Personalized Management for Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Clinical Entities
3. Imaging
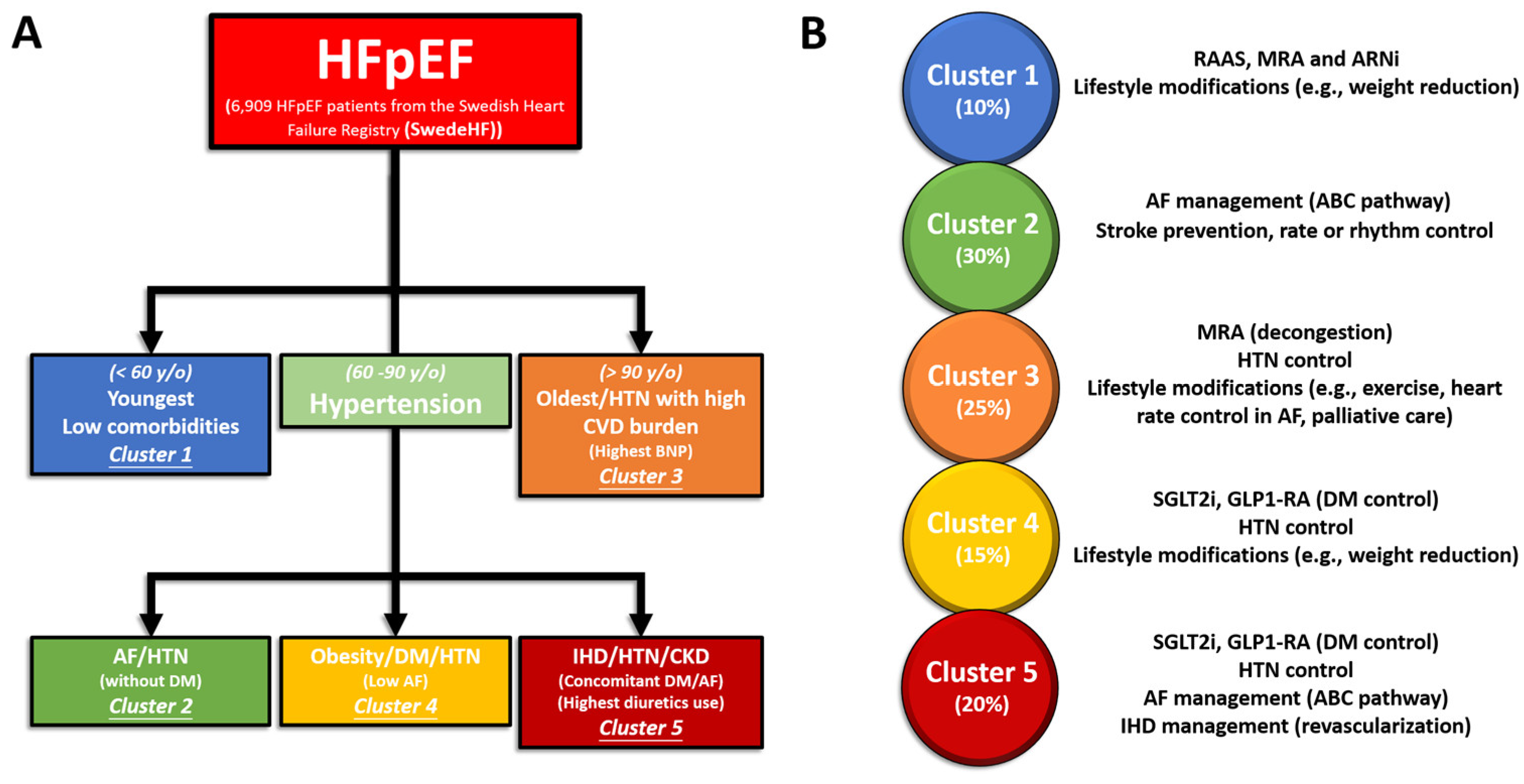
4. Management of HFpEF Phenotype Based on “SwedeHF” and “CHECK-HF” Registries
4.1. Cluster 1
4.2. Cluster 2
4.3. Cluster 3
4.4. Cluster 4
4.5. Cluster 5
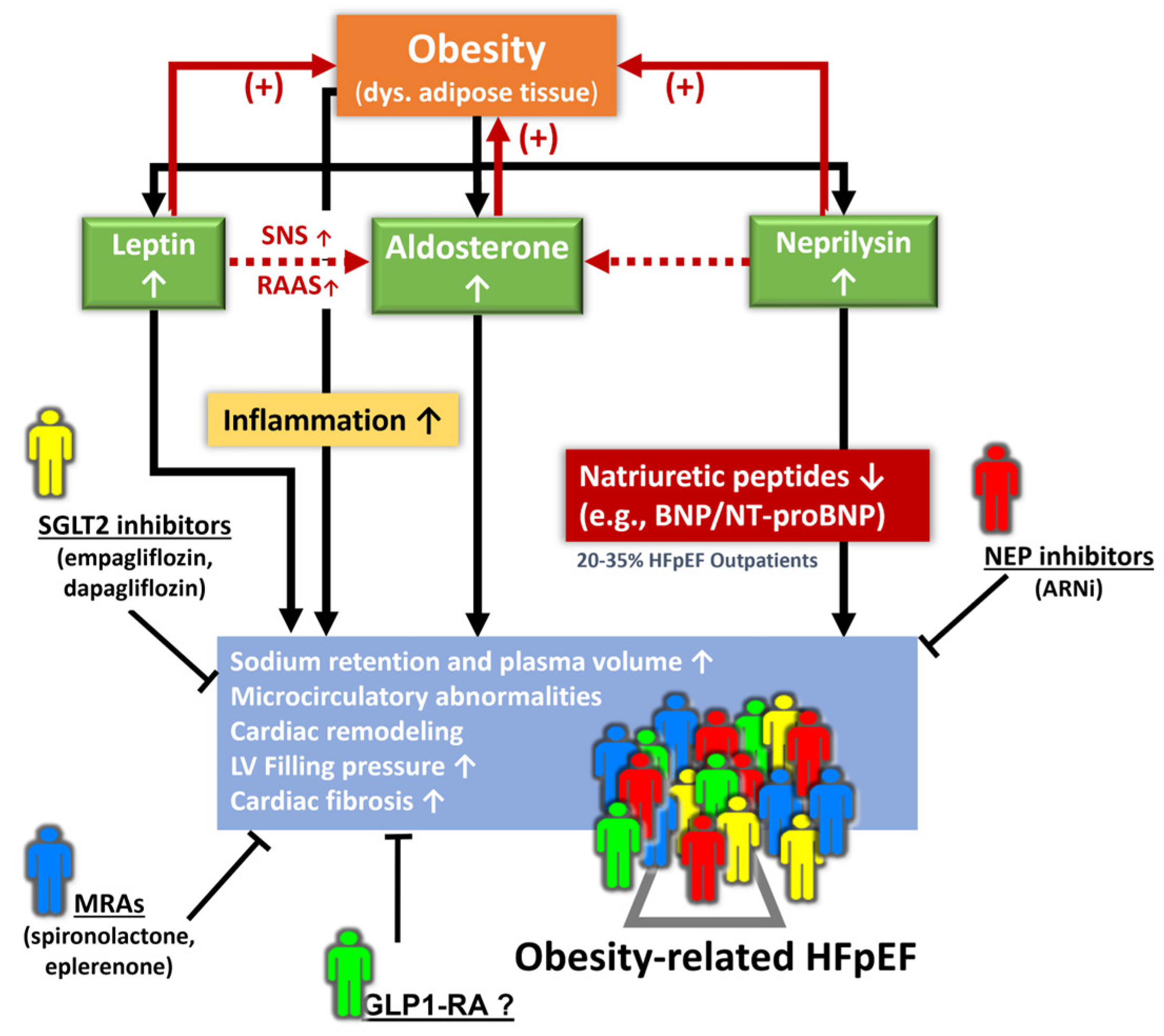
5. Management of Obesity-Related HFpEF Phenotype
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Kataria, R.; Zannad, F.; Sauer, A.J.; Damman, K.; Sharma, K.; Shah, S.J.; Van Spall, H.G.C. Heart failure with preserved ejection fraction: Recent concepts in diagnosis, mechanisms and management. Heart 2022, 108, 1342–1350. [Google Scholar] [CrossRef]
- Vasan, R.S.; Levy, D. Defining diastolic heart failure: A call for standardized diagnostic criteria. Circulation 2012, 131, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart failure with preserved ejection fraction in perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Dávila-Román, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; de las Fuentes, L.; Joseph, S.M.; Vader, J.; Hernandez, A.F.; et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: A RELAX trial ancillary study. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Jaiswal, A.; Ennezat, P.V.; Cassidy, M.; Le Jemtel, T.H. Clinical Phenotypes in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2016, 5, e002477. [Google Scholar] [CrossRef]
- Almengló, C.; Fu, X.; Flores-Arias, M.T.; Fernández, Á.L.; Viñuela, J.E.; Martínez-Cereijo, J.M.; Durán, D.; Rodríguez-Mañero, M.; González-Juanatey, J.R.; Eiras, S. Synergism between obesity and HFpEF on neutrophils phenotype and its regulation by adipose tissue-molecules and SGLT2i dapagliflozin. J. Cell. Mol. Med. 2022, 26, 4416–4427. [Google Scholar] [CrossRef]
- Wang, A.; Li, Z.; Zhuo, S.; Gao, F.; Zhang, H.; Zhang, Z.; Ren, G.; Ma, X. Mechanisms of Cardiorenal Protection with SGLT2 Inhibitors in Patients with T2DM Based on Network Pharmacology. Front. Cardiovasc. Med. 2022, 9, 857952. [Google Scholar] [CrossRef]
- Van Ham, W.B.; Kessler, E.L.; Oerlemans, M.I.F.J.; Handoko, M.L.; Sluijter, J.P.G.; van Veen, T.A.B.; den Ruijter, H.M.; de Jager, S.C.A. Clinical Phenotypes of Heart Failure with Preserved Ejection Fraction to Select Preclinical Animal Models. JACC Basic Transl. Sci. 2022, 7, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.C.; Deo, R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015, 131, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, S.; Duchateau, N.; Erdei, T.; Kunszt, G.; Aakhus, S.; Degiovanni, A.; Marino, P.; Carluccio, E.; Piella, G.; Fraser, A.G.; et al. Machine Learning Analysis of Left Ventricular Function to Characterize Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2018, 11, e007138. [Google Scholar] [CrossRef] [PubMed]
- Przewlocka-Kosmala, M.; Marwick, T.H.; Dabrowski, A.; Kosmala, W. Contribution of Cardiovascular Reserve to Prognostic Categories of Heart Failure with Preserved Ejection Fraction: A Classification Based on Machine Learning. J. Am. Soc. Echocardiogr. 2019, 32, 604–615.e6. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Hage, C.; Sharma, A.; Brosnan, M.J.; Buckbinder, L.; Gan, L.M.; Shah, S.J.; Linde, C.M.; Donal, E.; Daubert, J.-C.; et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart 2020, 106, 342–349. [Google Scholar] [CrossRef]
- Schrub, F.; Oger, E.; Bidaut, A.; Hage, C.; Charton, M.; Daubert, J.C.; Leclercq, C.; Linde, C.; Lund, L.; Donal, E. Heart failure with preserved ejection fraction: A clustering approach to a heterogenous syndrome. Arch. Cardiovasc. Dis. 2020, 113, 381–390. [Google Scholar] [CrossRef]
- Woolley, R.J.; Ceelen, D.; Ouwerkerk, W.; Tromp, J.; Figarska, S.M.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Zannad, F.; Metra, M.; et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 983–991, Correction in Eur. J. Heart Fail. 2021, 1802. [Google Scholar] [CrossRef]
- Gu, J.; Pan, J.A.; Lin, H.; Zhang, J.F.; Wang, C.Q. Characteristics, prognosis and treatment response in distinct phenogroups of heart failure with preserved ejection fraction. Int. J. Cardiol. 2021, 323, 148–154. [Google Scholar] [CrossRef]
- Kao, D.P.; Lewsey, J.D.; Anand, I.S.; Massie, B.M.; Zile, M.R.; Carson, P.E.; McKelvie, R.S.; Komajda, M.; McMurray, J.J.V.; Lindenfeld, J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur. J. Heart Fail. 2015, 17, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical Phenogroups in Heart Failure with Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Uijl, A.; Savarese, G.; Vaartjes, I.; Dahlström, U.; Brugts, J.J.; Linssen, G.C.M.; van Empel, V.; Brunner-La Rocca, H.P.; Asselbergs, F.W.; Lund, L.H.; et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Joseph, J. Defining the Phenotypes for Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail. Rep. 2022, 19, 445–457. [Google Scholar] [CrossRef]
- Kresoja, K.P.; Unterhuber, M.; Wachter, R.; Thiele, H.; Lurz, P. A cardiologist’s guide to machine learning in cardiovascular disease prognosis prediction. Basic Res. Cardiol. 2023, 118, 10. [Google Scholar] [CrossRef]
- Casebeer, A.; Horter, L.; Hayden, J.; Simmons, J.; Evers, T. Phenotypic clustering of heart failure with preserved ejection fraction reveals different rates of hospitalization. J. Cardiovasc. Med. 2021, 22, 45–52. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- Zawadzka, M.M.; Grabowski, M.; Kapłon-Cieślicka, A. Phenotyping in heart failure with preserved ejection fraction: A key to find effective treatment. Adv. Clin. Exp. Med. 2022, 31, 1163–1172. [Google Scholar] [CrossRef]
- Hwang, S.J.; Melenovsky, V.; Borlaug, B.A. Implications of coronary artery disease in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 64, 1702–1713. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H. Spironolactone Use and Improved Outcomes in Patients with Heart Failure with Preserved Ejection Fraction with Resistant Hypertension. J. Am. Heart Assoc. 2020, 9, e018827. [Google Scholar] [CrossRef]
- Jackson, A.M.; Jhund, P.S.; Anand, I.S.; Düngen, H.-D.; Lam, C.S.P.; Lefkowitz, M.P.; Linssen, G.; Lund, L.H.; Maggioni, A.P.; Pfeffer, M.A.; et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2021, 42, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining β-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People with Obesity. Circulation 2018, 137, 1614–1631. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, I.; AbouEzzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right ventricular function in heart failure with preserved ejection fraction: A community-based study. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef]
- Benameur, N.; Arous, Y.; Ben Abdallah, N.; Kraiem, T. Comparison between 3D Echocardiography and Cardiac Magnetic Resonance Imaging (CMRI) in the Measurement of Left Ventricular Volumes and Ejection Fraction. Curr. Med. Imaging Rev. 2019, 15, 654–660. [Google Scholar] [CrossRef]
- Liu, A.; Wijesurendra, R.S.; Liu, J.M.; Forfar, J.C.; Channon, K.M.; Jerosch-Herold, M.; Piechnik, S.K.; Neubauer, S.; Kharbanda, R.K.; Ferreira, V.M. Diagnosis of Microvascular Angina Using Cardiac Magnetic Resonance. J. Am. Coll. Cardiol. 2018, 71, 969–979, Retraction in J. Am. Coll. Cardiol. 2020, 76, 1916. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. Diastolic dysfunction and heart failure with preserved ejection fraction: Understanding mechanisms by using noninvasive methods. JACC Cardiovasc. Imaging 2019, 13, 245–257. [Google Scholar]
- Bolog, M.; Dumitrescu, M.; Luminiţa, M.; Romanoschi, F.; Păcuraru, E.; Râpă, A. Left Atrial Longitudinal Strain Evaluated by 2D Speckle Tracking Echocardiography Can Identify Patients with Heart Failure with Preserved Ejection Fraction. Intern. Med. 2019, 16, 7–19. [Google Scholar] [CrossRef]
- Aimo, A.; Senni, M.; Barison, A.; Panichella, G.; Passino, C.; Bayes-Genis, A.; Emdin, M. Management of heart failure with preserved ejection fraction: From neurohormonal antagonists to empagliflozin. Heart Fail. Rev. 2023, 28, 179–191. [Google Scholar] [CrossRef]
- Sotomi, Y.; Hikoso, S.; Nakatani, D.; Okada, K.; Dohi, T.; Sunaga, A.; Kida, H.; Sato, T.; Matsuoka, Y.; Kitamura, T.; et al. Medications for specific phenotypes of heart failure with preserved ejection fraction classified by a machine learning-based clustering model. Heart 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Bourg, C.; Kosmala, W.; Oger, E.; Donal, E. Phenomapping Heart Failure with Preserved Ejection Fraction Using Machine Learning Cluster Analysis: Prognostic and Therapeutic Implications. Heart Fail. Clin. 2021, 17, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Kitzman, D.W. Obesity-Related Heart Failure with a Preserved Ejection Fraction: The Mechanistic Rationale for Combining Inhibitors of Aldosterone, Neprilysin, and Sodium-Glucose Cotransporter-2. JACC Heart Fail. 2018, 6, 633–639. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Marwick, T.H. Obesity cardiomyopathy: Pathogenesis and pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 436–443. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Brosolo, G.; Novello, M.; Sechi, L.A. Aldosterone and left ventricular remodeling. Horm. Metab. Res. 2015, 47, 981–986. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Hundley, W.G.; Bluemke, D.A.; Jerosch-Herold, M.; Blankstein, R.; Petersen, S.E.; Rider, O.J.; Lima, J.A.; Allison, M.A.; Murthy, V.L.; et al. Visceral adiposity and left ventricular remodeling: The Multi-Ethnic Study of Atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 667–676. [Google Scholar] [CrossRef]
- Olivier, A.; Pitt, B.; Girerd, N.; Lamiral, Z.; Machu, J.L.; McMurray, J.J.V.; Swedberg, K.; van Veldhuisen, D.J.; Collier, T.J.; Pocock, S.J.; et al. Effect of eplerenone in patients with heart failure and reduced ejection fraction: Potential effect modification by abdominal obesity: Insight from the EMPHASIS-HF trial. Eur. J. Heart Fail. 2017, 19, 1186–1197. [Google Scholar] [CrossRef]
- Gruden, G.; Landi, A.; Bruno, G. Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes Care 2014, 37, 2899–2908. [Google Scholar] [CrossRef]
- Packer, M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Standeven, K.F.; Hess, K.; Carter, A.M.; Rice, G.I.; Cordell, P.A.; Balmforth, A.J.; Lu, B.; Scott, D.J.; Turner, A.J.; Hooper, N.M.; et al. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 2011, 35, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Petramala, L.; Marinelli, C.; Calvieri, C.; Zinnamosca, L.; Concistrè, A.; Iannucci, G.; De Toma, G.; Letizia, C. Epicardial fat thickness and primary aldosteronism. Horm. Metab. Res. 2016, 48, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Kresoja, K.-P.; Rommel, K.-P.; Wachter, R.; Henger, S.; Besler, C.; Klöting, N.; Schnelle, M.; Hoffmann, A.; Büttner, P.; Ceglarek, U.; et al. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 1633–1644. [Google Scholar] [CrossRef] [PubMed]

| Machine-Learning | |||
| Study | Number of Subjects | Classification | Characteristics |
| Shah et al., 2015 [13] | 397 | Phenogroup 1 | Natriuretic Peptide Deficiency Syndrome, young, obese, relatively fewer comorbidities |
| Phenogroup 2 | Extreme Cardiometabolic Syndrome, HTN, obesity (typically BMI > 35), DM | ||
| Phenogroup 3 | Right Ventricle-cardio-abdomino-renal Syndrome, CKD, PH, cardiorenal phenotype | ||
| Sanchez-Martinez et al., 2018 [14] | 156 | Cluster 1 | Healthy cluster |
| Cluster 2 | HFpEF: Older, higher NTproBNP, BMI, impaired exercise tolerance at 6MWT, LV hypertrophy, higher E/e’ ratio | ||
| Przewlocka-Kosmala et al., 2019 [15] | 228 | Cluster 1 | Normal CR/DR, normal increase in HR and diastolic function during exercise |
| Cluster 2 | Altered CR/DR, decreased exercise tolerance at CPET; chronotropic incompetence and diastolic dysfunction on exercise | ||
| Segar et al., 2020 [16] | 654 | Phenogroup 1 | Older, several CV risk factors: obesity; DM, HTN, worse renal function, significant LV concentric remodeling, LA dilatation, diastolic dysfunction |
| Phenogroup 2 | Low prevalence of CV risk factors, moderate LV concentric remodeling, moderate LA dilatation, and higher prevalence of moderate MR | ||
| Phenogroup 3 | Intermediate burden of CV risk factors, mainly DM and HTN, moderate LV concentric remodeling and LA dilatation | ||
| Hedman et al., 2020 [17] | 397 | Phenogroup 1 | HTN, IHD, DM, and CKD, marked LV concentric remodeling, modest electric remodeling (AF 37%) |
| Phenogroup 2 | Older age, HTN, significant LA dilatation and higher prevalence of RV failure, severe electric remodeling (AF 85%) | ||
| Phenogroup 3 | Younger, HTN, modest LV remodeling and electric remodeling (AF 48%) | ||
| Phenogroup 4 | HTN, significant LV and atrial remodeling, highest electrical remodeling (AF 90%) | ||
| Phenogroup 5 | HTN, IHD, moderate LV remodeling, moderate electrical remodeling (AF 43%) | ||
| Phenogroup 6 | Low BMI, severe LA remodeling, RV dysfunction; significant electric remodeling (AF 96%) | ||
| Schrub et al., 2020 [18] | 356 | Cluster 1 | Younger, HTN, DM, obesity, CKD, less electric remodeling, LV hypertrophy, lowest rate of severe MR |
| Cluster 2 | Intermediate age, HTN, less LV remodeling, but significant LA atrial dilatation and higher severe MR rate | ||
| Cluster 3 | Oldest, severe electrical remodeling (AF 87%), severe LA dilatation, higher prevalence of severe MR | ||
| Woolley et al., 2021 [19] | 429 | Cluster 1 | Highest frequency of CKD and DM |
| Cluster 2 | Elderly, high frequency of AF and HTN | ||
| Cluster 3 | Young, obese, fewest comorbidities | ||
| Cluster 4 | Highest rates of COPD, CAD, and smoking | ||
| Gu et al., 2021 [20] | 970 | Phenogroup 1 | Relatively preserved NYHA class and few to no comorbidities |
| Phenogroup 2 | Higher proportion of women and prevalence of AF | ||
| Phenogroup 3 | Highest BMI, highest prevalence of IHD, DM, and severe symptoms assessed with NYHA | ||
| Latent Class Analysis | |||
| Study | Number of Subjects | Classification | Characteristics |
| Kao et al., 2015 [21] | 4113 | Subgroup A | Median age 65, men, low rates of AF, CKD, valvular disease, and high rates of alcohol use |
| Subgroup B | Median age 65, women, low rates of AF, CKD, valvular disease, and high rates of anemia | ||
| Subgroup C | Median age 70, high rates of DM, obesity, HLD, CAD, CKD | ||
| Subgroup D | Median age 73, women, average rates of DM, obesity, HLD, CKD | ||
| Subgroup E | Median age 75, men, low BMI, high rates of AF, CAD | ||
| Subgroup F | Median age 82, women, low BMI, high rates of AF, valvular disease, CKD and anemia | ||
| Cohen et al., 2020 [22] | 3442 | Phenogroup 1 | Younger with mild symptoms lowest levels of NP, DM, CKD, and LV dysfunction, highest rates of smoking |
| Phenogroup 2 | Older with stiff arteries, small LVs and AF, women, highest rates of AF and CKD, low rates of obesity and DM | ||
| Phenogroup 3 | Obese diabetic with advanced symptoms, highest rates of obesity, DM, and high rates of CKD and depression | ||
| Uijl et al., 2021 [23] | 6909 | Cluster 1 | Median age 59, more males, fewest comorbidities, most had NYHA class I/ll and normal eGFR |
| Cluster 2 | Median age 77, higher rates of AF and HTN, relatively normal eGFR and lowest rate of DM | ||
| Cluster 3 | Median age 88, more females, highest rate of AF, lowest BMI values | ||
| Cluster 4 | Median age 71 years, most likely male, higher BMI and almost all patients had HTN and DM | ||
| Cluster 5 | Median age 82, most likely female, higher BMI values and NYHA III/IV, IHD, AF, all patients had HTN and most had lower eGFR values | ||
| Classification | Characteristics | Treatment Strategy |
|---|---|---|
| Cluster 1 | Younger with low comorbidity | Lifestyle modifications Risk factor screening |
| Cluster 2 | AF without T2DM | Restoration of normal sinus rhythm, anticoagulation, blood pressure control |
| Cluster 3 | Oldest with many cardiovascular comorbidities | Diuretics, mineralocorticoid receptor antagonists, lifestyle interventions |
| Cluster 4 | T2DM without AF | Glycemic control, SGLT2i |
| Cluster 5 | T2DM and AF | SGLT2i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Sung, H.-Y.; Chen, Y.-J.; Yeh, H.-I.; Hou, C.J.-Y.; Tsai, C.-T.; Hung, C.-L. Personalized Management for Heart Failure with Preserved Ejection Fraction. J. Pers. Med. 2023, 13, 746. https://doi.org/10.3390/jpm13050746
Lin C-Y, Sung H-Y, Chen Y-J, Yeh H-I, Hou CJ-Y, Tsai C-T, Hung C-L. Personalized Management for Heart Failure with Preserved Ejection Fraction. Journal of Personalized Medicine. 2023; 13(5):746. https://doi.org/10.3390/jpm13050746
Chicago/Turabian StyleLin, Chang-Yi, Heng-You Sung, Ying-Ju Chen, Hung-I. Yeh, Charles Jia-Yin Hou, Cheng-Ting Tsai, and Chung-Lieh Hung. 2023. "Personalized Management for Heart Failure with Preserved Ejection Fraction" Journal of Personalized Medicine 13, no. 5: 746. https://doi.org/10.3390/jpm13050746
APA StyleLin, C.-Y., Sung, H.-Y., Chen, Y.-J., Yeh, H.-I., Hou, C. J.-Y., Tsai, C.-T., & Hung, C.-L. (2023). Personalized Management for Heart Failure with Preserved Ejection Fraction. Journal of Personalized Medicine, 13(5), 746. https://doi.org/10.3390/jpm13050746






