andresen, M.; Bravo, S.; Basoalto, R.; Bachmann, M.C.; et al. Beta-Lactam Antibiotics Can Be Measured in the Exhaled Breath Condensate in Mechanically Ventilated Patients: A Pilot Study. J. Pers. Med. 2023, 13, 1146. https://doi.org/10.3390/jpm13071146
Escalona J, Soto D, Oviedo V, Rivas E, Severino N, Kattan E, Andresen M, Bravo S, Basoalto R, Bachmann MC, et al. Beta-Lactam Antibiotics Can Be Measured in the Exhaled Breath Condensate in Mechanically Ventilated Patients: A Pilot Study. Journal of Personalized Medicine. 2023; 13(7):1146. https://doi.org/10.3390/jpm13071146
Chicago/Turabian StyleEscalona, José, Dagoberto Soto, Vanessa Oviedo, Elizabeth Rivas, Nicolás Severino, Eduardo Kattan, Max Andresen, Sebastián Bravo, Roque Basoalto, María Consuelo Bachmann, and et al. 2023. "Beta-Lactam Antibiotics Can Be Measured in the Exhaled Breath Condensate in Mechanically Ventilated Patients: A Pilot Study" Journal of Personalized Medicine 13, no. 7: 1146. https://doi.org/10.3390/jpm13071146
APA StyleEscalona, J., Soto, D., Oviedo, V., Rivas, E., Severino, N., Kattan, E., Andresen, M., Bravo, S., Basoalto, R., Bachmann, M. C., Wong, K.-Y., Pavez, N., Bruhn, A., Bugedo, G., & Retamal, J. (2023). Beta-Lactam Antibiotics Can Be Measured in the Exhaled Breath Condensate in Mechanically Ventilated Patients: A Pilot Study. Journal of Personalized Medicine, 13(7), 1146. https://doi.org/10.3390/jpm13071146







