Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
- Less than 18 years of age;
- Pregnancy and/or breastfeeding;
- Therapy with immunosuppressive drugs and/or chemotherapy within six months prior to enrollment;
- Myocardial infarction within six weeks before recruitment;
- Chronic heart failure, classified as New York Heart Association stage IV;
- Human immunodeficiency virus (HIV) infection and/or hepatitis B/C infection;
- End-stage incurable disease;
- Persistent vegetative state (apallic syndrome);
- “Do Not Treat (DNT)” or “Do Not Resuscitate” (DNR) order;
- Participation in interventional studies and family member of a study-site employee.
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographics and Patient Baseline Characteristics
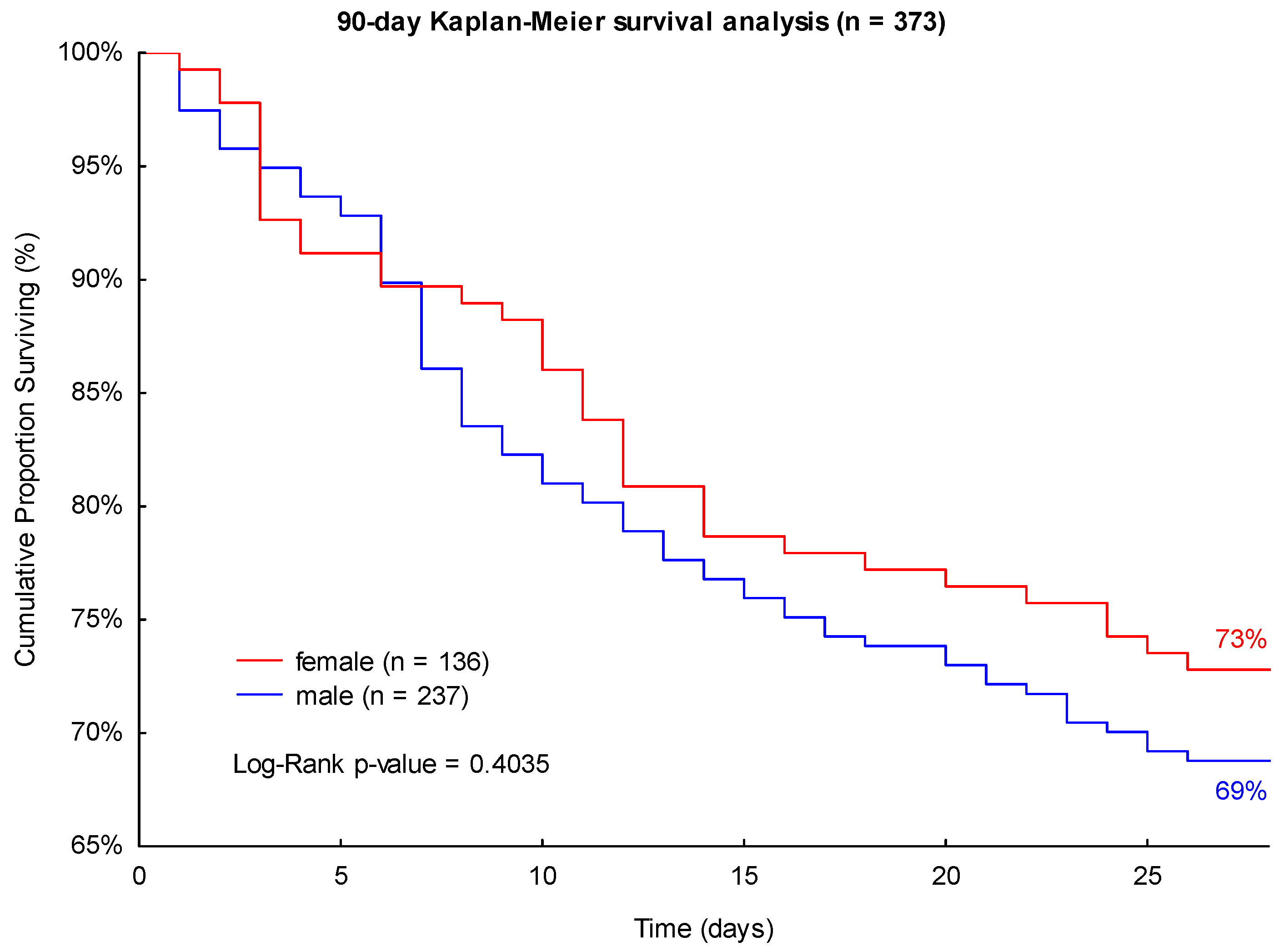
3.2. Survival Analyses
3.3. Disease Severity Analysis
3.4. Multivariate Cox Proportional Hazards Regression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and Burden of Sepsis Acquired in Hospitals and Intensive Care Units: A Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Rose, N.; Freytag, A.; Spoden, M.; Prescott, H.C.; Schettler, A.; Wedekind, L.; Ditscheid, B.; Storch, J.; Born, S.; et al. Epidemiology and Costs of Postsepsis Morbidity, Nursing Care Dependency, and Mortality in Germany, 2013 to 2017. JAMA Netw. Open 2021, 4, e2134290. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, N.; Taneja, A.; Kaleekal, T.; Tarima, S.; McGinley, E.; Jimenez, E.; Mohan, A.; Khan, R.A.; Whittle, J.; et al. Nationwide Trends of Severe Sepsis in the 21st Century (2000–2007). Chest 2011, 140, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of Severe Sepsis in the United States: Analysis of Incidence, Outcome, and Associated Costs of Care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Adrie, C.; Azoulay, E.; Francais, A.; Clec’h, C.; Darques, L.; Schwebel, C.; Nakache, D.; Jamali, S.; Goldgran-Toledano, D.; Garrouste-Orgeas, M.; et al. Influence of Gender on the Outcome of Severe Sepsis: A Reappraisal. Chest 2007, 132, 1786–1793. [Google Scholar] [CrossRef]
- Colbert, J.F.; Traystman, R.J.; Poisson, S.N.; Herson, P.S.; Ginde, A.A. Sex-Related Differences in the Risk of Hospital-Acquired Sepsis and Pneumonia Post Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2399–2404. [Google Scholar] [CrossRef]
- Xu, J.; Tong, L.; Yao, J.; Guo, Z.; Lui, K.Y.; Hu, X.; Cao, L.; Zhu, Y.; Huang, F.; Guan, X.; et al. Association of Sex With Clinical Outcome in Critically Ill Sepsis Patients: A Retrospective Analysis of the Large Clinical Database MIMIC-III. Shock 2019, 52, 146. [Google Scholar] [CrossRef]
- Sakr, Y.; Elia, C.; Mascia, L.; Barberis, B.; Cardellino, S.; Livigni, S.; Fiore, G.; Filippini, C.; Ranieri, V.M. The Influence of Gender on the Epidemiology of and Outcome from Severe Sepsis. Crit. Care 2013, 17, R50. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, T.D.; Pelletier, S.J.; Gleason, T.G.; Pruett, T.L.; Sawyer, R.G. Gender-Dependent Differences in Outcome after the Treatment of Infection in Hospitalized Patients. JAMA 1999, 282, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, A.P.; Glance, L.G.; Oakes, D.; Fisher, S.G. Gender Differences in Mortality in Patients with Severe Sepsis or Septic Shock. Gender Med. 2010, 7, 422–437. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Mewes, C.; Böhnke, C.; Alexander, T.; Büttner, B.; Hinz, J.; Popov, A.-F.; Ghadimi, M.; Beißbarth, T.; Raddatz, D.; Meissner, K.; et al. Favorable 90-Day Mortality in Obese Caucasian Patients with Septic Shock According to the Sepsis-3 Definition. J. Clin. Med. 2019, 9, 46. [Google Scholar] [CrossRef]
- Mewes, C.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.-F.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Jensen, O.; Runzheimer, J.; et al. CTLA-4 Genetic Variants Predict Survival in Patients with Sepsis. J. Clin. Med. 2019, 8, 70. [Google Scholar] [CrossRef]
- Mewes, C.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Shen-Orr, S.; Bergmann, I.; et al. The CTLA-4 Rs231775 GG Genotype Is Associated with Favorable 90-Day Survival in Caucasian Patients with Sepsis. Sci. Rep. 2018, 8, 15140. [Google Scholar] [CrossRef]
- Mewes, C.; Alexander, T.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.-F.; Ghadimi, M.; Beißbarth, T.; Tzvetkov, M.; Grade, M.; et al. TIM-3 Genetic Variants Are Associated with Altered Clinical Outcome and Susceptibility to Gram-Positive Infections in Patients with Sepsis. Int. J. Mol. Sci. 2020, 21, 8318. [Google Scholar] [CrossRef]
- Mewes, C.; Alexander, T.; Büttner, B.; Hinz, J.; Alpert, A.; Popov, A.-F.; Beißbarth, T.; Tzvetkov, M.; Grade, M.; Quintel, M.; et al. Effect of the Lymphocyte Activation Gene 3 Polymorphism Rs951818 on Mortality and Disease Progression in Patients with Sepsis-A Prospective Genetic Association Study. J. Clin. Med. 2021, 10, 5302. [Google Scholar] [CrossRef]
- Hinz, J.; Büttner, B.; Kriesel, F.; Steinau, M.; Frederik Popov, A.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Bergmann, I.; Mansur, A. The FER Rs4957796 TT Genotype Is Associated with Unfavorable 90-Day Survival in Caucasian Patients with Severe ARDS Due to Pneumonia. Sci. Rep. 2017, 7, 9887. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.E.; Simmons, J.; Choo, E.K.; Portelli, D.; McGregor, A.J.; Napoli, A.M. The DISPARITY Study: Do Gender Differences Exist in Surviving Sepsis Campaign Resuscitation Bundle Completion, Completion of Individual Bundle Elements, or Sepsis Mortality? J. Crit. Care 2014, 29, 473.e7–473.e11. [Google Scholar] [CrossRef] [PubMed]
- van Vught, L.A.; Scicluna, B.P.; Wiewel, M.A.; Hoogendijk, A.J.; Klein Klouwenberg, P.M.C.; Ong, D.S.Y.; Cremer, O.L.; Horn, J.; Franitza, M.; Toliat, M.R.; et al. Association of Gender With Outcome and Host Response in Critically Ill Sepsis Patients. Crit. Care Med. 2017, 45, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Finfer, S.R.; Woodward, M.; Leong, R.N.F.; Liu, B. Sex Differences in Sepsis Hospitalisations and Outcomes in Older Women and Men: A Prospective Cohort Study. J. Infect. 2022, 84, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Modra, L.J.; Higgins, A.M.; Abeygunawardana, V.S.; Vithanage, R.N.; Bailey, M.J.; Bellomo, R. Sex Differences in Treatment of Adult Intensive Care Patients: A Systematic Review and Meta-Analysis. Crit. Care Med. 2022, 50, 913. [Google Scholar] [CrossRef]
- Peng, J.; Tang, R.; Yu, Q.; Wang, D.; Qi, D. No Sex Differences in the Incidence, Risk Factors and Clinical Impact of Acute Kidney Injury in Critically Ill Patients with Sepsis. Front. Immunol. 2022, 13, 895018. [Google Scholar] [CrossRef]
- Lakbar, I.; Einav, S.; Lalevée, N.; Martin-Loeches, I.; Pastene, B.; Leone, M. Interactions between Gender and Sepsis—Implications for the Future. Microorganisms 2023, 11, 746. [Google Scholar] [CrossRef]
- Sperry, J.L.; Nathens, A.B.; Frankel, H.L.; Vanek, S.L.; Moore, E.E.; Maier, R.V.; Minei, J.P.; Inflammation and the Host Response to Injury Investigators. Characterization of the Gender Dimorphism after Injury and Hemorrhagic Shock: Are Hormonal Differences Responsible? Crit. Care Med. 2008, 36, 1838–1845. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of Immune Responses to Viral Vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender Differences in Sepsis: Cardiovascular and Immunological Aspects. Virulence 2014, 5, 12–19. [Google Scholar] [CrossRef]
- Diodato, M.D.; Knöferl, M.W.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Gender Differences in the Inflammatory Response and Survival Following Haemorrhage and Subsequent Sepsis. Cytokine 2001, 14, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Viñas, J.L.; Porter, C.J.; Douvris, A.; Spence, M.; Gutsol, A.; Zimpelmann, J.A.; Tailor, K.; Campbell, P.A.; Burns, K.D. Sex Diversity in Proximal Tubule and Endothelial Gene Expression in Mice with Ischemic Acute Kidney Injury. Clin. Sci. 2020, 134, 1887–1909. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen Protects Renal Endothelial Barrier Function from Ischemia-Reperfusion in Vitro and in Vivo. Am. J. Physiol. Renal Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Campo, S.; Falliti, G.; Caruso, D.; Gargano, R.; Giunta, E.; Monardo, P. Free Light Chains, High Mobility Group Box 1, and Mortality in Hemodialysis Patients. J. Clin. Med. 2022, 11, 6904. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | All (n = 737) | Male (n = 484) | Female (n = 253) | p-Value |
|---|---|---|---|---|
| Basic Conditions | ||||
| Age [years] | 63 ± 15 | 63 ± 15 | 63 ± 16 | 0.7029 |
| Body mass index [kg/m2] | 28 ± 7 | 28 ± 7 | 27 ± 7 | 0.0062 |
| Severity on Sepsis Onset (Day 1) | ||||
| SOFA score | 10 ± 4 | 10 ± 4 | 9 ± 4 | 0.0636 |
| APACHE II-score | 22 ± 7 | 22 ± 7 | 22 ± 7 | 0.4484 |
| Use of vasopressor [%] | 70 | 69 | 72 | 0.4433 |
| Mechanical ventilation [%] | 87 | 87 | 87 | 0.9973 |
| Renal replacement therapy [%] | 10 | 11 | 9 | 0.5351 |
| Comorbidities [%] | ||||
| Arterial hypertension | 53 | 53 | 53 | 0.8619 |
| COPD | 15 | 16 | 12 | 0.1050 |
| Bronchial asthma | 2 | 2 | 3 | 0.3601 |
| Renal dysfunction | 10 | 11 | 8 | 0.1156 |
| NIDDM | 8 | 10 | 5 | 0.0206 |
| IDDM | 10 | 11 | 8 | 0.2232 |
| Chronic liver disease | 6 | 6 | 4 | 0.2527 |
| History of myocardial infarction | 6 | 7 | 4 | 0.0859 |
| History of stroke | 5 | 6 | 5 | 0.5533 |
| History of cancer | 14 | 15 | 12 | 0.3671 |
| Medication on Sepsis Onset [%] | ||||
| Statins | 23 | 26 | 19 | 0.0234 |
| Beta-blocker | 37 | 37 | 36 | 0.9559 |
| ACE inhibitor | 29 | 32 | 23 | 0.0097 |
| Bronchodilator | 10 | 10 | 10 | 0.8481 |
| Diuretics | 33 | 35 | 30 | 0.2007 |
| Anticoagulation during the last 6 months | 26 | 27 | 23 | 0.2449 |
| Recent Surgical History [%] | ||||
| Elective surgery | 27 | 26 | 30 | |
| Emergency surgery | 52 | 53 | 50 | 0.5379 |
| No surgery | 21 | 21 | 20 | |
| Site of Infection [%] | ||||
| Lung | 63 | 67 | 56 | |
| Abdomen | 19 | 17 | 22 | |
| Bone or soft tissue | 4 | 3 | 5 | |
| Surgical wound | 2 | 1 | 2 | 0.0838 |
| Urogenital | 2 | 2 | 4 | |
| Primary bacteremia | 6 | 6 | 5 | |
| Other | 4 | 4 | 6 |
| Characteristics | All (n = 737) | Male (n = 484) | Female (n = 253) | p-Value |
|---|---|---|---|---|
| Sepsis Severity | ||||
| SOFA score | 7.2 ± 3.7 | 7.4 ± 3.6 | 6.9 ± 3.8 | 0.0265 |
| Days in septic shock | 1 (0, 2) | 0 (0, 2) | 1 (0, 2) | 0.1087 |
| ICU length of stay | 21 ± 16 | 20 ± 15 | 21 ± 19 | 0.6748 |
| Hospital length of stay | 39 ± 29 | 40 ± 30 | 37 ± 27 | 0.5416 |
| Inflammatory Values | ||||
| Leukocytes [1000/µL] | 13.2 ± 5 | 13.1 ± 5.1 | 13.6 ± 4.9 | 0.1291 |
| C-reactive protein [mg/L] (n = 380) | 150.9 ± 85.7 | 153.9 ± 86,2 | 144 ± 84.3 | 0.3088 |
| Procalcitonin [ng/dL] (n = 657) | 1 (0.3, 3.4) | 1 (0.3, 3.7) | 0.8 (0.3, 2.8) | 0.1571 |
| Respiratory Values | ||||
| SOFA respiratory subscore | 2.0 ± 0.8 | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.0091 |
| Ventilated days | 11 ± 8 | 11 ± 8 | 11 ± 9 | 0.2803 |
| Patients with mechanical ventilation [%] | 94 | 94 | 93 | 0.4787 |
| Ventilation days/observation days [%] | 68 ± 32 | 69 ± 31 | 66 ± 32 | 0.2674 |
| Coagulation | ||||
| SOFA coagulation subscore | 0 (0, 0.5) | 0 (0, 0.5) | 0 (0, 0.5) | 0.2398 |
| Thrombocytes [1000/µL] | 292 ± 150 | 293 ± 154 | 290 ± 141 | 0.7498 |
| Liver Values | ||||
| SOFA hepatic subscore | 0 (0, 0.4) | 0 (0, 0.5) | 0 (0, 0.3) | 0.1423 |
| Bilirubin [mg/dL] | 0.6 (0.4, 1.1) | 0.7 (0.5, 1.2) | 0.5 (0.4, 0.9) | <0.001 |
| AST [IU/L] (n = 483) | 57 (35, 112) | 58 (35, 112) | 56 (32, 110) | 0.8264 |
| ALT [IU/L] (n = 713) | 46 (23, 92) | 47 (23, 88) | 43 (22, 100) | 0.3322 |
| Cardiovascular Values | ||||
| SOFA cardiovascular subscore | 1.6 ± 1 | 1.6 ± 1 | 1.7 ± 1.1 | 0.7080 |
| Vasopressor days | 4 (1, 8) | 4 (2, 8) | 4 (1, 8) | 0.9163 |
| Patients with vasopressor treatment [%] | 81 | 81 | 82 | 0.6856 |
| Vasopressor days/observation days [%] | 29 (11, 57) | 29 (11, 56) | 31 (11, 57) | 0.5938 |
| Central Nervous System | ||||
| SOFA central nervous system | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.1 ± 1.1 | 0.9434 |
| Glasgow Coma Scale (GCS) | 10 ± 3 | 10 ± 3 | 10 ± 3 | 0.9965 |
| Renal Values | ||||
| SOFA renal subscore | 0.2 (0, 1.2) | 0.3 (0, 1.4) | 0 (0, 0.8) | <0.001 |
| Creatinine [mg/dL] | 1.2 ± 0.9 | 1.4 ± 1 | 0.9 ± 0.6 | <0.001 |
| Urine output [mL/d] | 2904 ± 1341 | 2878 ± 1334 | 2954 ± 1357 | 0.3841 |
| Urine output [mL/kg/d] | 1.5 ± 0.8 | 1.4 ± 0.7 | 1.7 ± 0.9 | <0.001 |
| Dialysed days | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.2947 |
| Patients with renal replacement therapy [%] | 22 | 24 | 19 | 0.1078 |
| Dialysis days/observation days [%] | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.2348 |
| Characteristics | All (n = 373) | Male (n = 237) | Female (n = 136) | p-Value |
|---|---|---|---|---|
| Sepsis Severity | ||||
| Sequential Organ Failure Assessment (SOFA) | 9 ± 3.9 | 9.2 ± 3.8 | 8.7 ± 4 | 0.1264 |
| Days in septic shock | 2 (1, 3) | 2 (1, 3) | 2 (1, 4) | 0.1425 |
| ICU length of stay | 24 ± 19 | 23 ± 16 | 25 ± 24 | 0.9666 |
| Hospital length of stay | 42 ± 31 | 43 ± 31 | 40 ± 31 | 0.4649 |
| Inflammatory Values | ||||
| Leukocytes [1000/µL] | 13.9 ± 5.5 | 13.8 ± 5.7 | 14.2 ± 5.2 | 0.3576 |
| C-reactive protein [mg/L] (n = 219) | 156 ± 79 | 160 ± 79 | 147 ± 78 | 0.2204 |
| Procalcitonin [ng/dL] (n = 352) | 1.8 (0.7, 5.9) | 2.2 (0.9, 6.1) | 1.4 (0.5, 5.4) | 0.0804 |
| Respiratory Values | ||||
| SOFA respiratory subscore | 2.2 ± 0.7 | 2.3 ± 0.8 | 2.1 ± 0.7 | 0.1089 |
| Ventilated days | 13 ± 9 | 13 ± 9 | 13 ± 9 | 0.9130 |
| Patients with mechanical ventilation [%] | 97 | 97 | 96 | 0.8788 |
| Ventilation days/observation days [%] | 74 ± 29 | 75 ± 29 | 73 ± 30 | 0.8123 |
| Coagulation | ||||
| SOFA coagulation subscore | 0.2 (0, 0.9) | 0.2 (0, 1) | 0.3 (0, 0.9) | 0.9773 |
| Thrombocytes [1000/µL] | 251 ± 142 | 257 ± 154 | 240 ± 118 | 0.7232 |
| Liver Values | ||||
| SOFA hepatic subscore | 0.1 (0, 1) | 0.1 (0, 0.9) | 0.1 (0, 1) | 0.6701 |
| Bilirubin [mg/dL] | 0.8 (0.5, 1.6) | 0.8 (0.5, 1.6) | 0.7 (0.4, 1.7) | 0.1118 |
| AST (GOT) [IU/L] (n = 291) | 69 (40, 140) | 71 (41, 160) | 67 (38, 121) | 0.6234 |
| ALT (GPT) [IU/L] (n = 362) | 44 (22, 101) | 49 (25, 98) | 37 (19, 106) | 0.0964 |
| Cardiovascular Values | ||||
| SOFA cardiovascular subscore | 2.1 ± 1 | 2.1 ± 1 | 2.1 ± 1 | 0.7594 |
| Vasopressor days | 8 ± 6 | 8 ± 6 | 9 ± 7 | 0.7382 |
| Patients with vasopressor treatment [%] | 100 | 100 | 100 | 1.0 |
| Vasopressor days/observation days [%] | 52 ± 30 | 51 ± 30 | 53 ± 30 | 0.5564 |
| Central Nervous System | ||||
| SOFA central nervous system | 2.3 ± 1 | 2.3 ± 1 | 2.3 ± 1 | 0.8255 |
| Glasgow Coma Scale (GCS) | 9 ± 3 | 9 ± 3 | 9 ± 3 | 0.9277 |
| Renal Values | ||||
| SOFA renal subscore | 0.6 (0.1, 2) | 0.8 (0.2, 2.3) | 0.3 (0, 1.3) | 0.0021 |
| Creatinine [mg/dL] | 1.4 ± 1 | 1.6 ± 1 | 1.1 ± 0.7 | <0.001 |
| Urine output [mL/d] | 2661 ± 1540 | 2661 ± 1549 | 2661 ± 1531 | 0.9924 |
| Urine output [mL/kg/d] | 1.4 ± 0.8 | 1.3 ± 0.8 | 1.5 ± 0.9 | 0.0326 |
| Dialysed days | 0 (0, 4) | 0 (0, 4) | 0 (0, 3) | 0.1682 |
| Patients with renal replacement therapy [%] | 39 | 43 | 32 | 0.0503 |
| Dialysis days/observation days [%] | 0 (0, 28) | 0 (0, 33) | 0 (0, 18) | 0.1027 |
| 28-Day Mortality | 90-Day Mortality | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value |
| Male sex | 1.25 | 0.89–1.76 | 0.2012 | 1.09 | 0.83–1.44 | 0.5236 |
| Age | 1.04 | 1.02–1.05 | <0.001 | 1.04 | 1.02–1.05 | <0.001 |
| BMI | 0.98 | 0.95–1.01 | 0.1439 | 1 | 0.97–1.02 | 0.7119 |
| NIDDM | 1.13 | 0.64–2.02 | 0.6702 | 0.99 | 0.64–1.55 | 0.9748 |
| Statins | 0.73 | 0.49–1.08 | 0.1120 | 0.84 | 0.62–1.16 | 0.2897 |
| ACE inhibitor | 1.05 | 0.73–1.5 | 0.7996 | 1.04 | 0.78–1.4 | 0.7868 |
| 28-Day Mortality | 90-Day Mortality | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%-CI | p-Value | HR | 95%-CI | p-Value |
| Sex | 1.25 | 0.84–1.87 | 0.2699 | 1.15 | 0.82–1.6 | 0.4142 |
| Age | 1.03 | 1.01–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| BMI | 0.97 | 0.93–1 | 0.0753 | 0.99 | 0.97–1.01 | 0.3743 |
| NIDDM | 0.94 | 0.5–1.78 | 0.8483 | 1 | 0.58–1.73 | 0.9893 |
| Statins | 0.79 | 0.49–1.28 | 0.3437 | 0.92 | 0.62–1.36 | 0.6684 |
| ACE inhibitor | 1.11 | 0.71–1.73 | 0.6454 | 1.12 | 0.78–1.62 | 0.5665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mewes, C.; Runzheimer, J.; Böhnke, C.; Büttner, B.; Hinz, J.; Quintel, M.; Mansur, A. Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock. J. Pers. Med. 2023, 13, 836. https://doi.org/10.3390/jpm13050836
Mewes C, Runzheimer J, Böhnke C, Büttner B, Hinz J, Quintel M, Mansur A. Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock. Journal of Personalized Medicine. 2023; 13(5):836. https://doi.org/10.3390/jpm13050836
Chicago/Turabian StyleMewes, Caspar, Julius Runzheimer, Carolin Böhnke, Benedikt Büttner, José Hinz, Michael Quintel, and Ashham Mansur. 2023. "Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock" Journal of Personalized Medicine 13, no. 5: 836. https://doi.org/10.3390/jpm13050836
APA StyleMewes, C., Runzheimer, J., Böhnke, C., Büttner, B., Hinz, J., Quintel, M., & Mansur, A. (2023). Association of Sex Differences with Mortality and Organ Dysfunction in Patients with Sepsis and Septic Shock. Journal of Personalized Medicine, 13(5), 836. https://doi.org/10.3390/jpm13050836






