Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
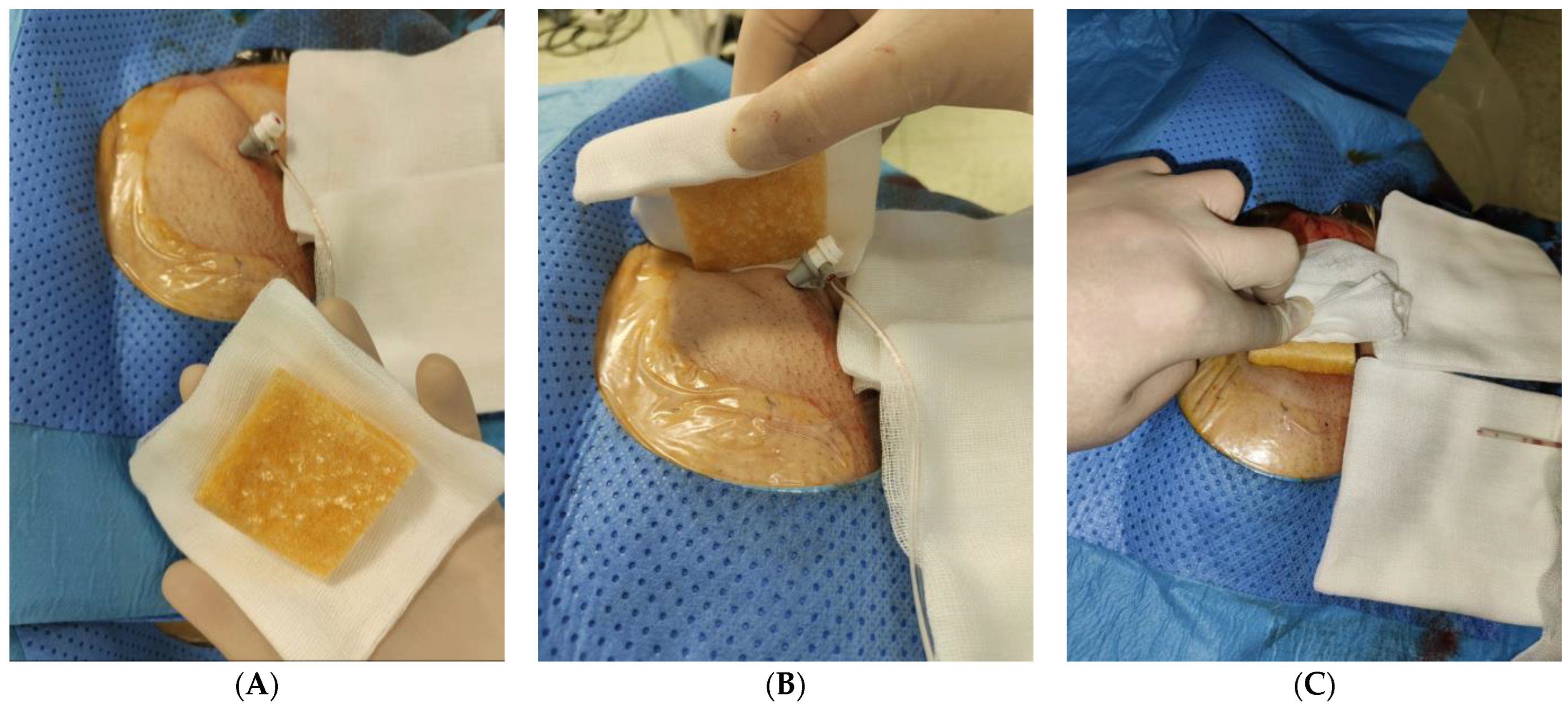
2.2. Treatment
2.3. Outcomes and Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Procedure Data
3.3. Outcomes
3.4. Sheath Size 4–6 Fr vs. Sheath Size 7–8 Fr
3.5. Predictors of Bleeding-Related VASCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kettenbach, J.; Ittrich, H.; Gaubert, J.Y.; Gebauer, B.; Vos, J.A. CIRSE Standards of Practice on Bronchial Artery Embolisation. Cardiovasc. Interv. Radiol. 2022, 45, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Burrel, M.; Guiu, B.; de Rubeis, G.; van Delden, O.; Helmberger, T. CIRSE Standards of Practice on Hepatic Transarterial Chemoembolisation. Cardiovasc. Interv. Radiol. 2021, 44, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Memarian, S.; Krokidis, M.; O’Sullivan, G.; Peynircioglu, B.; Rossi, M.; Kashef, E. CIRSE Standards of Practice on Arterial Access for Interventions. Cardiovasc. Interv. Radiol. 2023, 46, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Del Giudice, C.; Manzi, M.; Reppas, L.; Rodt, T.; Uberoi, R. CIRSE Standards of Practice on Below-the-Knee Revascularisation. Cardiovasc. Interv. Radiol. 2021, 44, 1309–1322. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Ierardi, A.M.; Petullà, M.; Bracale, U.M.; Carrafiello, G.; Laganà, D. Thoracic Endovascular Repair for Blunt Traumatic Thoracic Aortic Injury: Long-Term Results. Vascular 2022. [Google Scholar] [CrossRef]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Giglio, E.; Minici, M.; Melina, M.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization as a Bridging Therapy in Patients with Early Stage Hepatocellular Carcinoma and Child-Pugh Stage B Eligible for Liver Transplant. Front. Pharmacol. 2021, 12, 634084. [Google Scholar] [CrossRef]
- Minici, R.; Ammendola, M.; Talarico, M.; Luposella, M.; Minici, M.; Ciranni, S.; Guzzardi, G.; Laganà, D. Endovascular Recanalization of Chronic Total Occlusions of the Native Superficial Femoral Artery after Failed Femoropopliteal Bypass in Patients with Critical Limb Ischemia. CVIR Endovasc. 2021, 4, 68. [Google Scholar] [CrossRef]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Minici, M.; Komaei, I.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization (DSM-TACE) in the Downstaging of Intermediate-Stage Hepatocellular Carcinoma (HCC) in Patients With a Child-Pugh Score of 8–9. Front. Pharmacol. 2021, 12, 634087. [Google Scholar] [CrossRef]
- Bracale, U.M.; Peluso, A.; Panagrosso, M.; Cecere, F.; Del Guercio, L.; Minici, R.; Giannotta, N.; Ielapi, N.; Licastro, N.; Serraino, G.F.; et al. Ankle-Brachial Index evaluation in totally percutaneous approach vs. femoral artery cutdown for endovascular aortic repair of abdominal aortic aneurysms. Chirurgia 2022, 35, 349–354. [Google Scholar] [CrossRef]
- Gedney, R.; Villacreses, C.F.; Wooster, M.D.; Genovese, E.A. Percutaneous, Antegrade Access of the Superficial Femoral Artery Can Be Safely Used in Procedures for the Treatment of Lower Extremity Limb Ischemia. Ann. Vasc. Surg. 2023, 91, 218–222. [Google Scholar] [CrossRef]
- Chivot, C.; Deramond, H.; Bouzerar, R.; Yzet, T. Safety and Efficacy of Femoral Artery Closure with the FemoSeal Device After Cerebral Thrombectomy Using an 8 French Sheath. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 55, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.E.; Moore, E.E.; Morrison, J.J.; Lyon, R.F.; DuBose, J.J.; Ross, J.D. Femoral Vascular Access for Endovascular Resuscitation. J. Trauma Acute Care Surg. 2021, 91, e104. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.; Blair, L.; Huntington, C.; Lincourt, A.; Sing, R.; Heniford, B.T. Systematic Review of Randomized Controlled Trials Comparing Manual Compression to Vascular Closure Devices for Diagnostic and Therapeutic Arterial Procedures. Surg. Technol. Int. 2015, 27, 32–44. [Google Scholar] [PubMed]
- Baim, D.S.; Knopf, W.D.; Hinohara, T.; Schwarten, D.E.; Schatz, R.A.; Pinkerton, C.A.; Cutlip, D.E.; Fitzpatrick, M.; Ho, K.K.; Kuntz, R.E. Suture-Mediated Closure of the Femoral Access Site after Cardiac Catheterization: Results of the Suture to Ambulate aNd Discharge (STAND I and STAND II) Trials. Am. J. Cardiol. 2000, 85, 864–869. [Google Scholar] [CrossRef]
- Noguchi, T.; Miyazaki, S.; Yasuda, S.; Baba, T.; Sumida, H.; Morii, I.; Daikoku, S.; Goto, Y.; Nonogi, H. A Randomised Controlled Trial of Prostar Plus for Haemostasis in Patients after Coronary Angioplasty. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2000, 19, 451–455. [Google Scholar] [CrossRef]
- Kussmaul, W.G.; Buchbinder, M.; Whitlow, P.L.; Aker, U.T.; Heuser, R.R.; King, S.B.; Kent, K.M.; Leon, M.B.; Kolansky, D.M.; Sandza, J.G. Rapid Arterial Hemostasis and Decreased Access Site Complications after Cardiac Catheterization and Angioplasty: Results of a Randomized Trial of a Novel Hemostatic Device. J. Am. Coll. Cardiol. 1995, 25, 1685–1692. [Google Scholar] [CrossRef]
- SEAL Trial Study Team Assessment of the Safety and Efficacy of the DUETT Vascular Hemostasis Device: Final Results of the Safe and Effective Vascular Hemostasis (SEAL) Trial. Am. Heart J. 2002, 143, 612–619. [CrossRef]
- Pang, N.; Gao, J.; Zhang, B.; Guo, M.; Zhang, N.; Sun, M.; Wang, R. Vascular Closure Devices versus Manual Compression in Cardiac Interventional Procedures: Systematic Review and Meta-Analysis. Cardiovasc. Ther. 2022, 2022, 8569188. [Google Scholar] [CrossRef]
- Gewalt, S.M.; Helde, S.M.; Ibrahim, T.; Mayer, K.; Schmidt, R.; Bott-Flügel, L.; Hoppe, K.; Ott, I.; Hieber, J.; Morath, T.; et al. Comparison of Vascular Closure Devices Versus Manual Compression After Femoral Artery Puncture in Women. Circ. Cardiovasc. Interv. 2018, 11, e006074. [Google Scholar] [CrossRef]
- Freitas, B.; Steiner, S.; Bausback, Y.; Staab, H.; Branzan, D.; Banning-Eichenseer, U.; Schmidt, A.; Scheinert, D. Single-Center Experience with Vascular Closure Devices in Real-World Endovascular Peripheral Interventions. J. Cardiovasc. Surg. 2018, 59, 797–803. [Google Scholar] [CrossRef]
- Schulz-Schüpke, S.; Helde, S.; Gewalt, S.; Ibrahim, T.; Linhardt, M.; Haas, K.; Hoppe, K.; Böttiger, C.; Groha, P.; Bradaric, C.; et al. Comparison of Vascular Closure Devices vs Manual Compression after Femoral Artery Puncture: The ISAR-CLOSURE Randomized Clinical Trial. JAMA 2014, 312, 1981–1987. [Google Scholar] [CrossRef]
- Munich, S.A.; Vakharia, K.; McPheeters, M.J.; Waqas, M.; Tso, M.K.; Levy, E.I.; Snyder, K.V.; Siddiqui, A.H.; Davies, J.M. Transition to Transradial Access for Mechanical Thrombectomy-Lessons Learned and Comparison to Transfemoral Access in a Single-Center Case Series. Oper. Neurosurg. 2020, 19, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Reekers, J.A.; Müller-Hülsbeck, S.; Libicher, M.; Atar, E.; Trentmann, J.; Goffette, P.; Borggrefe, J.; Zeleňák, K.; Hooijboer, P.; Belli, A.-M. CIRSE Vascular Closure Device Registry. Cardiovasc. Interv. Radiol. 2011, 34, 50–53. [Google Scholar] [CrossRef]
- Tavris, D.R.; Gallauresi, B.A.; Lin, B.; Rich, S.E.; Shaw, R.E.; Weintraub, W.S.; Brindis, R.G.; Hewitt, K. Risk of Local Adverse Events Following Cardiac Catheterization by Hemostasis Device Use and Gender. J. Invasive Cardiol. 2004, 16, 459–464. [Google Scholar] [PubMed]
- Kabeer, M.; Venugopalan, P.P.; Subhash, V.C. Pre-Hospital Hemorrhagic Control Effectiveness of Axiostat® Dressing Versus Conventional Method in Acute Hemorrhage Due to Trauma. Cureus 2019, 11, e5527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kale, T.P.; Balihallimath, L.J.; Motimath, A. Evaluating Effectiveness of Axiostat Hemostatic Material in Achieving Hemostasis and Healing of Extraction Wounds in Patients on Oral Antiplatelet Drugs. J. Contemp. Dent. Pract. 2017, 18, 802–806. [Google Scholar] [CrossRef]
- Rajendra, K.; Vempalli, S.; Kadiyala, M.; Sharma, V.; Karipineni, S.; Gunturu, S.; Patil, D.B. Effect of Platelet-Rich Fibrin versus Chitosan-Based Axiostat Hemostatic Agent Following Dental Extraction in Cardiac Patients on Antiplatelet Therapy: A Comparative Study. Natl. J. Maxillofac. Surg. 2021, 12, 361–366. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of Chitosan as a Biomaterial: Studies on Its Safety and Hemostatic Potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Malette, W.G.; Quigley, H.J.; Gaines, R.D.; Johnson, N.D.; Rainer, W.G. Chitosan: A New Hemostatic. Ann. Thorac. Surg. 1983, 36, 55–58. [Google Scholar] [CrossRef]
- Eldibany, R.M. Platelet Rich Fibrin versus Hemcon Dental Dressing Following Dental Extraction in Patients under Anticoagulant Therapy. Tanta Dent. J. 2014, 11, 75–84. [Google Scholar] [CrossRef]
- Anchan, R.; Venturini, J.; Larsen, P.; Lee, L.; Fernandez, C.; Besser, S.A.; Kalathiya, R.; Paul, J.; Blair, J.; Nathan, S. Safe and Rapid Radial Hemostasis Achieved Using a Novel Topical Hemostatic Patch: Results of a First-in-Human Pilot Study Using Hydrophobically Modified Polysaccharide-Chitosan. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 99, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.Z.; Aijaz, S.; Sheikh, S.; Sattar, S. Randomized Trial Comparing Radial Hemostasis Techniques; Catechol Conjugated Chitosan Pad (InnoSEAL) versus Pneumatic Compression Band. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, E181–E187. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S.; Niu, J.; Pastor-Cervantes, J.A. Comparison of Hemostasis Times with a Chitosan-Based Hemostatic Pad (Clo-SurPlus RadialTM) vs Mechanical Compression (TR Band®) Following Transradial Access: A Pilot Study. Cardiovasc. Revascularization Med. Mol. Interv. 2019, 20, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Han, D.; Kim, S.; Yoon, C.-H.; Park, J.-J.; Suh, J.-W.; Cho, Y.-S.; Youn, T.-J.; Chae, I.-H. Hemostasis Pad Combined with Compression Device after Transradial Coronary Procedures: A Randomized Controlled Trial. PLoS ONE 2017, 12, e0181099. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Xu, D.; Hou, L.; Peng, W.; Wei, Y.; Xu, Y. A Comparison of 2 Devices for Radial Artery Hemostasis after Transradial Coronary Intervention. J. Cardiovasc. Nurs. 2015, 30, 192–196. [Google Scholar] [CrossRef]
- Aijaz, S.; Sheikh, S.; Pathan, A. Combination of InnoSEAL plus TR Band Compared with TR Band Alone for Radial Artery Outcomes in Patients Undergoing Transradial Coronary Intervention (InnoSEAL-II): An Open-Label Randomised Controlled Trial (Protocol). BMJ Open 2020, 10, e042101. [Google Scholar] [CrossRef]
- Hadi, M.; Walker, C.; Desborough, M.; Basile, A.; Tsetis, D.; Hunt, B.; Müller-Hüllsbeck, S.; Rand, T.; van Delden, O.; Uberoi, R. CIRSE Standards of Practice on Peri-Operative Anticoagulation Management During Interventional Radiology Procedures. Cardiovasc. Interv. Radiol. 2021, 44, 523–536. [Google Scholar] [CrossRef]
- Morton Kern, M.D. Back to Basics: Femoral Artery Access and Hemostasis. Cath Lab Dig. 2013, 21. [Google Scholar]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Stoner, M.C.; Calligaro, K.D.; Chaer, R.A.; Dietzek, A.M.; Farber, A.; Guzman, R.J.; Hamdan, A.D.; Landry, G.J.; Yamaguchi, D.J. on behalf of Society for Vascular Surgery. Reporting Standards of the Society for Vascular Surgery for Endovascular Treatment of Chronic Lower Extremity Peripheral Artery Disease. J. Vasc. Surg. 2016, 64, e1–e21. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Kleiman, N.S.; Kereiakes, D.J.; Feit, F.; Bittl, J.A.; Jackman, J.D.; Sarembock, I.J.; Cohen, D.J.; Spriggs, D.; Ebrahimi, R.; et al. Long-Term Efficacy of Bivalirudin and Provisional Glycoprotein IIb/IIIa Blockade vs Heparin and Planned Glycoprotein IIb/IIIa Blockade during Percutaneous Coronary Revascularization: REPLACE-2 Randomized Trial. JAMA 2004, 292, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Guiu, B.; D’Athis, P.; Mezzetta, L.; Gagnaire, A.; Jouve, J.-L.; Ortega-Deballon, P.; Cheynel, N.; Cercueil, J.-P.; Krausé, D. Arterial Embolotherapy for Endoscopically Unmanageable Acute Gastroduodenal Hemorrhage: Predictors of Early Rebleeding. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2009, 7, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Serra, R.; Giurdanella, M.; Talarico, M.; Siciliano, M.A.; Carrafiello, G.; Laganà, D. Efficacy and Safety of Distal Radial Access for Transcatheter Arterial Chemoembolization (TACE) of the Liver. J. Pers. Med. 2023, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Talarico, M.; Schepis, F.; Coppi, F.; Sgura, F.A.; Monopoli, D.E.; Minici, R.; Boriani, G. Effects of Sildenafil on Right Ventricle Remodelling in Portopulmonary Hypertension. Pulm. Pharmacol. Ther. 2021, 70, 102071. [Google Scholar] [CrossRef]
- Rossi, R.; Talarico, M.; Pascale, A.; Pascale, V.; Minici, R.; Boriani, G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics 2022, 12, 2572. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Iannò, B.; Galasso, O.; Gasparini, G.; Laganà, D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthcare 2023, 11, 724. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; De Rosi, N.; Ciranni, S.; Talarico, M.; Petullà, M.; Guzzardi, G.; Fontana, F.; Laganà, D. Endovascular treatment of femoro-popliteal occlusions with retrograde tibial access after failure of the antegrade approach. Catheter. Cardiovasc. Interv. 2023. [Google Scholar] [CrossRef]
- Martin, J.L.; Pratsos, A.; Magargee, E.; Mayhew, K.; Pensyl, C.; Nunn, M.; Day, F.; Shapiro, T. A Randomized Trial Comparing Compression, Perclose Proglide and Angio-Seal VIP for Arterial Closure Following Percutaneous Coronary Intervention: The CAP Trial. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2008, 71, 1–5. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Chen, J.; Shi, H.; Pan, J.; Zhang, X.; Jin, Z. Safety and Effectiveness of Repeat Arterial Closure Using the StarClose Vascular Closure Device in Patients with Hepatic Malignancy. Clin. Radiol. 2013, 68, e498–e501. [Google Scholar] [CrossRef]
- Sekhar, A.; Sutton, B.S.; Raheja, P.; Mohsen, A.; Anggelis, E.; Anggelis, C.N.; Keith, M.C.; Dawn, B.; Straton, S.; Flaherty, M.P. Femoral Arterial Closure Using ProGlide® Is More Efficacious and Cost-Effective When Ambulating Early Following Cardiac Catheterization. Int. J. Cardiol. Heart Vasc. 2016, 13, 6–13. [Google Scholar] [CrossRef]
- Pieper, C.C.; Thomas, D.; Nadal, J.; Willinek, W.A.; Schild, H.H.; Meyer, C. Patient Satisfaction After Femoral Arterial Access Site Closure Using the ExoSeal(®) Vascular Closure Device Compared to Manual Compression: A Prospective Intra-Individual Comparative Study. Cardiovasc. Interv. Radiol. 2016, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.; Mehran, R.; Kokolis, S.; Feldman, D.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Lansky, A.J.; Stone, G.W.; Leon, M.B. Vascular Complications after Percutaneous Coronary Interventions Following Hemostasis with Manual Compression versus Arteriotomy Closure Devices. J. Am. Coll. Cardiol. 2001, 38, 638–641. [Google Scholar] [CrossRef]
- Pate, G.; Broderick, B. Variations in the Usage and Composition of a Radial Cocktail during Radial Access Coronary Angiography Procedures. Ir. Med. J. 2011, 104, 280–281. [Google Scholar] [PubMed]
- Robertson, L.; Andras, A.; Colgan, F.; Jackson, R. Vascular Closure Devices for Femoral Arterial Puncture Site Haemostasis. Cochrane Database Syst. Rev. 2016, 3, CD009541. [Google Scholar] [CrossRef]
- Jakobsen, L.; Holm, N.R.; Maeng, M.; Thim, T.; Kristensen, S.D.; Mogensen, L.H.; Christiansen, E.H. Comparison of MynxGrip Vascular Closure Device and Manual Compression for Closure after Femoral Access Angiography: A Randomized Controlled Trial: The Closure Devices Used in Every Day Practice Study, CLOSE-UP III Trial. BMC Cardiovasc. Disord. 2022, 22, 68. [Google Scholar] [CrossRef]
- Minici, R.; Venturini, M.; Fontana, F.; Guzzardi, G.; Pingitore, A.; Piacentino, F.; Serra, R.; Coppola, A.; Santoro, R.; Laganà, D. Efficacy and Safety of Ethylene-Vinyl Alcohol (EVOH) Copolymer-Based Non-Adhesive Liquid Embolic Agents (NALEAs) in Transcatheter Arterial Embolization (TAE) of Acute Non-Neurovascular Bleeding: A Multicenter Retrospective Cohort Study. Medicina 2023, 59, 710. [Google Scholar] [CrossRef]
- Minici, R.; Fontana, F.; Venturini, M.; Guzzardi, G.; Siciliano, A.; Piacentino, F.; Serra, R.; Coppola, A.; Guerriero, P.; Apollonio, B.; et al. Transcatheter arterial embolization (TAE) in the management of bleeding in the COVID-19 patient. Medicina, 2023; in press. [Google Scholar]
- Wong, S.C.; Bachinsky, W.; Cambier, P.; Stoler, R.; Aji, J.; Rogers, J.H.; Hermiller, J.; Nair, R.; Hutman, H.; Wang, H.; et al. A Randomized Comparison of a Novel Bioabsorbable Vascular Closure Device versus Manual Compression in the Achievement of Hemostasis after Percutaneous Femoral Procedures: The ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc. Interv. 2009, 2, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.G.; Janardhanapillai, R.K.; Dabas, A.K.; Chadha, D.S.; Swamy, A.J.; Chadha, A.S. Femoral Artery Access Site Closure with Perclose Suture Mediated Device in Coronary Interventions. Indian Heart J. 2021, 73, 180–184. [Google Scholar] [CrossRef]
- Owens, J.T.; Bhatty, S.; Donovan, R.J.; Tordini, A.; Danyi, P.; Patel, K.; Wegelin, J.A.; Jovin, I.S. Usefulness of a Nonsuture Closure Device in Patients Undergoing Diagnostic Coronary and Peripheral Angiography. Int. J. Angiol. 2017, 26, 228–233. [Google Scholar] [CrossRef]
- Minici, R.; Paone, S.; Talarico, M.; Zappia, L.; Abdalla, K.; Petullà, M.; Laganà, D. Percutaneous Treatment of Vascular Access-Site Complications: A Ten Years’ Experience in Two Centres. CVIR Endovasc. 2020, 3, 29. [Google Scholar] [CrossRef]
- Nikolsky, E.; Mehran, R.; Halkin, A.; Aymong, E.D.; Mintz, G.S.; Lasic, Z.; Negoita, M.; Fahy, M.; Krieger, S.; Moussa, I.; et al. Vascular Complications Associated with Arteriotomy Closure Devices in Patients Undergoing Percutaneous Coronary Procedures: A Meta-Analysis. J. Am. Coll. Cardiol. 2004, 44, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients (n = 120) |
|---|---|
| Age (years) | 68.3 (±14) |
| Sex (M/F) | 59 (49.2%)/61 (50.8%) |
| BMI | 24.7 (±5.4) |
| INR | 1.2 (±0.2) |
| aPTT (s) | 35.8 (±4.2) |
| PLT (×103/µL) | 404.6 (±32) |
| Coagulopathy | 12 (10%) |
| Hypercoagulable state | 8 (6.7%) |
| Diabetes mellitus | 71 (59.2%) |
| Coronary artery disease | 47 (39.2%) |
| Congestive heart failure | 31 (25.8%) |
| Cerebrovascular disease | 9 (7.5%) |
| Smoking history | 65 (54.2%) |
| Current smoker | 39 (32.5%) |
| Hypertension | 61 (50.8%) |
| Hyperlipidaemia | 80 (66.7%) |
| Chronic renal insufficiency (eGFR < 60 mL/min) | 23 (19.2%) |
| Antiplatelet therapy | 42 (35%) |
| Anticoagulant therapy | 34 (28.3%) |
| Antiplatelet OR Anticoagulant therapy | 70 (58.3%) |
| Antiplatelet AND Anticoagulant therapy | 3 (2.5%) |
| Variables | All Patients (n = 120) |
|---|---|
| Introducer sheath size | |
| - 4F | 19 (15.8%) |
| - 5F | 46 (38.3%) |
| - 6F | 27 (22.5%) |
| - 7F | 15 (12.5%) |
| - 8F | 13 (10.8%) |
| Number of punctures of vascular access-site | 1.3 (±0.6) |
| Intraoperative Unfractionated Heparin | 2000 (0–2000) |
| Intraoperative thrombolytic agent | 8 (6.7%) |
| Intraoperative Antiplatelet therapy | 10 (8.3%) |
| Systolic Pressure (mmHg) | 134.4 (±26.3) |
| Diastolic Pressure (mmHg) | 85.5 (±16.8) |
| Mean Arterial Pressure (mmHg) | 110 (±20.6) |
| Variables | All Patients (n = 120) | |
|---|---|---|
| Primary technical success | 110 (91.7%) | |
| Secondary technical success | 120 (100%) | |
| Time-to-hemostasis (min) | 8.9 (±3.9) | |
| Time-to-ambulation (min) | 462 (±199) | |
| Clinical success | 113 (94.2%) | |
| Vascular access-site complications (VASCs), no/yes | 110 (91.7%)/10 (8.3%) | |
| Haematoma | 2 (1.7%) | |
| Pseudoaneurysm | 5 (4.1%) | |
| Dissection | 3 (2.5%) | |
| AV Fistula | 0 (0%) | |
| Major bleeding | 0 (0%) | |
| Thrombosis | 0 (0%) | |
| Infection | 0 (0%) | |
| Neuropathy | 0 (0%) | |
| Bleeding-related VASCs | 7 (5.8%) | |
| SVS Grading VASCs | ||
| Grade 1 | 1 (0.8%) | |
| Grade 2 | 9 (7.5%) | |
| Grade 3 | 0 (0%) | |
| CIRSE Grading VASCs | ||
| Grade 1 | 0 (0%) | |
| Grade 2 | 2 (1.7%) | |
| Grade 3 | 8 (6.6%) | |
| Grade > 3 | 0 (0%) | |
| Required treatment | ||
| Medical | 9 (7.5%) | |
| Percutaneous | 1 (0.8%) | |
| Surgical | 0 (0%) |
| Variables | Group 1 (n = 92) Sheath Size 4–5–6 Fr | Group 2 (n = 28) Sheath Size 7–8 Fr | p Value |
|---|---|---|---|
| Age (years) | 69.2 (±13.7) | 65.5 (±15.1) | 0.317 |
| Coagulopathy | 11 (11.9%) | 1 (3.6%) | 0.291 |
| INR | 1.2 (±0.2) | 1.2 (±0.2) | 0.937 |
| Intraoperative thrombolytic agent | 1 (1.1%) | 7 (25%) | <0.001 |
| Antiplatelet OR Anticoagulant therapy | 51 (55.4%) | 19 (67.8%) | 0.343 |
| Systolic pressure ≥ 180 mmHg | 10 (10.8%) | 6 (21.4%) | 0.201 |
| Primary technical success | 90 (97.8%) | 20 (71.4%) | <0.001 |
| VASCs | 9 (9.8%) | 1 (3.6%) | 0.450 |
| Bleeding-related VASCs | 6 (6.5%) | 1 (3.6%) | 1 |
| Predictors | Coeff. | Std. Err. | Z | p > |z| |
|---|---|---|---|---|
| Coagulopathy | 2.08/8.87/4.55 | 0.99/13.44/2.63 | 2.09/0.66/1.73 | 0.036/0.509/0.084 |
| INR | 3.33/−2.70 | 1.74/4.36 | 1.91/−0.62 | 0.056/0.536 |
| Antiplatelet OR Anticoagulant therapy | 0.12/−3.15 | 0.94/11.14 | 0.13/−0.28 | 0.999/0.777 |
| Systolic pressure | 0.08/0.35/0.12 | 0.03/0.36/0.06 | 2.68/0.99/2.18 | 0.007/0.321/0.029 |
| Primary technical success | −0.82/8.89 | 1.17/13.61 | −0.70/0.65 | 0.482/0.513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minici, R.; Serra, R.; Maglia, C.; Guzzardi, G.; Spinetta, M.; Fontana, F.; Venturini, M.; Laganà, D. Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. J. Pers. Med. 2023, 13, 812. https://doi.org/10.3390/jpm13050812
Minici R, Serra R, Maglia C, Guzzardi G, Spinetta M, Fontana F, Venturini M, Laganà D. Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. Journal of Personalized Medicine. 2023; 13(5):812. https://doi.org/10.3390/jpm13050812
Chicago/Turabian StyleMinici, Roberto, Raffaele Serra, Claudio Maglia, Giuseppe Guzzardi, Marco Spinetta, Federico Fontana, Massimo Venturini, and Domenico Laganà. 2023. "Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers" Journal of Personalized Medicine 13, no. 5: 812. https://doi.org/10.3390/jpm13050812
APA StyleMinici, R., Serra, R., Maglia, C., Guzzardi, G., Spinetta, M., Fontana, F., Venturini, M., & Laganà, D. (2023). Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. Journal of Personalized Medicine, 13(5), 812. https://doi.org/10.3390/jpm13050812









