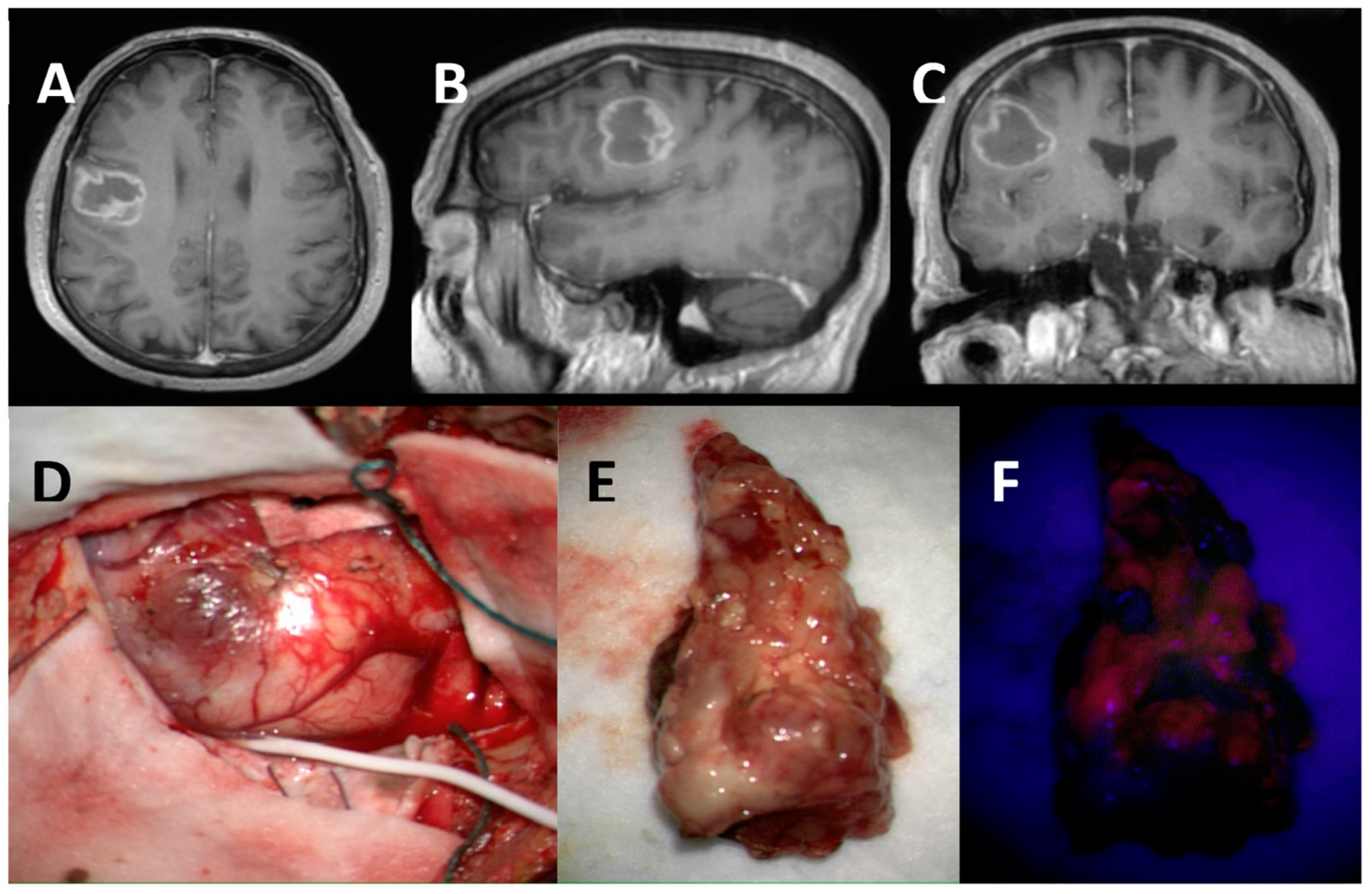
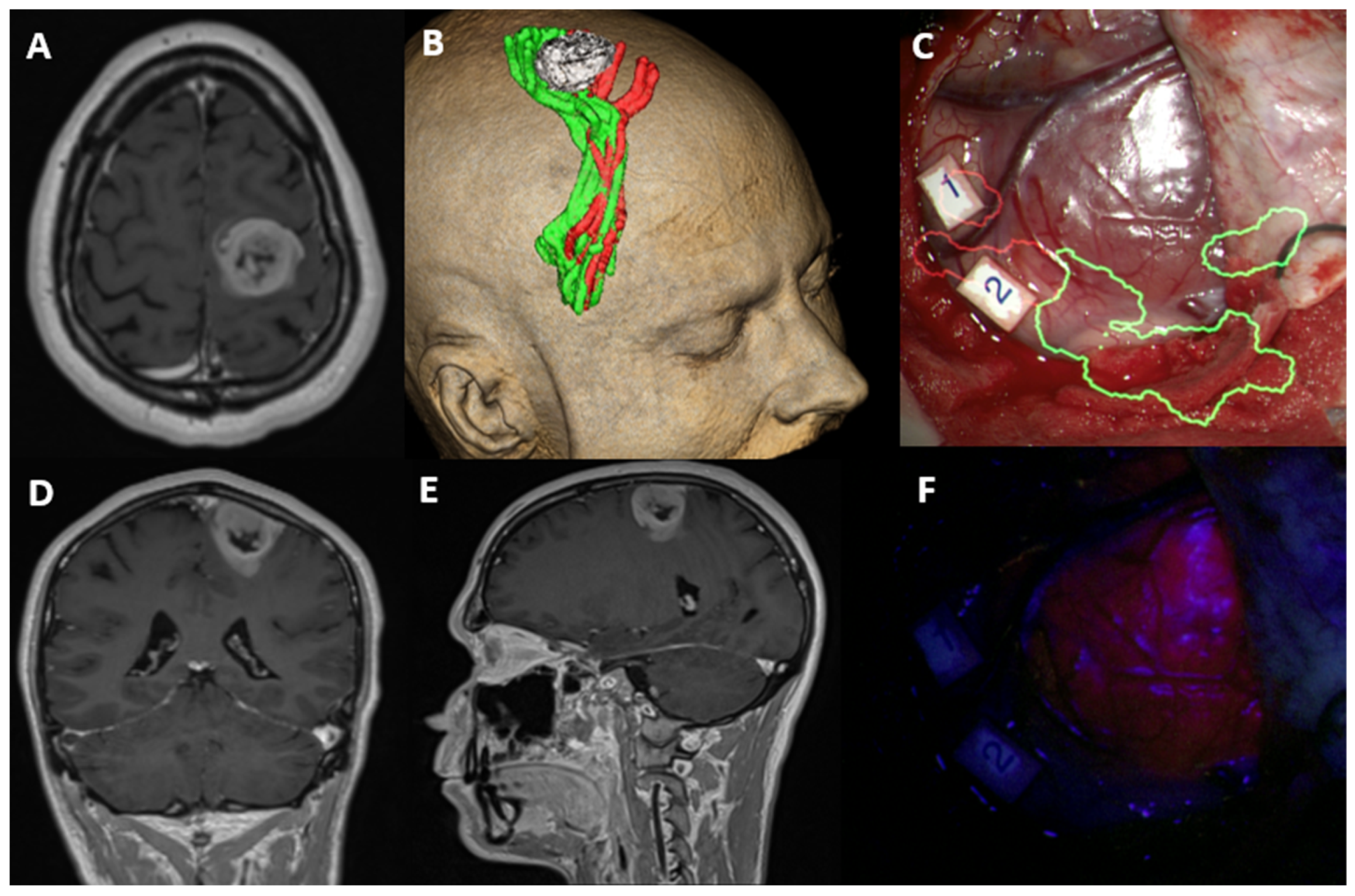
MGMT Promoter Methylation: Prognostication beyond Treatment Response
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Author | Years Included | Total Patients Enrolled in the Study | Patients with Viable Methylation Testing | Patients with Unmethylated MGMT Promoter Region | Technique Used to Test Methylation Status | Median Age (Years) | Extent of Resection | Treatment Post-Surgical Resection | Overall Median Survival (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Minniti et al., 2011 [24] | 2005–2009 | 83 | 83 | 41 | MSP | 73.2 | GTR—19 pts | Focal RT + concomitant TMZ, followed by adjuvant TMZ | 10.2 |
| STR—32 pts | ||||||||||
| Partial Resection—32 pts | ||||||||||
| 2 | Gerstner et al., 2009 [20] | 1998–2009 | 64 | 64 | 27 | MSP | 74 | GTR—10 pts STR—17 pts | Treated with TMZ—16 pts | 7.4 |
| Not Treated with TMZ—7 pts | ||||||||||
| Unknown/no Treatment—4 pts | ||||||||||
| 3 | Reifenberger et al., 2011 [33] | 2004–2010 | 233 | 233 | 99 | MSP and PSQ | 74 | GTR—90 pts | RT—61 pts | 8.8 |
| STR—87 pts | RT + TMZ—90 pts RT + nitrosourea—1 pt | 10.4 | ||||||||
| Partial Resection—13 pts | No therapy—66 pts | 2 | ||||||||
| Biopsy—37 pts | TMZ alone—14 pts Procarbacine + Lomustine alone—2 pts | 2.6 | ||||||||
| 4 | Hegi et al., 2005 [21] | N/A | 573 | 206 | 114 | MSP | N/A | N/A | RT only—54 pts | 11.8 |
| RT + TMZ—60 pts | 12.7 | |||||||||
| 5 | Brigliadori et al., 2016 [36] | 2008–2013 | 106 | 106 | 50 | PSQ | 61 | GTR—46 pts | RT + TMZ | 15.2 |
| STR—48 pts | ||||||||||
| Biopsy—11 pts | ||||||||||
| 6 | Dunn et al., 2009 [29] | 2004–2007 | 109 | 109 | 51 | PSQ | 55 | Resection—83 pts | RT + Concurrent TMZ, followed by RT and adjuvant TMZ | 11.1 |
| Biopsy—26 pts | ||||||||||
| 7 | Poon et al., 2021 [48] | 2012–2020 | 414 | 414 | 223 | PSQ | 61 | GTR—168 pts | RT + Concomitant and adjuvant TMZ—66 pts | 24.7 |
| STR—88 pts | ||||||||||
| Biopsy only—158 pts | ||||||||||
| 8 | Gilbert et al., 2013 [25] | 2006–2008 | 833 | 762 | 517 | MSP | 50 | GTR—451 pts | Standard Dose TMZ—254 pts Dose- Dense TMZ—263 pts | 16.6 15.4 |
| STR–355 pts | ||||||||||
| Biopsy only—27 pts | ||||||||||
| 9 | Malmstrom et al., 2012 [23] | 2000–2009 | 342 | 203 | 112 | MSP | 70 | Total/Partial Resection—214 pts Biopsy only—77 pts | TMZ | 7 |
| Hypo fractionated RT Standard RT | 6.8 | |||||||||
| 10 | Thon et al., 2017 [22] | 2006–2008 | 56 | 56 | 26 | MSP | 70 | Unresectable—56 pts | RT + Concomitant TMZ + Adjuvant TMZ—40 pts | 7.3 |
| RT + Concomitant TMZ—16 pts | ||||||||||
| 11 | Rivera et al., 2010 [19] | N/A | 225 | 183 | 138 | MSP | 58.1 | GTR—111 pts | RT + Adjuvant TMZ—53 pts | 12.8 |
| STR/Biopsy—114 pts | RT—172 pts | |||||||||
| 12 | Caccese et al., 2022 [15] | 2005–2018 | 883 | 591 | N/A | PSQ | 60 | Radical Resection—343 pts | RT + Concomitant TMZ (Stupp Protocol)—591 pts | 14.8 |
| Partial Resection—246 pts | ||||||||||
| 13 | Shen et al., 2014 [49] | 2008–2012 | 128 | 128 | 53 | PSQ | 56 | GTR—36 pts | RT + TMZ | 6.4 |
| STR—92 pts | ||||||||||
| 14 | Li et al., 2021 [34] | 2013–2018 | 312 | 312 | 182 | PSQ | 50 | GTR—165 pts | RT + Concomitant TMZ (Stupp Protocol) | 16 |
| STR—127 pts | ||||||||||
| Partial Resection—20 pts | ||||||||||
| 15 | Gurrieri et al., 2017 [33] | 2008–2013 | 108 | 108 | 51 | PSQ | 61 | Radical Resection—101 pts Partial Resection—7 pts | RT or none—20 | 13.2 |
| RT + TMZ or TMZ only—24 | ||||||||||
| RT + Concomitant TMZ (Stupp Protocol)—64 |
| No. | Author | Years Included | Total Patients Enrolled in the Study | Patients with Viable Methylation Testing | Patients with Methylated MGMT Promoter Region | Technique Used to Test Methylation Status | Median Age | Extent of Resection | Extent of Methylation | Treatment Post Surgical Resection | Overall Median Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Minniti et al., 2011 [24] | 2005–2009 | 83 | 83 | 42 | MSP | 73.2 | GTR—19 pts | Not Measured | Focal RT + concomitant TMZ, followed by adjuvant TMZ | 15.3 |
| STR—32 pts | |||||||||||
| Partial Resection—32 pts | |||||||||||
| 2 | Gerstner et al., 2009 [20] | 1998–2009 | 64 | 64 | 37 | MSP | 74 | GTR—14 pts STR—23 pts | Not Measured | Treated with TMZ—24 pts | 11.5 |
| Not Treated with TMZ—7 pts | |||||||||||
| Unknown/no Treatment—6 pts | |||||||||||
| 3 | Reifenberger et al., 2011 [33] | 2004–2010 | 233 | 233 | 134 | MSP and PSQ | 74 | GTR—90 pts | >8% | RT—61 pts | 7.8 |
| STR—87 pts | RT + TMZ—90 pts RT + nitrosourea—1 pt | 13.1 | |||||||||
| Partial Resection—13 pts | No therapy—66 pts | 2.3 | |||||||||
| Biopsy—37 pts | TMZ alone—14 pts Procarbacine + Lomustine alone—2 pts | 7.2 | |||||||||
| 4 | Hegi et al., 2005 [21] | N/A | 307 | 206 | 92 | MSP | N/A | N/A | Not Measured | RT—46 pts | 15.3 |
| RT + Temzolomide—46 pts | 21.7 | ||||||||||
| 5 | Brigliadori et al., 2016 [36] | 2008–2013 | 106 | 106 | 55 | PSQ | 61 | GTR—46 pts | 10–29%—14 pts 30–100%—41 pts | RT + TMZ | 15.2 25.2 |
| STR—48 pts | |||||||||||
| Biopsy—11 pts | |||||||||||
| 6 | Dunn et al., 2009 [29] | 2004–2007 | 109 | 109 | 58 | PSQ | 55 | Resection—83 pts Biopsy—26 pts | 9–20%—20 pts | RT + Concurrent TMZ, followed by RT and adjuvant TMZ | 11.3 |
| >20–35%—19 pts | 15.5 | ||||||||||
| >35%—19 pts | 26.2 | ||||||||||
| 7 | Poon et al., 2021 [48] | 2012–2020 | 414 | 414 | 191 | PSQ | 61 | GTR—168 pts | Median extent of methylation was 30% Threshold of 6.4% was used | RT + Concomitant and adjuvant TMZ—66 pts | High methylation group: 36.3 Low Methylation group: 37.0 |
| STR—88 pts | |||||||||||
| Biopsy only—158 pts | |||||||||||
| 8 | Gilbert et al., 2013 [25] | 2006—2009 | 833 | 762 | 245 | MSP | N/A | GTR—451 pts | Not Measured | Standard Dose TMZ—122 Dose- Dense TMZ—123 | 23.5 21.9 |
| STR—355 pts | |||||||||||
| Biopsy only—27 pts | |||||||||||
| 9 | Malmstrom et al., 2012 [32] | 2000–2009 | 342 | 203 | 91 | MSP | 70 | Total/Partial Resection—214 pts Biopsy only—77 pts | Not Measured | TMZ | 9.7 |
| Hypofractionated RT Standard RT | 8.2 | ||||||||||
| 10 | Thon et al., 2017 [22] | 2006–2008 | 56 | 56 | 30 | MSP | 70 | Unresectable—56 patients | Not Measured | RT + Concomitant TMZ + Adjuvant TMZ—40 pts RT + Concomitant TMZ—16 pts | 20.3 |
| 11 | Rivera et al., 2010 [19] | N/A | 225 | 183 | 45 | MSP | 58.1 | GTR—111 pts STR/Biopsy—114 pts | Not Specified | RT—172 pts | 15.7 |
| 12 | Caccese et al., 2022 [15] | 2005–2018 | 883 | 591 | N/A | PSQ | 60 | Radical Resection—343 pts Partial Resection—246 pts | 4–40% | RT + Concomitant TMZ (Stupp Protocol)—591 pts | 18.9 |
| >40–100% | 29.9 | ||||||||||
| 13 | Shen et al., 2014 [49] | 2008–2012 | 128 | 128 | 53 | PSQ | 56 | GTR—36 pts | 0–10% | RT + TMZ | 10.3 |
| STR—92 pts | >10–100% | 12.6 | |||||||||
| 14 | Li et al., 2021 [34] | 2013–2018 | 312 | 312 | 130 | PSQ | 50 | GTR—165 pts | >12% threshold | RT + Concomitant TMZ (Stupp Protocol) | 35 |
| STR—127 pts | |||||||||||
| Partial Resection—20 pts | |||||||||||
| 15 | Gurrieri et al., 2017 [35] | 2008–2013 | 108 | 108 | 54 | PSQ | 61 | Radical Resection—101 pts Partial Resection—7 pts | 9–29%—24 pts > 29%—33 pts | RT or none—20 | 15.8 19.5 |
| RT + TMZ or TMZ only—24 | |||||||||||
| RT + Concomitant TMZ (Stupp Protocol)—64 |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. Exon Publ. 2017, 143–153. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-Oncology 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved Survival Time Trends for Glioblastoma Using the SEER 17 Population-Based Registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Brodbelt, A.; Greenberg, D.; Winters, T.; Williams, M.; Vernon, S.; Collins, V.P. Glioblastoma in England: 2007–2011. Eur. J. Cancer 2015, 51, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Rock, P.; Gaillard, F. Stupp Protocol for Glioblastoma. Radiopaedia.org 2016. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in Glioblastoma: A Review on the Impact of Treatment Modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of Temozolomide Resistance in Glioblastoma—A Comprehensive Review. Cancer Drug Resist. 2020, 4, 17. [Google Scholar] [CrossRef]
- Everhard, S.; Tost, J.; El Abdalaoui, H.; Crinière, E.; Busato, F.; Marie, Y.; Gut, I.G.; Sanson, M.; Mokhtari, K.; Laigle-Donadey, F.; et al. Identification of Regions Correlating MGMT Promoter Methylation and Gene Expression in Glioblastomas. Neuro-Oncology 2009, 11, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Herman, J.G. Generating Mutations but Providing Chemosensitivity: The Role of O6-Methylguanine DNA Methyltransferase in Human Cancer. Oncogene 2004, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Graff, J.R.; Myöhänen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-Specific PCR: A Novel PCR Assay for Methylation Status of CpG Islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef]
- Caccese, M.; Simonelli, M.; Villani, V.; Rizzato, S.; Ius, T.; Pasqualetti, F.; Russo, M.; Rudà, R.; Amoroso, R.; Bellu, L.; et al. Definition of the Prognostic Role of MGMT Promoter Methylation Value by Pyrosequencing in Newly Diagnosed IDH Wild-Type Glioblastoma Patients Treated with Radiochemotherapy: A Large Multicenter Study. Cancers 2022, 14, 2425. [Google Scholar] [CrossRef]
- Christians, A.; Hartmann, C.; Benner, A.; Meyer, J.; von Deimling, A.; Weller, M.; Wick, W.; Weiler, M. Prognostic Value of Three Different Methods of MGMT Promoter Methylation Analysis in a Prospective Trial on Newly Diagnosed Glioblastoma. PLoS ONE 2012, 7, e33449. [Google Scholar] [CrossRef] [PubMed]
- Quillien, V.; Lavenu, A.; Sanson, M.; Legrain, M.; Dubus, P.; Karayan-Tapon, L.; Mosser, J.; Ichimura, K.; Figarella-Branger, D. Outcome-Based Determination of Optimal Pyrosequencing Assay for MGMT Methylation Detection in Glioblastoma Patients. J. Neurooncol. 2014, 116, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Karayan-Tapon, L.; Quillien, V.; Guilhot, J.; Wager, M.; Fromont, G.; Saikali, S.; Etcheverry, A.; Hamlat, A.; Loussouarn, D.; Campion, L.; et al. Prognostic Value of O6-Methylguanine-DNA Methyltransferase Status in Glioblastoma Patients, Assessed by Five Different Methods. J. Neurooncol. 2010, 97, 311–322. [Google Scholar] [CrossRef]
- Rivera, A.L.; Pelloski, C.E.; Gilbert, M.R.; Colman, H.; De La Cruz, C.; Sulman, E.P.; Bekele, B.N.; Aldape, K.D. MGMT Promoter Methylation Is Predictive of Response to Radiotherapy and Prognostic in the Absence of Adjuvant Alkylating Chemotherapy for Glioblastoma. Neuro-Oncology 2010, 12, 116–121. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Yip, S.; Wang, D.L.; Louis, D.N.; Iafrate, A.J.; Batchelor, T.T. MGMT methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology 2009, 73, 1509–1510. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Thon, N.; Thorsteinsdottir, J.; Eigenbrod, S.; Schüller, U.; Lutz, J.; Kreth, S.; Belka, C.; Tonn, J.-C.; Niyazi, M.; Kreth, F.W. Outcome in Unresectable Glioblastoma: MGMT Promoter Methylation Makes the Difference. J. Neurol. 2017, 264, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus Standard 6-Week Radiotherapy versus Hypofractionated Radiotherapy in Patients Older than 60 Years with Glioblastoma: The Nordic Randomised, Phase 3 Trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Minniti, G.; Salvati, M.; Arcella, A.; Buttarelli, F.; D’Elia, A.; Lanzetta, G.; Esposito, V.; Scarpino, S.; Maurizi Enrici, R.; Giangaspero, F. Correlation between O6-Methylguanine-DNA Methyltransferase and Survival in Elderly Patients with Glioblastoma Treated with Radiotherapy plus Concomitant and Adjuvant Temozolomide. J. Neurooncol. 2011, 102, 311–316. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT Promoter Methylation Status Testing to Guide Therapy for Glioblastoma: Refining the Approach Based on Emerging Evidence and Current Challenges. Neuro-Oncology 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Butler, M.; Pongor, L.; Su, Y.-T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef]
- Nguyen, N.; Redfield, J.; Ballo, M.; Michael, M.; Sorenson, J.; Dibaba, D.; Wan, J.; Ramos, G.D.; Pandey, M. Identifying the Optimal Cutoff Point for MGMT Promoter Methylation Status in Glioblastoma. CNS Oncol. 2021, 10, CNS74. [Google Scholar] [CrossRef]
- Dunn, J.; Baborie, A.; Alam, F.; Joyce, K.; Moxham, M.; Sibson, R.; Crooks, D.; Husband, D.; Shenoy, A.; Brodbelt, A.; et al. Extent of MGMT Promoter Methylation Correlates with Outcome in Glioblastomas given Temozolomide and Radiotherapy. Br. J. Cancer 2009, 101, 124–131. [Google Scholar] [CrossRef]
- Hegi, M.E.; Genbrugge, E.; Gorlia, T.; Stupp, R.; Gilbert, M.R.; Chinot, O.L.; Nabors, L.B.; Jones, G.; Van Criekinge, W.; Straub, J.; et al. MGMT Promoter Methylation Cutoff with Safety Margin for Selecting Glioblastoma Patients into Trials Omitting Temozolomide: A Pooled Analysis of Four Clinical Trials. Clin. Cancer Res. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Brandner, S.; McAleenan, A.; Kelly, C.; Spiga, F.; Cheng, H.-Y.; Dawson, S.; Schmidt, L.; Faulkner, C.L.; Wragg, C.; Jefferies, S.; et al. MGMT Promoter Methylation Testing to Predict Overall Survival in People with Glioblastoma Treated with Temozolomide: A Comprehensive Meta-Analysis Based on a Cochrane Systematic Review. Neuro-Oncology 2021, 23, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Häni, L.; Kopcic, M.; Branca, M.; Schütz, A.; Murek, M.; Söll, N.; Vassella, E.; Raabe, A.; Hewer, E.; Schucht, P. Quantitative Analysis of the MGMT Methylation Status of Glioblastomas in Light of the 2021 WHO Classification. Cancers 2022, 14, 3149. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Hentschel, B.; Felsberg, J.; Schackert, G.; Simon, M.; Schnell, O.; Westphal, M.; Wick, W.; Pietsch, T.; Loeffler, M.; et al. Predictive Impact of MGMT Promoter Methylation in Glioblastoma of the Elderly. Int. J. Cancer 2012, 131, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dong, G.; Zhang, W.; Ren, X.; Jiang, H.; Yang, C.; Zhao, X.; Zhu, Q.; Li, M.; Chen, H.; et al. Combining MGMT Promoter Pyrosequencing and Protein Expression to Optimize Prognosis Stratification in Glioblastoma. Cancer Sci. 2021, 112, 3699–3710. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, L.; De Carlo, E.; Gerratana, L.; De Maglio, G.; Macerelli, M.; Pisa, F.E.; Masiero, E.; Aprile, G.; Follador, A.; Puglisi, F.; et al. MGMT Pyrosequencing-Based Cut-off Methylation Level and Clinical Outcome in Patients with Glioblastoma Multiforme. Future Oncol. 2018, 14, 699–707. [Google Scholar] [CrossRef]
- Brigliadori, G.; Foca, F.; Dall’Agata, M.; Rengucci, C.; Melegari, E.; Cerasoli, S.; Amadori, D.; Calistri, D.; Faedi, M. Defining the Cutoff Value of MGMT Gene Promoter Methylation and Its Predictive Capacity in Glioblastoma. J. Neurooncol. 2016, 128, 333–339. [Google Scholar] [CrossRef]
- Wick, W.; Weller, M.; van den Bent, M.; Sanson, M.; Weiler, M.; von Deimling, A.; Plass, C.; Hegi, M.; Platten, M.; Reifenberger, G. MGMT Testing—The Challenges for Biomarker-Based Glioma Treatment. Nat. Rev. Neurol. 2014, 10, 372–385. [Google Scholar] [CrossRef]
- McAleenan, A.; Kelly, C.; Spiga, F.; Kernohan, A.; Cheng, H.-Y.; Dawson, S.; Schmidt, L.; Robinson, T.; Brandner, S.; Faulkner, C.L.; et al. Prognostic Value of Test(s) for O6-methylguanine–DNA Methyltransferase (MGMT) Promoter Methylation for Predicting Overall Survival in People with Glioblastoma Treated with Temozolomide. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Ricciardi, L.; Sturiale, C.L.; Scerrati, A.; Stifano, V.; Somma, T.; Ius, T.; Trungu, S.; Acqui, M.; Raco, A.; Miscusi, M.; et al. 5-Aminolevulinic Acid False-Positive Rates in Newly Diagnosed and Recurrent Glioblastoma: Do Pseudoprogression and Radionecrosis Play a Role? A Meta-Analysis. Front. Oncol. 2022, 12, 848036. [Google Scholar] [CrossRef]
- Baig Mirza, A.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic Acid-Guided Resection Improves the Overall Survival of Patients with Glioblastoma—A Comparative Cohort Study of 343 Patients. Neuro-Oncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef] [PubMed]
- Mareike, M.; Franziska, S.-B.; Julia, E.; Daniel, H.; Michael, S.; Jörg, F.; Marion, R. Does Positive MGMT Methylation Outbalance the Limitation of Subtotal Resection in Glioblastoma IDH-Wildtype Patients? J. Neurooncol. 2021, 153, 537–545. [Google Scholar] [CrossRef]
- Organisation Mondiale de la Santé, Centre International de Recherche sur le Cancer (Ed.) Central Nervous System Tumours, 5th ed.; World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Brigliadori, G.; Goffredo, G.; Bartolini, D.; Tosatto, L.; Gurrieri, L.; Mercatali, L.; Ibrahim, T. Influence of Intratumor Heterogeneity on the Predictivity of MGMT Gene Promoter Methylation Status in Glioblastoma. Front. Oncol. 2020, 10, 533000. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Wölfer, J.; Ewelt, C.; Holling, M.; Hasselblatt, M.; Niederstadt, T.; Zoubi, T.; Weckesser, M.; Stummer, W. The Value of 5-Aminolevulinic Acid in Low-Grade Gliomas and High-Grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumor Molecular Factors. Neurosurgery 2016, 78, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yu, Z.; Liang, R. Effect of long-term adjuvant temozolomide chemotherapy on primary glioblastoma patient survival. BMC Neurol. 2021, 21, 424. [Google Scholar] [CrossRef]
- Bhandari, M.; Gandhi, A.K.; Devnani, B.; Kumar, P.; Sharma, D.N.; Julka, P.K. Comparative Study of Adjuvant Temozolomide Six Cycles Versus Extended 12 Cycles in Newly Diagnosed Glioblastoma Multiforme. J. Clin. Diagn. Res. 2017, 11, XC04–XC08. [Google Scholar] [CrossRef]
- Poon, M.T.C.; Keni, S.; Vimalan, V.; Ip, C.; Smith, C.; Erridge, S.; Weir, C.J.; Brennan, P.M. Extent of MGMT Promoter Methylation Modifies the Effect of Temozolomide on Overall Survival in Patients with Glioblastoma: A Regional Cohort Study. Neuro-Oncol. Adv. 2021, 3, vdab171. [Google Scholar] [CrossRef]
- Shen, D.; Liu, T.; Lin, Q.; Lu, X.; Wang, Q.; Lin, F.; Mao, W. MGMT Promoter Methylation Correlates with an Overall Survival Benefit in Chinese High-Grade Glioblastoma Patients Treated with Radiotherapy and Alkylating Agent-Based Chemotherapy: A Single-Institution Study. PLoS ONE 2014, 9, e107558. [Google Scholar] [CrossRef]
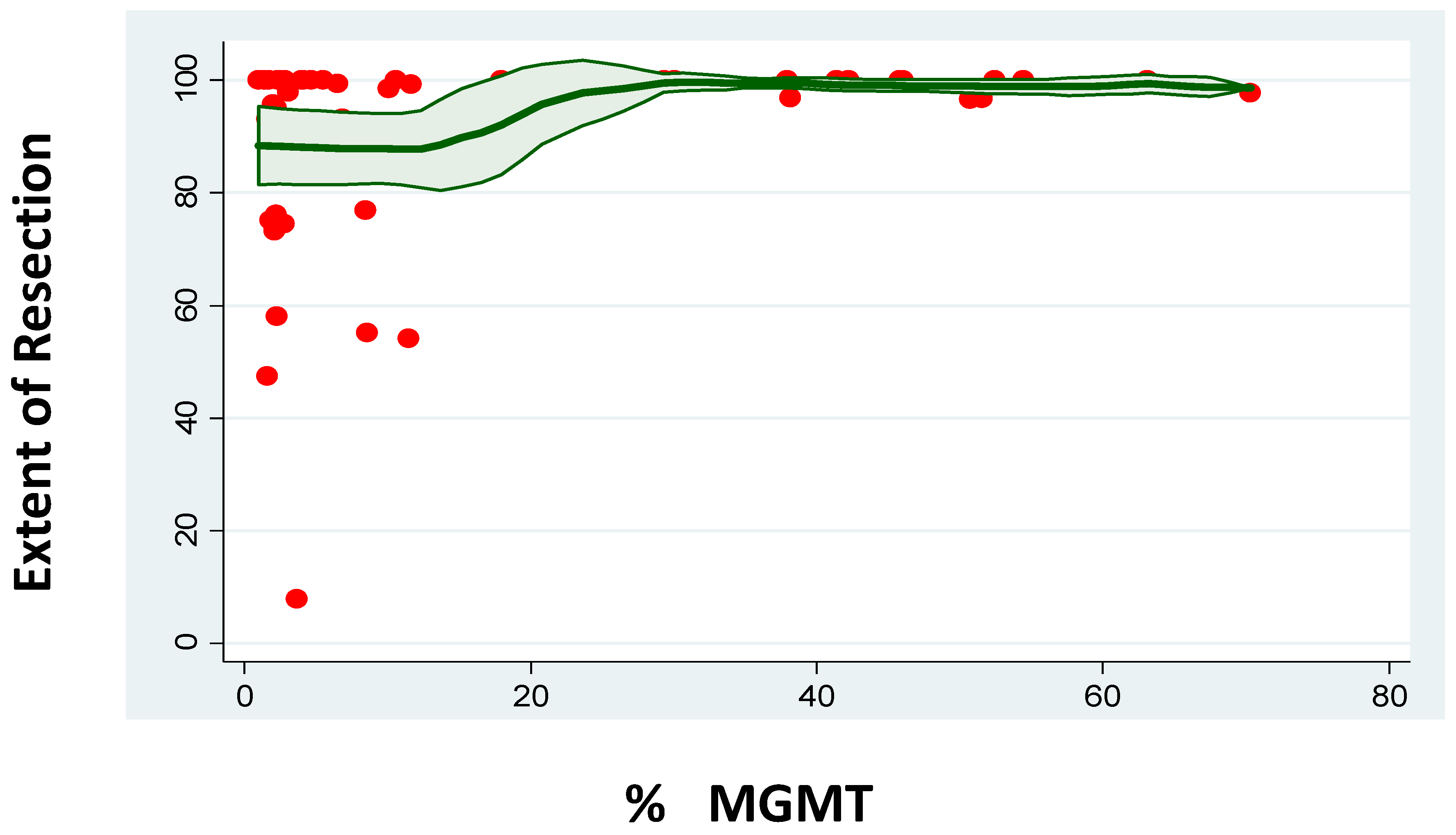
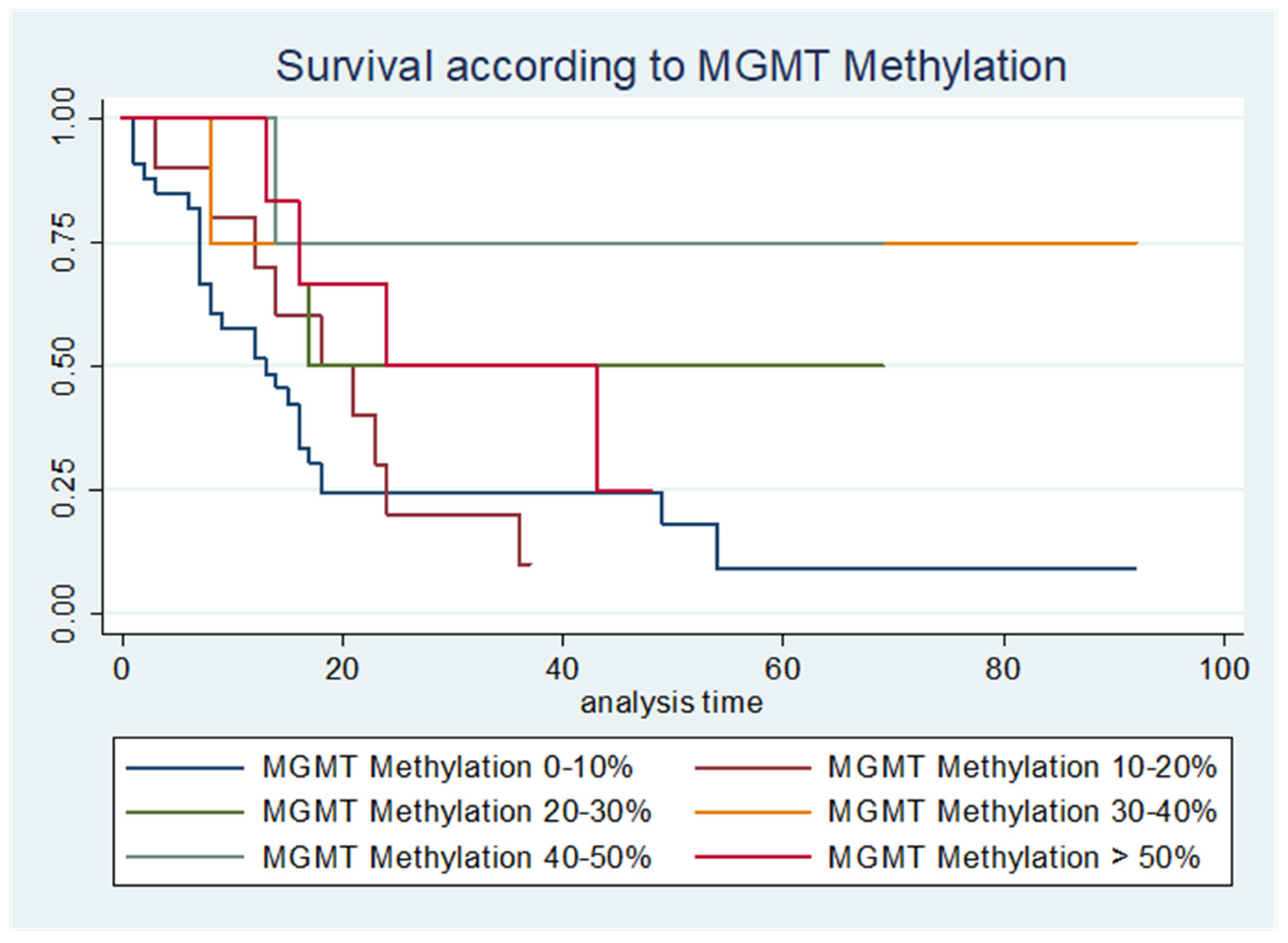
| PFS | OS | n | |
|---|---|---|---|
| MGMT Promoter Methylation 0–10% (months) | 9 | 13 | 34 |
| MGMT Promoter Methylation 10–20% (months) | 16 | 18 | 10 |
| MGMT Promoter Methylation 20–30% (months) | 19 | 17 | 6 |
| MGMT Promoter Methylation 30–40% (months) | 31 | - | 4 |
| MGMT Promoter Methylation 40–50% (months) | 37 | - | 4 |
| MGMT Promoter Methylation <50% (months) | 21 | 24 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkan, K.; Baig Mirza, A.; Soumpasis, C.; Syrris, C.; Kalaitzoglou, D.; Sharma, C.; James, Z.J.; Khoja, A.K.; Ahmed, R.; Vastani, A.; et al. MGMT Promoter Methylation: Prognostication beyond Treatment Response. J. Pers. Med. 2023, 13, 999. https://doi.org/10.3390/jpm13060999
Ashkan K, Baig Mirza A, Soumpasis C, Syrris C, Kalaitzoglou D, Sharma C, James ZJ, Khoja AK, Ahmed R, Vastani A, et al. MGMT Promoter Methylation: Prognostication beyond Treatment Response. Journal of Personalized Medicine. 2023; 13(6):999. https://doi.org/10.3390/jpm13060999
Chicago/Turabian StyleAshkan, Keyoumars, Asfand Baig Mirza, Christos Soumpasis, Christoforos Syrris, Dimitrios Kalaitzoglou, Chaitanya Sharma, Zachariah Joseph James, Abbas Khizar Khoja, Razna Ahmed, Amisha Vastani, and et al. 2023. "MGMT Promoter Methylation: Prognostication beyond Treatment Response" Journal of Personalized Medicine 13, no. 6: 999. https://doi.org/10.3390/jpm13060999
APA StyleAshkan, K., Baig Mirza, A., Soumpasis, C., Syrris, C., Kalaitzoglou, D., Sharma, C., James, Z. J., Khoja, A. K., Ahmed, R., Vastani, A., Bartram, J., Chia, K., Al-Salihi, O., Swampilai, A., Brazil, L., Laxton, R., Reisz, Z., Bodi, I., King, A., ... Lavrador, J. P. (2023). MGMT Promoter Methylation: Prognostication beyond Treatment Response. Journal of Personalized Medicine, 13(6), 999. https://doi.org/10.3390/jpm13060999