Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pharmacokinetic Measurements
2.3. Pharmacodynamic Measurements
2.4. Estimation of Individual Plasma Exposure to Dapagliflozin and Empagliflozin
2.5. Evaluation of Association between Demographics and Plasma Exposure
2.6. Evaluation of the Association between Exposure and Response
3. Results
3.1. Dapagliflozin Exposure
3.2. Empagliflozin Exposure
3.3. Evaluation of Association between Demographics and Plasma Exposure
3.4. Evaluation of the Association between Exposure and Treatment Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. 2), S165–S171. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, N.H. Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics. Diabetes Metab. J. 2022, 46, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Heerspink, H.J.L.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; Hantel, S.; Woerle, H.J.; Broedl, U.C.; von Eynatten, M.; et al. Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA-REG OUTCOME Trial. J. Am. Soc. Nephrol. 2018, 29, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, E.J.M.; Muskiet, M.H.A.; van Baar, M.J.B.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.H.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, R.A.; Hesp, A.C.; Mosterd, C.M.; Geurts, F.; Hoorn, E.J.; Touw, D.J.; Krebber, M.M.; Joles, J.A.; Heerspink, H.J.L.; van Raalte, D.H. Kidney Hemodynamic Effects of Angiotensin Receptor Blockade, Sodium-Glucose Cotransporter-2 Inhibition Alone, and Their Combination: A Crossover Randomized Trial in People With Type 2 Diabetes. Circulation 2022, 146, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Petrykiv, S.I.; Laverman, G.D.; de Zeeuw, D.; Heerspink, H.J.L. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes. Metab. 2017, 19, 1363–1370. [Google Scholar] [CrossRef]
- Levey, A.S.; Gansevoort, R.T.; Coresh, J.; Inker, L.A.; Heerspink, H.L.; Grams, M.E.; Greene, T.; Tighiouart, H.; Matsushita, K.; Ballew, S.H.; et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration With the US Food and Drug Administration and European Medicines Agency. Am. J. Kidney Dis. 2020, 75, 84–104. [Google Scholar] [CrossRef]
- Kroonen, M.Y.A.M.; Koomen, J.V.; Petrykiv, S.I.; Laverman, G.D.; Heerspink, H.J.L.; Stevens, J. Exposure-response relationships for the sodium-glucose co-transporter-2 inhibitor dapagliflozin with regard to renal risk markers. Diabetes Obes. Metab. 2020, 22, 916–921. [Google Scholar] [CrossRef]
- van der Aart-van der Beek, A.B.; Koomen, J.V.; Dekkers, C.C.J.; Barbour, S.J.; Boulton, D.W.; Gansevoort, R.T.; Greasley, P.J.; Abdul Gafor, A.H.; Laverman, G.D.; Li, Q.; et al. Evaluation of the Pharmacokinetics and Exposure-Response Relationship of Dapagliflozin in Patients without Diabetes and with Chronic Kidney Disease. Clin. Pharmacokinet. 2021, 60, 517–525. [Google Scholar] [CrossRef]
- Koomen, J.V.; Stevens, J.; Heerspink, H.J.L. Exposure-response relationships of dapagliflozin on cardiorenal risk markers and adverse events: A pooled analysis of 13 phase II/III trials. Br. J. Clin. Pharmacol. 2020, 86, 2192–2203. [Google Scholar] [CrossRef]
- Baron, K.T.; Macha, S.; Broedl, U.C.; Nock, V.; Retlich, S.; Riggs, M. Population Pharmacokinetics and Exposure-Response (Efficacy and Safety/Tolerability) of Empagliflozin in Patients with Type 2 Diabetes. Diabetes Ther. 2016, 7, 455–471. [Google Scholar] [CrossRef]
- Van der Aart-Van der Beek, A.B.; Wessels, A.M.A.; Heerspink, H.J.L.; Touw, D.J. Simple, fast and robust LC-MS/MS method for the simultaneous quantification of canagliflozin, dapagliflozin and empagliflozin in human plasma and urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1152, 122257. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Smits, M.M.; Muskiet, M.H.; Hoekstra, T.; Kramer, M.H.; Danser, A.H.; Ter Wee, P.M.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Renal Effects of DPP-4 Inhibitor Sitagliptin or GLP-1 Receptor Agonist Liraglutide in Overweight Patients With Type 2 Diabetes: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care 2016, 39, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Hoekstra, T.; Kramer, M.H.H.; Danser, A.H.J.; Diamant, M.; Joles, J.A.; van Raalte, D.H. Postprandial renal haemodynamic effect of lixisenatide vs once-daily insulin-glulisine in patients with type 2 diabetes on insulin-glargine: An 8-week, randomised, open-label trial. Diabetes Obes. Metab. 2017, 19, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Kasichayanula, S.; Liu, X.; Lacreta, F.; Griffen, S.C.; Boulton, D.W. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin. Pharmacokinet. 2014, 53, 17–27. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Liu, X.; Zhang, W.; Pfister, M.; LaCreta, F.P.; Boulton, D.W. Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: An open-label, parallel-group, single-dose study. Clin. Ther. 2011, 33, 1798–1808. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Liu, X.; Pe Benito, M.; Yao, M.; Pfister, M.; LaCreta, F.P.; Humphreys, W.G.; Boulton, D.W. The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus. Br. J. Clin. Pharmacol. 2013, 76, 432–444. [Google Scholar] [CrossRef]
- Macha, S.; Mattheus, M.; Halabi, A.; Pinnetti, S.; Woerle, H.J.; Broedl, U.C. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes. Metab. 2014, 16, 215–222. [Google Scholar] [CrossRef]
- Laffel, L.M.B.; Tamborlane, W.V.; Yver, A.; Simons, G.; Wu, J.; Nock, V.; Hobson, D.; Hughan, K.S.; Kaspers, S.; Marquard, J. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: A randomized trial. Diabet. Med. 2018, 35, 1096–1104. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Chang, M.; Hasegawa, M.; Liu, X.; Yamahira, N.; LaCreta, F.P.; Imai, Y.; Boulton, D.W. Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium-glucose co-transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus. Diabetes Obes. Metab. 2011, 13, 357–365. [Google Scholar] [CrossRef]
- Devineni, D.; Curtin, C.R.; Polidori, D.; Gutierrez, M.J.; Murphy, J.; Rusch, S.; Rothenberg, P.L. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J. Clin. Pharmacol. 2013, 53, 601–610. [Google Scholar] [CrossRef]
- Riggs, M.M.; Seman, L.J.; Staab, A.; MacGregor, T.R.; Gillespie, W.; Gastonguay, M.R.; Woerle, H.J.; Macha, S. Exposure-response modelling for empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in patients with type 2 diabetes. Br. J. Clin. Pharmacol. 2014, 78, 1407–1418. [Google Scholar] [CrossRef] [PubMed]

| RED (n = 23) | RECOLAR (n = 20) | |
|---|---|---|
| Age (yr) | 63 ± 7 | 66 ± 6.3 |
| Female sex, n (%) | 5 (22) | 2 (10) |
| Body weight (kg) | 96.6 ± 18.3 | 97.9 ± 16.4 |
| Body mass index (kg/m2) | 30.8 ± 4.0 | 31.1 ± 4.0 |
| Systolic blood pressure (mmHg) | 137.9 ± 13.9 | 136.2 ± 9.7 † |
| Hemoglobin (mmol/L) | 8.5 ± 0.7 | 8.5 ± 0.9 |
| mGFR (mL/min) | 113.6 ± 20.0 | 108.4 ± 21.1 |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 84.8 ± 13.7 | 87.4 ± 11.8 |
| HbA1c (mmol/mol) | 57.6 ± 7.2 | 57.3 ± 9.9 |
| Albumin (g/L) | 38.0 ± 2.5 | 37.5 ± 2.0 |
| Alkaline phosphatase (U/L) | 76.6 ± 21.6 | NA |
| Gamma-glutamyl transferase (U/L) | 33.0 [19.5, 53.0] | 31 [22.0, 50.5] |
| Aspartate aminotransferase (U/L) | 21.0 [17.3, 26.0] | 21 [19.3, 28.3] † |
| Alanine aminotransferase (U/L) | 30.6 ± 14.5 | NA |
| Albuminuria (mg/24 h) | 11.0 [7.5, 16.9] | 12.1 [6.1, 22.0] |
| UACR (mg/mmol) | 0.81 [0.51, 1.16] | 1.18 [0.66, 1.44] |
| Diabetes duration (yr) | 9.7 ± 4.2 | 12.5 ± 5.7 |
| Metformin, n (%) | 23 (100) | 20 (100) |
| Sulfonylurea derivative, n (%) | 0 | 9 (45) |
| RAS inhibitor, n (%) | 15 (65.2) | 0 (0.0) |
| RED (n = 23) | RECOLAR (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dapagliflozin 10 mg | Empagliflozin 10 mg | |||||||
| Univariate | Multivariable | Univariate | Multivariable | |||||
| β (% Change) | p | β | p | β (% Change) | p | β | p | |
| Age (yr) | −1.37 | 0.63 | 2.47 | 0.18 | ||||
| Female sex | −57.72 | 0.06 | −49.89 | 0.13 | −13.43 | 0.71 | ||
| Body weight (kg) | 0.55 | 0.62 | −1.68 | 0.01 | −1.39 | 0.008 | ||
| eGFR (CKD-EPI) (mL/min/1.73 m2) | −1.05 | 0.47 | −2.60 | 0.003 | −1.41 | 0.06 | ||
| mGFR (mL/min) | −0.31 | 0.77 | −0.73 | 0.18 | ||||
| UACR (%) | −0.025 | 0.89 | 0.21 | 0.04 | ||||
| HbA1c (mmol/mol) | −0.57 † | 0.73 | 0.94 | 0.44 | ||||
| Serum albumin (g/L) | −6.41 | 0.40 | 11.64 | 0.052 | 10.36 | 0.002 | ||
| Alkaline phosphatase (U/L) | −1.14 | 0.21 | NA | |||||
| Gamma-glutamyl transferase (%) | −0.44 † | 0.049 | 0.015 †,‡ | 0.93 | ||||
| Aspartate aminotransferase (%) | −0.56 † | 0.36 | 0.35 † | 0.30 | ||||
| Alanine aminotransferase (U/L) | −2.43 | 0.06 | −1.94 | 0.13 | NA | |||
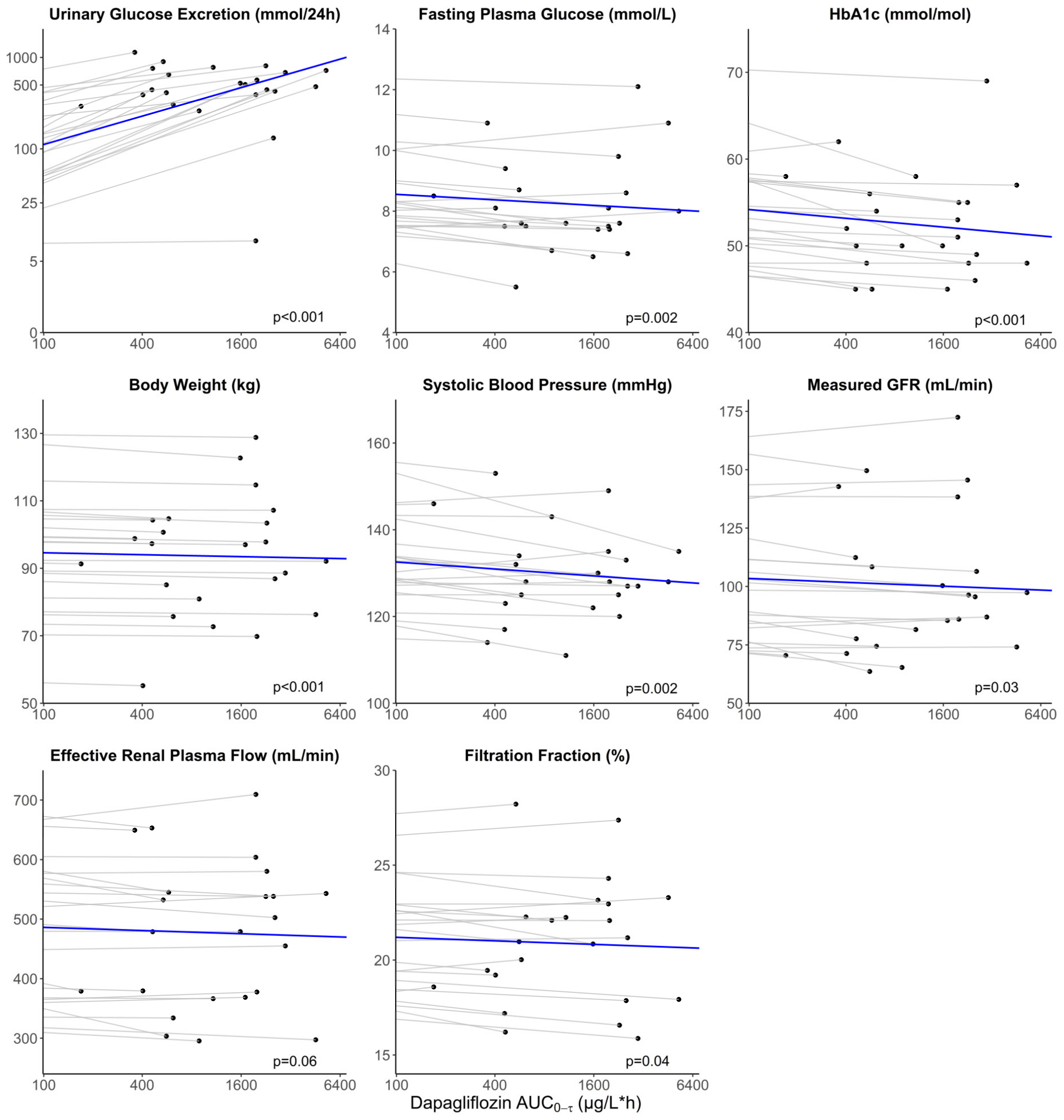
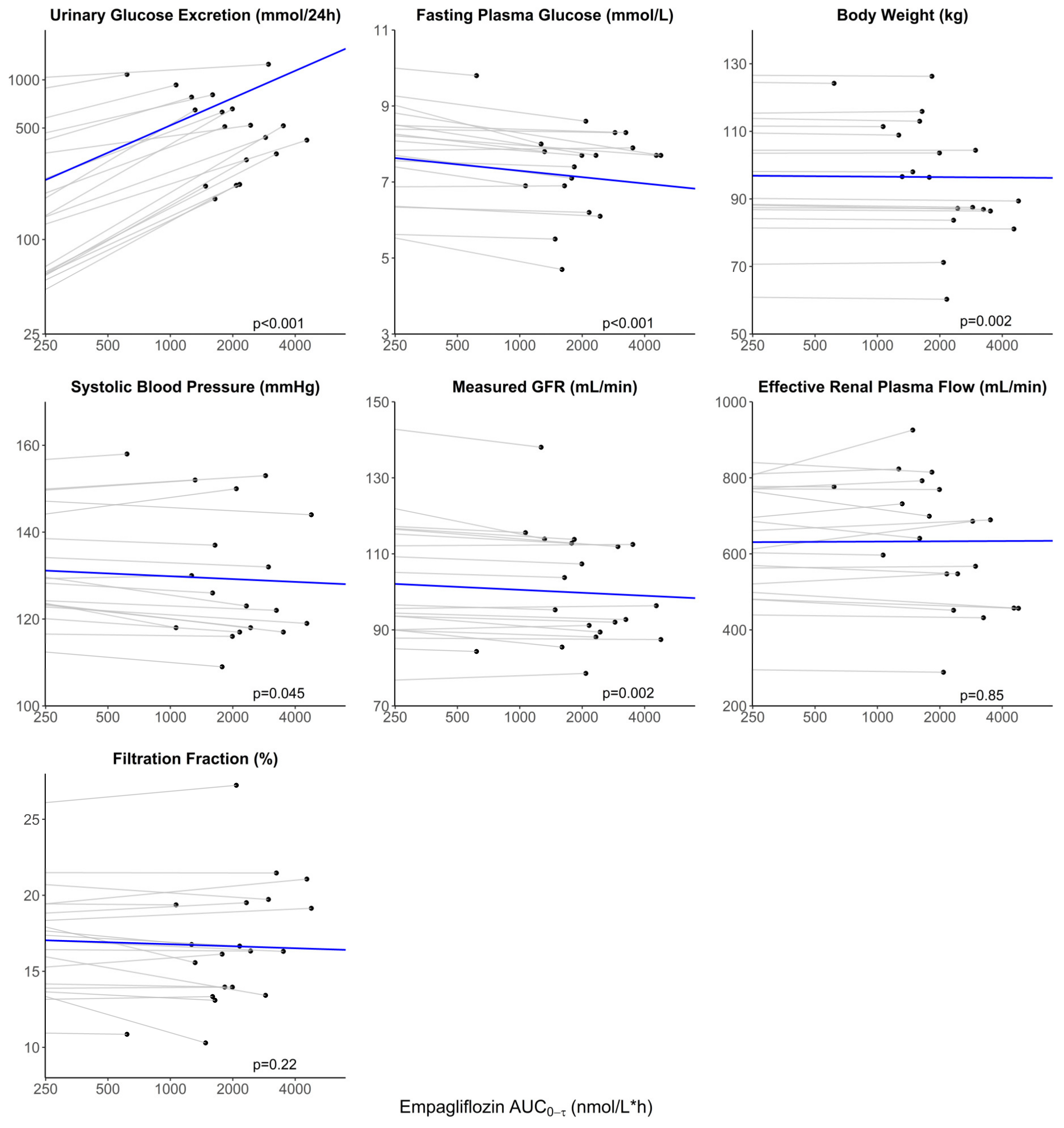
| RED Study (n = 23) | RECOLAR Study (n = 20) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dapagliflozin 10 mg | Empagliflozin 10 mg | |||||||||||
| Baseline | End of Treatment | Change | p | β per Doubling AUC | p | Baseline | End of Treatment | Change | p | β per Doubling AUC | p | |
| Body weight (kg) | 96.6 ± 18.3 | 93.6 ± 17.3 | −3.0 (−4.0, −2.0) | <0.001 | −0.29 (−0.39, −0.19) | <0.001 | 97.9 ± 17.1 | 96.6 ± 17.1 | −1.3 (−2.0, −0.6) | 0.002 | −0.13 (−0.20, −0.06) | 0.002 |
| SBP (mmHg) | 137.9 ± 13.9 | 129.8 ± 10.6 | −8.1 (−12.9, −3.2) | 0.003 | −0.80 (−1.27, −0.34) | 0.002 | 136.2 ± 9.7 † | 130.3 ± 14.9 ‡ | −6.1 (−12.3, 0.04) | 0.06 | −0.65 (−1.26, −0.03) | 0.045 |
| HbA1c (mmol/mol) | 57.6 ± 7.2 | 52.3 ± 5.9 | −5.3 (−7.5, −3.0) | <0.001 | −0.51 (−0.73, −0.29) | <0.001 | NA | NA | NA | NA | NA | NA |
| FPG (mmol/L) | 9.20 ± 1.54 | 8.20 ± 1.55 | −1.00 (−1.5, −0.5) | <0.001 | −0.09 (−0.14, −0.04) | 0.002 | 9.4 ± 2.6 | 7.4 ± 1.2 ‡ | −1.6 (−2.2, −1.0) | <0.001 | −0.16 (−0.22, −0.01) | <0.001 |
| UGE (mmol/24 h) | 7.6 [0.8–39.7] | 480 [391–703] | 409 (170, 974) § | <0.001 | 48.69 (35.88, 62.71) | <0.001 | 6.1 [0.8, 60.9] | 517 [331, 719] ‡ | 477 (212, 1017) § | <0.001 | 48.05 (36.82, 60.20) | <0.001 |
| mGFR (mL/min) ¶ | 109.4 ± 27.8 | 100.1 ± 30.4 | −9.3 (−16.5, −2.0) | 0.01 | −0.83 (−1.53, −0.12) | 0.03 | 108.4 ± 21.1 | 100.5 ± 14.8 | −7.9 (−12.2, −3.6) | 0.002 | −0.78 (−1.22, −0.33) | 0.002 |
| ERPF (mL/min) ¶ | 506.7 ± 124.7 | 474.3 ± 122.6 | −32.3 (−60.4, −4.3) | 0.03 | −2.69 (−5.46, 0.08) | 0.06 | 623.2 ± 169.6 | 634.7 ± 164.5 | 11.5 (−60.9, 83.9) | 0.75 | 0.69 (−6.65, 8.03) | 0.85 |
| FF (%) | 21.8 ± 2.9 | 20.9 ± 3.3 | −1.0 (−1.9, −0.04) | 0.04 | −0.09 (−0.18, −0.005) | 0.04 | 18.2 ± 4.1 | 16.7 ± 4.0 | −1.5 (−3.5, 0.6) | 0.16 | −0.13 (−0.34, 0.08) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Hoek, S.; Koomen, J.V.; van Bommel, E.J.M.; Mosterd, C.M.; Scholtes, R.A.; Hesp, A.C.; Stevens, J.; van Raalte, D.H.; Heerspink, H.J.L. Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes. J. Pers. Med. 2023, 13, 747. https://doi.org/10.3390/jpm13050747
van der Hoek S, Koomen JV, van Bommel EJM, Mosterd CM, Scholtes RA, Hesp AC, Stevens J, van Raalte DH, Heerspink HJL. Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes. Journal of Personalized Medicine. 2023; 13(5):747. https://doi.org/10.3390/jpm13050747
Chicago/Turabian Stylevan der Hoek, Sjoukje, Jeroen V. Koomen, Erik J. M. van Bommel, Charlotte M. Mosterd, Rosalie A. Scholtes, Anne C. Hesp, Jasper Stevens, Daniel H. van Raalte, and Hiddo J. L. Heerspink. 2023. "Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes" Journal of Personalized Medicine 13, no. 5: 747. https://doi.org/10.3390/jpm13050747
APA Stylevan der Hoek, S., Koomen, J. V., van Bommel, E. J. M., Mosterd, C. M., Scholtes, R. A., Hesp, A. C., Stevens, J., van Raalte, D. H., & Heerspink, H. J. L. (2023). Exposure–Response Analysis of the Sodium–Glucose Cotransporter-2 Inhibitors Dapagliflozin and Empagliflozin on Kidney Hemodynamics in Patients with Type 2 Diabetes. Journal of Personalized Medicine, 13(5), 747. https://doi.org/10.3390/jpm13050747






