ARIA Care Pathways 2019: Next-Generation Allergic Rhinitis Care and Allergen Immunotherapy in Malaysia
Abstract
1. Introduction
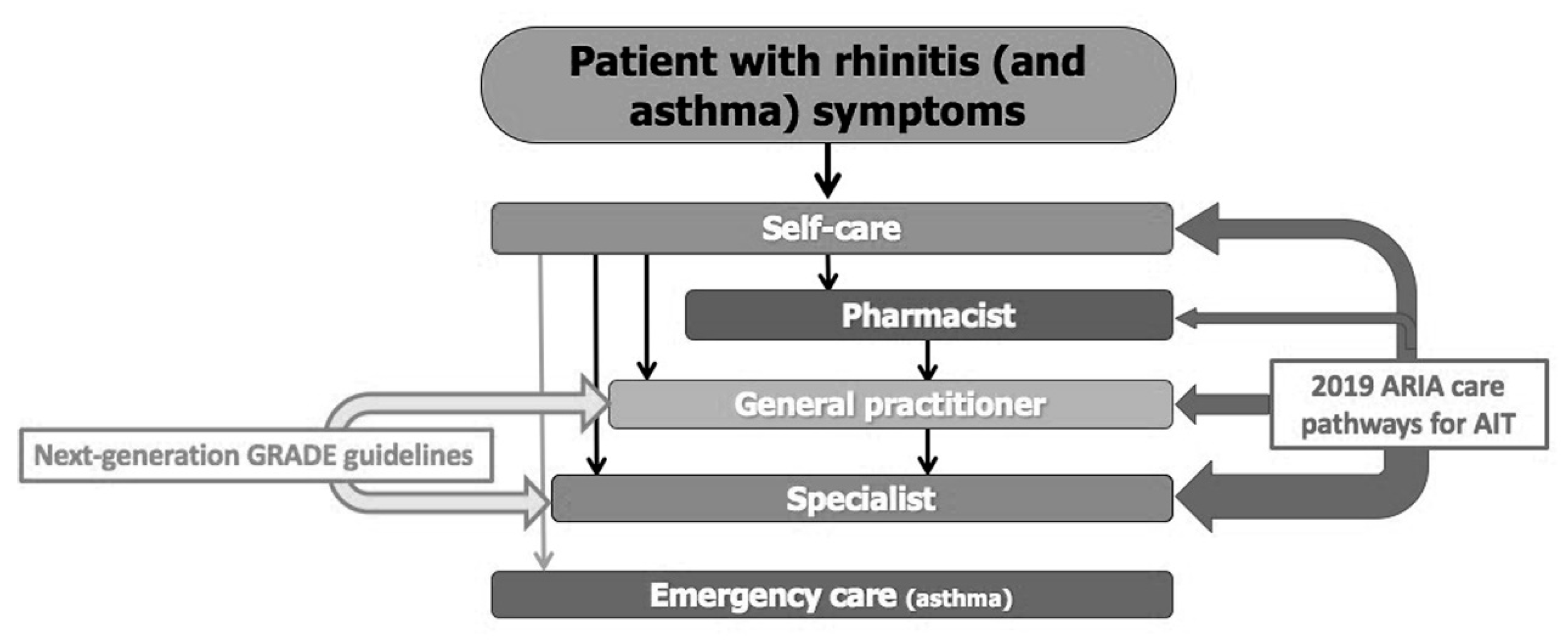
2. Management of AR in the Health Care System of Malaysia
3. Next-Generation ARIA-GRADE Guidelines
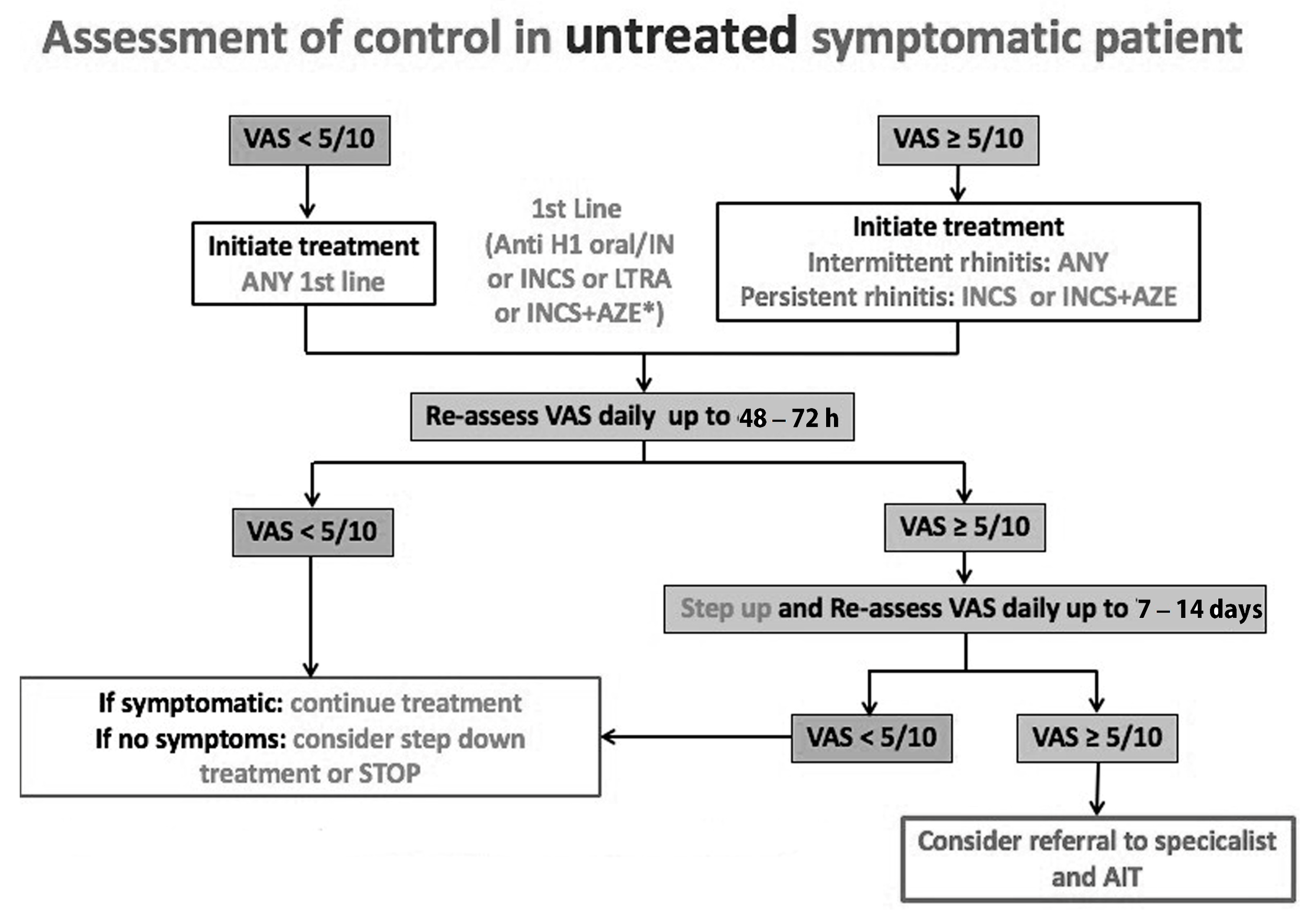
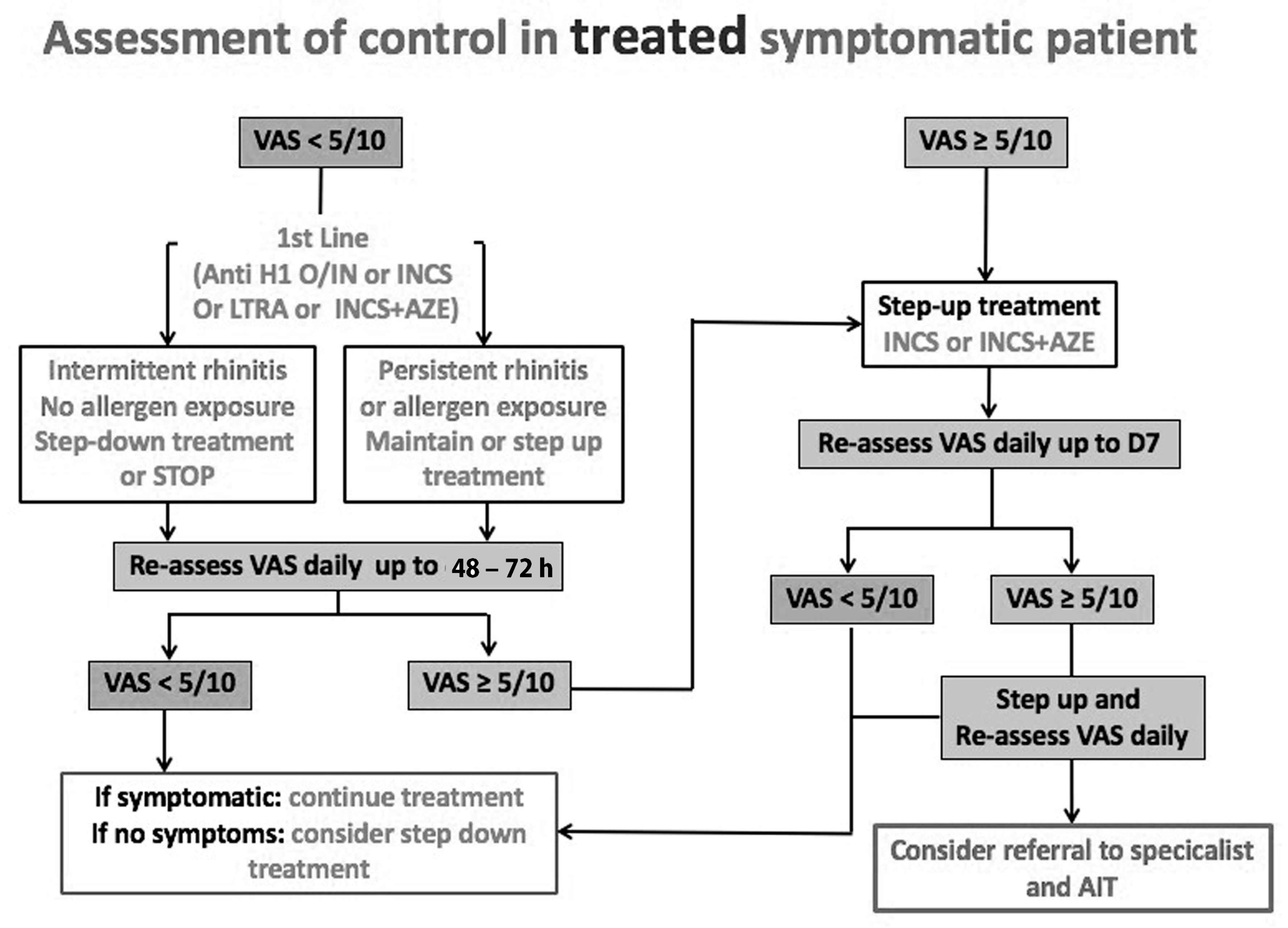
4. Development of ARIA Integrated Care Pathways for Pharmacologic Treatment
5. Next-Generation ARIA-GRADE Guidelines Algorithm
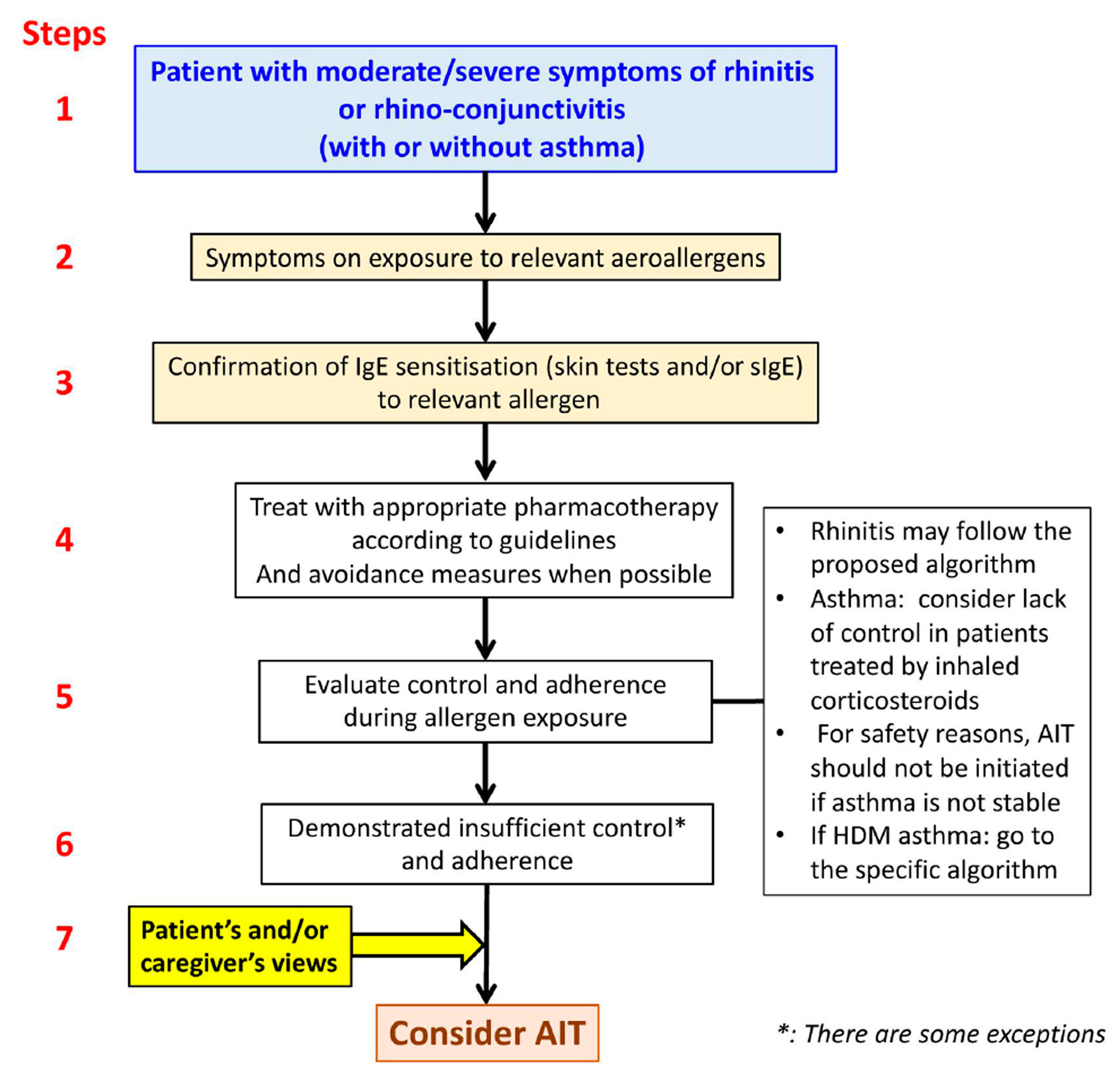
6. Allergen-Specific Immunotherapy
7. Safety of Subcutaneous Immunotherapy (SCIT)
8. Safety of Sublingual Immunotherapy (SLIT)
9. Shared Decision Making in Deciding on AIT
10. Patient Stratification in AIT
11. mHealth in the AIT Precision Medicine Approach
12. Challenges and Opportunities for Malaysia
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, B.; Abdul Latiff, A.H.; Manuel, A.M.; Mohamed Jamli, F.; Dalip Singh, H.S.; Ismail, I.H.; Jahendran, J.; Saniasiaya, J.; Kee Chen, K.W.; Khoo, P.C.; et al. Pharmacological Management of Allergic Rhinitis: A Consensus Statement from the Malaysian Society of Allergy and Immunology. J. Asthma Allergy 2022, 15, 983–1003. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Blaiss, M.S.; Nathan, R.A.; Doherty, D.E.; Murphy, K.R.; Stoloff, S.W. Asthma Burden in the United States: Results of the 2009 Asthma Insight and Management Survey. Allergy Asthma Proc. 2012, 33, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Quah, B.S.; Razif, A.; Razak, A.; Hashim, M.; Hassan, M. Prevalence of Asthma, Rhinitis and Eczema among Schoolchildren in Kelantan, Malaysia. Pediatr. Int. 1997, 39, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Naidu, R. Clinical Manifestation and Sensitization of Allergic Children from Malaysia. Asia Pac. Allergy 2015, 5, 78–83. [Google Scholar] [CrossRef]
- Prasad, V.; Abdullah, M.; Nordin, F.; Subha, S.T. Prevalence, Causes and Treatments of Allergic Rhinitis in Malaysia: A Literature Review. Egypt. J. Otolaryngol. 2022, 38, 170. [Google Scholar] [CrossRef]
- Bousquet, J.J.; Schünemann, H.J.; Togias, A.; Erhola, M.; Hellings, P.W.; Zuberbier, T.; Agache, I.; Ansotegui, I.J.; Anto, J.M.; Bachert, C.; et al. Next-Generation ARIA Care Pathways for Rhinitis and Asthma: A Model for Multimorbid Chronic Diseases. Clin. Transl. Allergy 2019, 9, 44. [Google Scholar] [CrossRef]
- Valiulis, A.; Bousquet, J.; Veryga, A.; Suprun, U.; Sergeenko, D.; Cebotari, S.; Borelli, D.; Pietikainen, S.; Banys, J.; Agache, I.; et al. Vilnius Declaration on Chronic Respiratory Diseases: Multisectoral Care Pathways Embedding Guided Self-Management, MHealth and Air Pollution in Chronic Respiratory Diseases. Clin. Transl. Allergy 2019, 9, 7. [Google Scholar] [CrossRef]
- Rannan-Eliya, R.P.; Anuranga, C.; Manual, A.; Sararaks, S.; Jailani, A.S.; Hamid, A.J.; Razif, I.M.; Tan, E.H.; Darzi, A. Improving Health Care Coverage, Equity, and Financial Protection through a Hybrid System: Malaysia’s Experience. Health Aff. 2016, 35, 838. [Google Scholar] [CrossRef]
- Mohd-Tahir, N.-A.; Paraidathathu, T.; Li, S.-C. Quality Use of Medicine in a Developing Economy: Measures to Overcome Challenges in the Malaysian Healthcare System. SAGE Open Med. 2015, 3, 2050312115596864. [Google Scholar] [CrossRef]
- Loh, P.; Chua, S.S.; Karuppannan, M. The Extent and Barriers in Providing Pharmaceutical Care Services by Community Pharmacists in Malaysia: A Cross-Sectional Study. BMC Health Serv. Res. 2021, 21, 822. [Google Scholar] [CrossRef]
- Bousquet, J.; Arnavielhe, S.; Bedbrook, A.; Bewick, M.; Laune, D.; Mathieu-Dupas, E.; Murray, R.; Onorato, G.L.; Pépin, J.L.; Picard, R.; et al. MASK 2017: ARIA Digitally-Enabled, Integrated, Person-Centred Care for Rhinitis and Asthma Multimorbidity Using Real-World-Evidence. Clin. Transl. Allergy 2018, 8, 45. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Annesi-Maesano, I.; Dedeu, T.; Dupas, E.; Pépin, J.-L.; Eyindanga, L.S.Z.; Arnavielhe, S.; Ayache, J.; Basagana, X.; et al. POLLAR: Impact of Air POLLution on Asthma and Rhinitis; a European Institute of Innovation and Technology Health (EIT Health) Project. Clin. Transl. Allergy 2018, 8, 36. [Google Scholar] [CrossRef]
- Bousquet, J.; Addis, A.; Adcock, I.; Agache, I.; Agusti, A.; Alonso, A.; Annesi-Maesano, I.; Anto, J.M.; Bachert, C.; Baena-Cagnani, C.E.; et al. Integrated Care Pathways for Airway Diseases (AIRWAYS-ICPs). Eur. Respir. J. 2014, 44, 304–323. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Baena-Cagnani, C.E.; Bonini, S.; Canonica, G.W.; Casale, T.B.; van Wijk, R.G.; Ohta, K.; Zuberbier, T.; Schünemann, H.J. Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines: 2010 Revision. J. Allergy Clin. Immunol. 2010, 126, 466. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines—2016 Revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Dykewicz, M.S.; Wallace, D.V.; Baroody, F.; Bernstein, J.; Craig, T.; Finegold, I.; Huang, F.; Larenas-Linnemann, D.; Meltzer, E.; Steven, G.; et al. Treatment of Seasonal Allergic Rhinitis. Ann. Allergy Asthma Immunol. 2017, 119, 489–511.e41. [Google Scholar] [CrossRef]
- Bousquet, J.; Devillier, P.; Anto, J.M.; Bewick, M.; Haahtela, T.; Arnavielhe, S.; Bedbrook, A.; Murray, R.; van Eerd, M.; Fonseca, J.A.; et al. Daily Allergic Multimorbidity in Rhinitis Using Mobile Technology: A Novel Concept of the MASK Study. Allergy 2018, 73, 1622–1631. [Google Scholar] [CrossRef]
- Bousquet, J.; Devillier, P.; Arnavielhe, S.; Bedbrook, A.; Alexis-Alexandre, G.; van Eerd, M.; Murray, R.; Canonica, G.W.; Illario, M.; Menditto, E.; et al. Treatment of Allergic Rhinitis Using Mobile Technology with Real-World Data: The MASK Observational Pilot Study. Allergy 2018, 73, 1763–1774. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Hellings, P.W.; Arnavielhe, S.; Bachert, C.; Bedbrook, A.; Bergmann, K.-C.; Bosnic-Anticevich, S.; Brozek, J.; Calderon, M.; et al. MACVIA Clinical Decision Algorithm in Adolescents and Adults with Allergic Rhinitis. J. Allergy Clin. Immunol. 2016, 138, 367–374.e2. [Google Scholar] [CrossRef]
- Glacy, J.; Putnam, K.; Godfrey, S.; Falzon, L.; Mauger, B.; Samson, D.; Aronson, N. Treatments for Seasonal Allergic Rhinitis; AHRQ Comparative Effectiveness Reviews: Rockville, MD, USA, 2013. [Google Scholar]
- Draft Guidance for Industry: Allergic Rhinitis: Clinical Development Programs for Drug Products; Food and Drug Administration, CDER: Silver Spring, MD, USA, 2000; Available online: http://wwwfdagov/cder/guidance/indexhtm (accessed on 12 January 2023).
- Allergic Rhinitis: Developing Drug Products for Treatment. Guidance for Industry; February 2016 Clinical/Medical Revision; U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2016; Available online: https://wwwfdagov/downloads/drugs/guidances/ucm071293pdf (accessed on 10 January 2023).
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-Generation Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines for Allergic Rhinitis Based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and Real-World Evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef]
- Bousquet, J.; Hellings, P.W.; Agache, I.; Bedbrook, A.; Bachert, C.; Bergmann, K.C.; Bewick, M.; Bindslev-Jensen, C.; Bosnic-Anticevitch, S.; Bucca, C.; et al. ARIA 2016: Care Pathways Implementing Emerging Technologies for Predictive Medicine in Rhinitis and Asthma across the Life Cycle. Clin. Transl. Allergy 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Guerriero, F.; Orlando, V.; Crola, C.; Di Somma, C.; Illario, M.; Morisky, D.E.; Colao, A. Self-Assessment of Adherence to Medication: A Case Study in Campania Region Community-Dwelling Population. J. Aging Res. 2015, 2015, 682503. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, B.; Snidvongs, K.; Recto, M.; Lestari Poerbonegoro, N.; Wang, D.Y. Primary Care Management of Allergic Rhinitis: A Cross-Sectional Study in Four ASEAN Countries. Multidiscip. Respir. Med. 2020, 15, 726. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Basagaña, X.; Anto, J.M.; Garcia-Aymerich, J.; Devillier, P.; Arnavielhe, S.; Bedbrook, A.; Onorato, G.L.; Czarlewski, W.; Murray, R.; et al. Mobile Technology Offers Novel Insights into the Control and Treatment of Allergic Rhinitis: The MASK Study. J. Allergy Clin. Immunol. 2019, 144, 135–143.e6. [Google Scholar] [CrossRef]
- Halken, S.; Larenas-Linnemann, D.; Roberts, G.; Calderón, M.A.; Angier, E.; Pfaar, O.; Ryan, D.; Agache, I.; Ansotegui, I.J.; Arasi, S.; et al. EAACI Guidelines on Allergen Immunotherapy: Prevention of Allergy. Pediatr. Allergy Immunol. 2017, 28, 728–745. [Google Scholar] [CrossRef]
- Bonertz, A.; Roberts, G.; Slater, J.E.; Bridgewater, J.; Rabin, R.L.; Hoefnagel, M.; Timon, M.; Pini, C.; Pfaar, O.; Sheikh, A.; et al. Allergen Manufacturing and Quality Aspects for Allergen Immunotherapy in Europe and the United States: An Analysis from the EAACI AIT Guidelines Project. Allergy 2018, 73, 816–826. [Google Scholar] [CrossRef]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; Gerth van Wijk, R.; Halken, S.; Larenas-Linnemann, D.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic Rhinoconjunctivitis. Allergy 2017, 73, 765–798. [Google Scholar] [CrossRef]
- Ryan, D.; Gerth van Wijk, R.; Angier, E.; Kristiansen, M.; Zaman, H.; Sheikh, A.; Cardona, V.; Vidal, C.; Warner, A.; Agache, I.; et al. Challenges in the Implementation of the EAACI AIT Guidelines: A Situational Analysis of Current Provision of Allergen Immunotherapy. Allergy 2017, 73, 827–836. [Google Scholar] [CrossRef]
- Pfaar, O.; Bachert, C.; Bufe, A.; Buhl, R.; Ebner, C.; Eng, P.; Friedrichs, F.; Fuchs, T.; Hamelmann, E.; Hartwig-Bade, D.; et al. Guideline on Allergen-Specific Immunotherapy in IgE-Mediated Allergic Diseases. Allergo J. Int. 2014, 23, 282–319. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Halken, S.; Agache, I.; Angier, E.; Fernandez-Rivas, M.; Gerth van Wijk, R.; Jutel, M.; Lau, S.; Pajno, G.; et al. EAACI Guidelines on Allergen Immunotherapy: Executive Statement. Allergy 2018, 73, 739–743. [Google Scholar] [CrossRef]
- Zielen, S.; Devillier, P.; Heinrich, J.; Richter, H.; Wahn, U. Sublingual Immunotherapy Provides Long-Term Relief in Allergic Rhinitis and Reduces the Risk of Asthma: A Retrospective, Real-World Database Analysis. Allergy 2018, 73, 165–177. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Van Weel, C.; et al. Allergic Rhinitis and Its Impact on Asthma (ARIA) 2008*. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef]
- Bousquet, J.; Pfaar, O.; Togias, A.; Schünemann, H.J.; Ansotegui, I.; Papadopoulos, N.G.; Tsiligianni, I.; Agache, I.; Anto, J.M.; Bachert, C.; et al. 2019 ARIA Care Pathways for Allergen Immunotherapy. Allergy 2019, 74, 2087–2102. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CPMP). Guideline on Allergen Products: Production and Quality Issues; EMEA/CHMP/BWP/304831/London, 20 November 2008; European Medicines Agency: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Passalacqua, G.; Baena-Cagnani, C.E.; Bousquet, J.; Canonica, G.W.; Casale, T.B.; Cox, L.; Durham, S.R.; Larenas-Linnemann, D.; Ledford, D.; Pawankar, R.; et al. Grading Local Side Effects of Sublingual Immunotherapy for Respiratory Allergy: Speaking the Same Language. J. Allergy Clin. Immunol. 2013, 132, 93–98. [Google Scholar] [CrossRef]
- Pitsios, C.; Dietis, N. Ways to Increase Adherence to Allergen Immunotherapy. Curr. Med. Res. Opin. 2018, 35, 1027–1031. [Google Scholar] [CrossRef]
- Bender, B.G.; Lockey, R.F. Solving the Problem of Nonadherence to Immunotherapy. Immunol. Allergy Clin. N. Am. 2016, 36, 205–213. [Google Scholar] [CrossRef]
- Bosnic-Anticevich, S.; Costa, E.; Menditto, E.; Lourenço, O.; Novellino, E.; Bialek, S.; Briedis, V.; Buonaiuto, R.; Chrystyn, H.; Cvetkovski, B.; et al. ARIA Pharmacy 2018 “Allergic Rhinitis Care Pathways for Community Pharmacy”: AIRWAYS ICPs Initiative (European Innovation Partnership on Active and Healthy Ageing, DG CONNECT and DG Santé) POLLAR (Impact of Air POLLution on Asthma and Rhinitis) GARD Demonstration Project. Allergy 2019, 74, 1219–1236. [Google Scholar] [CrossRef]
- Canonica, G.; Bachert, C.; Hellings, P.; Ryan, D.; Valovirta, E.; Wickman, M.; De Beaumont, O.; Bousquet, J. Allergen Immunotherapy (AIT): A Prototype of Precision Medicine. World Allergy Organ. J. 2015, 8, 31. [Google Scholar] [CrossRef]
- Pitsios, C.; Demoly, P.; Bilò, M.B.; Gerth van Wijk, R.; Pfaar, O.; Sturm, G.J.; Rodriguez del Rio, P.; Tsoumani, M.; Gawlik, R.; Paraskevopoulos, G.; et al. Clinical Contraindications to Allergen Immunotherapy: An EAACI Position Paper. Allergy 2015, 70, 897–909. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Available online: www.ginasthma.org (accessed on 10 January 2023).
- Summary of Product Characteristics. Acarizax 12 SQ-HDM Oral Lyophilisate. European Medicines Agency. 2016. Available online: https://www.medicines.org.uk/emc/product/12905/smpc#gref (accessed on 5 January 2023).
- Amaral, R.; Fonseca, J.A.; Jacinto, T.; Pereira, A.M.; Malinovschi, A.; Janson, C.; Alving, K. Having Concomitant Asthma Phenotypes Is Common and Independently Relates to Poor Lung Function in NHANES 2007–2012. Clin. Transl. Allergy 2018, 8, 13. [Google Scholar] [CrossRef]
- Masuyama, K.; Okamoto, Y.; Okamiya, K.; Azuma, R.; Fujinami, T.; Riis, B.; Ohashi-Doi, K.; Natsui, K.; Imai, T.; Okubo, K. Efficacy and Safety of SQ House Dust Mite Sublingual Immunotherapy-Tablet in Japanese Children. Allergy 2018, 73, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Penagos, M.; Eifan, A.O.; Durham, S.R.; Scadding, G.W. Duration of Allergen Immunotherapy for Long-Term Efficacy in Allergic Rhinoconjunctivitis. Curr. Treat. Options Allergy 2018, 5, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Valovirta, E.; Petersen, T.H.; Piotrowska, T.; Laursen, M.K.; Andersen, J.S.; Sørensen, H.F.; Klink, R.; Varga, E.-M.; Huttegger, I.; Agertoft, L.; et al. Results from the 5-Year SQ Grass Sublingual Immunotherapy Tablet Asthma Prevention (GAP) Trial in Children with Grass Pollen Allergy. J. Allergy Clin. Immunol. 2018, 141, 529–538.e13. [Google Scholar] [CrossRef]
- Möller, C.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Jacobsen, L.; Koivikko, A.; Koller, D.Y.; Niggemann, B.; Norberg, L.A.; et al. Pollen Immunotherapy Reduces the Development of Asthma in Children with Seasonal Rhinoconjunctivitis (the PAT-Study). J. Allergy Clin. Immunol. 2002, 109, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Demoly, P.; Gerth van Wijk, R.; Bonini, S.; Bousquet, J.; Canonica, G.W.; Durham, S.R.; Jacobsen, L.; Malling, H.J.; Mösges, R.; et al. Recommendations for the Standardization of Clinical Outcomes Used in Allergen Immunotherapy Trials for Allergic Rhinoconjunctivitis: An EAACI Position Paper. Allergy 2014, 69, 854–867. [Google Scholar] [CrossRef]
- Kristiansen, M.; Dhami, S.; Netuveli, G.; Halken, S.; Muraro, A.; Roberts, G.; Larenas-Linnemann, D.; Calderón, M.A.; Penagos, M.; Du Toit, G.; et al. Allergen Immunotherapy for the Prevention of Allergy: A Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. 2016, 28, 18–29. [Google Scholar] [CrossRef]
- Bożek, A.; Kołodziejczyk, K.; Kozłowska, R.; Canonica, G.W. Evidence of the Efficacy and Safety of House Dust Mite Subcutaneous Immunotherapy in Elderly Allergic Rhinitis Patients: A Randomized, Double-Blind Placebo-Controlled Trial. Clin. Transl. Allergy 2017, 7, 43. [Google Scholar] [CrossRef]
- Bousquet, J.; Arnavielhe, S.; Bedbrook, A.; Fonseca, J.; Morais Almeida, M.; Todo Bom, A.; Annesi-Maesano, I.; Caimmi, D.; Demoly, P.; Devillier, P.; et al. The Allergic Rhinitis and Its Impact on Asthma (ARIA) Score of Allergic Rhinitis Using Mobile Technology Correlates with Quality of Life: The MASK Study. Allergy 2017, 73, 505–510. [Google Scholar] [CrossRef]
- Courbis, A.; Murray, R.B.; Arnavielhe, S.; Caimmi, D.; Bedbrook, A.; Van Eerd, M.; De Vries, G.; Dray, G.; Agache, I.; Morais-Almeida, M.; et al. Electronic Clinical Decision Support System for Allergic Rhinitis Management: MASK E-CDSS. Clin. Exp. Allergy 2018, 48, 1640–1653. [Google Scholar] [CrossRef]
- Navarro-Locsin, C.G.; Romualdez, J.A. Attitudes, Practices on Allergic Rhinitis of Generalists and Specialists in Philippine National Capital Region. Asia Pac. Allergy 2015, 5, 203–209. [Google Scholar] [CrossRef]
- Blaiss, M.S.; Fromer, L.M.; Jacob-Nara, J.A.; Long, R.M.; Mannion, K.M.; Lauersen, L.A. Current Allergic Rhinitis Experiences Survey (CARES): Health-Care Practitioners’ Awareness, Attitudes and Practices. Allergy Asthma Proc. 2014, 35, 316–322. [Google Scholar] [CrossRef]
- Sherman, J.; Patel, P.; Hutson, A.; Chesrown, S.; Hendeles, L. Adherence to Oral Montelukast and Inhaled Fluticasone in Children with Persistent Asthma. Pharmacotherapy 2001, 21, 1464–1467. [Google Scholar] [CrossRef]
- Manjit Singh, P.K.; Krishnan, E.K.; Mat Lazim, N.; Yaacob, N.M.; Abdullah, B. Medication Adherence to Intranasal Corticosteroids in Allergic Rhinitis Patients with Comorbid Medical Conditions. Pharmaceutics 2022, 14, 2459. [Google Scholar] [CrossRef]
- Abdullah, B.; Pawankar, R.; Abdul Latiff, A.H.; Woo, K.C.K.; Wüstenberg, E.; Khalid, M.A.F.; Xiang, Y.Z.; Husain, S.; Mohammad, N.; Md Shukri, N. Malaysian Society of Allergy and Immunology Consensus Statement on Sublingual Immunotherapy in Allergic Rhinitis. J. Clin. Med. 2023, 12, 1151. [Google Scholar] [CrossRef]
- Hellings, P.W.; Fokkens, W.J.; Akdis, C.; Bachert, C.; Cingi, C.; Dietz De Loos, D.; Gevaert, P.; Hox, V.; Kalogjera, L.; Lund, V.; et al. Uncontrolled Allergic Rhinitis and Chronic Rhinosinusitis: Where Do We Stand Today? Allergy 2013, 68, 1–7. [Google Scholar] [CrossRef]
- Abdullah, B.; Singh, S. Surgical Interventions for Inferior Turbinate Hypertrophy: A Comprehensive Review of Current Techniques and Technologies. Int. J. Environ. Res. Public Health 2021, 18, 3441. [Google Scholar] [CrossRef]
- Karatzanis, A.D. Septoplasty Outcome in Patients with and without Allergic Rhinitis. Rhinol. J. 2009, 47, 444. [Google Scholar] [CrossRef]
- Simmonds, J.C.; Paz-Lansberg, M.; Scangas, G.; Metson, R. Endoscopic Sinus Surgery for Chronic Rhinosinusitis: 22-item Sino-Nasal Outcome Test 5-year Results. Int. Forum. Allergy Rhinol. 2022, 12, 257–265. [Google Scholar] [CrossRef]
- Pfaar, O.; Gehrt, F.; Li, H.; Rudhart, S.A.; Nastev, A.; Stuck, B.A.; Hoch, S. Anti-IgE: A Treatment Option in Allergic Rhinitis? Allergol. Sel. 2021, 5, 119–127. [Google Scholar] [CrossRef]
- World Health Organization & International Telecommunication Union. Be He@lthy, Be Mobile: A Handbook on How to Implement mBreatheFreely, mHealth for COPD and Asthma. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/274575 (accessed on 15 January 2023).
- ICT Facts and Figures ICT Data and Statistics Division Telecommunication Development Bureau International Telecommunication Union Place des Nations 1211 Geneva 20—Switzerland. Available online: https://www.itu.int/dms_pub/itu-d/opb/ind/d-ind-ict_mdd-2022-pdf-e.pdf (accessed on 15 January 2023).
- Abdullah, B.; Snidvongs, K.; Poerbonegoro, N.L.; Sutikno, B. Reshaping the Management of Allergic Rhinitis in Primary Care: Lessons from the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 13632. [Google Scholar] [CrossRef]
- Bourret, R.; Bousquet, J.; Mercier, J.; Camuzat, T.; Bedbrook, A.; Demoly, P.; Caimmi, D.; Laune, D.; Arnavielhe, S. MASK rhinitis, a single tool for integrated care pathways in allergic rhinitis. World Hosp. Health Serv. 2015, 51, 36–39. [Google Scholar] [PubMed]
- Bousquet, J.; Schunemann, H.J.; Fonseca, J.; Samolinski, B.; Bachert, C.; Canonica, G.W.; Casale, T.; Cruz, A.A.; Demoly, P.; Hellings, P.; et al. MACVIA-ARIA Sentinel NetworK for Allergic Rhinitis (MASK-Rhinitis): The New Generation Guideline Implementation. Allergy 2015, 70, 1372–1392. [Google Scholar] [CrossRef] [PubMed]
- Cingi, C.; Gevaert, P.; Mösges, R.; Rondon, C.; Hox, V.; Rudenko, M.; Muluk, N.B.; Scadding, G.; Manole, F.; Hupin, C.; et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology Task Force Report. Clin. Transl. Allergy 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.; Kurup, R.; Laba, T.L.; Santo, K.; Thiagalingam, A.; Rodgers, A.; Woodward, M.; Redfern, J.; Chow, C.K. Mobile telephone text messaging for medication adherence in chronic disease: A meta-analysis. JAMA Intern. Med. 2016, 176, 340–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Latiff, A.H.; Husain, S.; Abdullah, B.; Suppiah, P.; Tan, V.; Ing Ping, T.; Woo, K.; Yap, Y.-Y.; Bachert, C.; J. Schunemann, H.; et al. ARIA Care Pathways 2019: Next-Generation Allergic Rhinitis Care and Allergen Immunotherapy in Malaysia. J. Pers. Med. 2023, 13, 835. https://doi.org/10.3390/jpm13050835
Abdul Latiff AH, Husain S, Abdullah B, Suppiah P, Tan V, Ing Ping T, Woo K, Yap Y-Y, Bachert C, J. Schunemann H, et al. ARIA Care Pathways 2019: Next-Generation Allergic Rhinitis Care and Allergen Immunotherapy in Malaysia. Journal of Personalized Medicine. 2023; 13(5):835. https://doi.org/10.3390/jpm13050835
Chicago/Turabian StyleAbdul Latiff, Amir Hamzah, Salina Husain, Baharudin Abdullah, Palaniappan Suppiah, Vincent Tan, Tang Ing Ping, Kent Woo, Yoke-Yeow Yap, Claus Bachert, Holger J. Schunemann, and et al. 2023. "ARIA Care Pathways 2019: Next-Generation Allergic Rhinitis Care and Allergen Immunotherapy in Malaysia" Journal of Personalized Medicine 13, no. 5: 835. https://doi.org/10.3390/jpm13050835
APA StyleAbdul Latiff, A. H., Husain, S., Abdullah, B., Suppiah, P., Tan, V., Ing Ping, T., Woo, K., Yap, Y.-Y., Bachert, C., J. Schunemann, H., Bedbrook, A., Czarlewski, W., & Bousquet, J. (2023). ARIA Care Pathways 2019: Next-Generation Allergic Rhinitis Care and Allergen Immunotherapy in Malaysia. Journal of Personalized Medicine, 13(5), 835. https://doi.org/10.3390/jpm13050835