The Impact of Venous Invasion on the Postoperative Recurrence of pT1–3N0cM0 Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pathological Examination
2.3. Statistical Analysis
3. Results
3.1. Clinicopathologic Features of Patients with pT1–3N0cM0 GC and Associations of the VI Grade with Recurrence
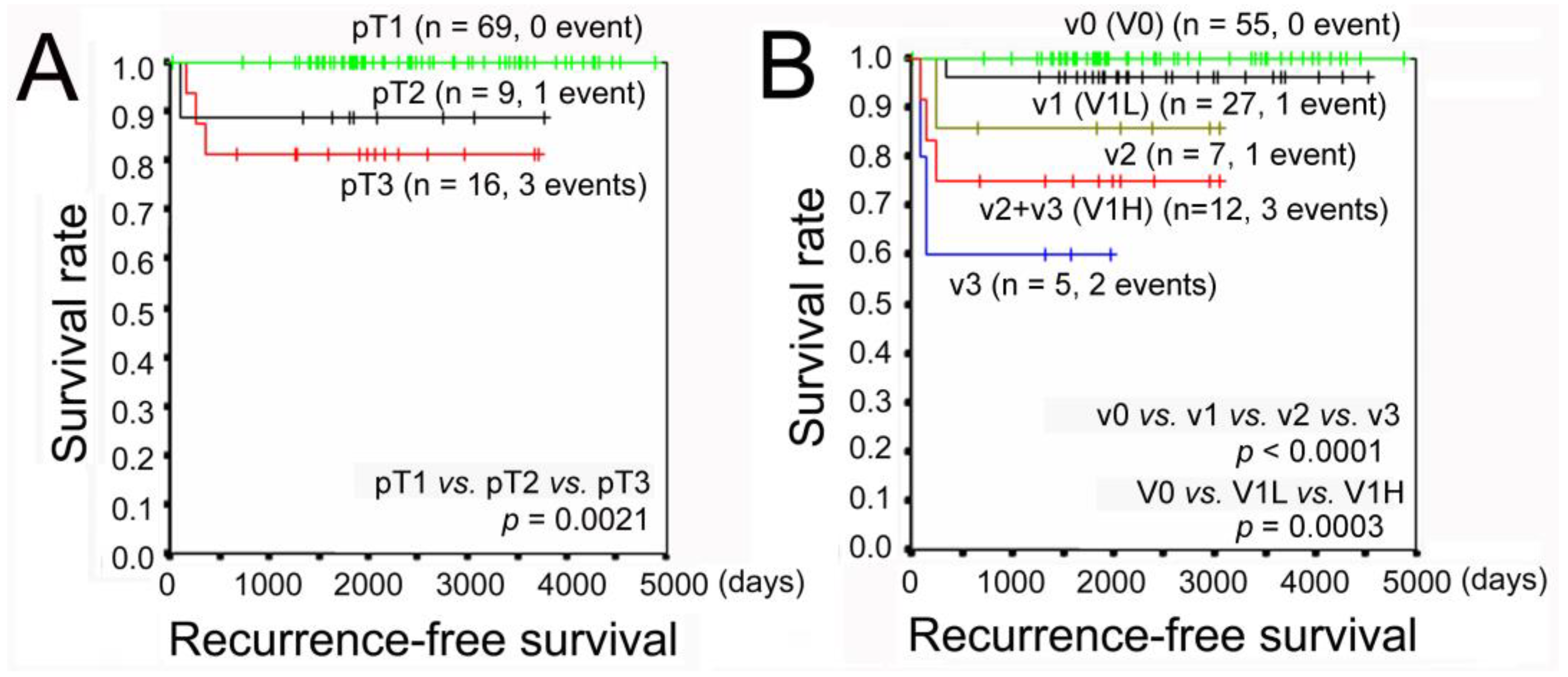
3.2. Recurrence-Free Survival Analysis Using the Kaplan–Meier Method
3.3. Multivariate Cox Regression Analysis of the Recurrence Predictive Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Stomach Cancer Survival Rates. Available online: https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 15 March 2022).
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), fiGastric Cancer, Version 1.2021. 9 February 2021. Available online: http://www.amoydxmed.com/uploadfile/2021/0421/20210421041509309.pdf (accessed on 23 July 2022).
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Union for International Cancer Control (UICC). Introduction and Digestive System Tumors. In TNM Classification of Malignant Tumours, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C., Eds.; Wiley Blackwell: Oxford, UK, 2017; pp. 28, 75–79. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 15th ed.; Kanehara: Tokyo, Japan, 2017; p. 40. (In Japanese) [Google Scholar]
- Siewert, J.R.; Stein, H.J. Adenocarcinoma of the gastroesophageal junction: Classification, pathology and extent of resection. Dis. Esophagus 1996, 9, 173–182. [Google Scholar]
- Hironaka, S.; Zenda, S.; Boku, N.; Fukutomi, A.; Yoshino, T.; Onozawa, Y. Weekly paclitaxel as second-line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 2006, 9, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, M.; Rugge, M.; Washington, M.K. Tumours of the stomach. In WHO Classification of Tumours, Digestive System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2018; pp. 59–109. [Google Scholar]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [PubMed]
- Sanjeevaiah, A.; Cheedella, N.; Hester, C.; Porembka, M.R. Gastric cancer: Recent molecular classification advances, racial disparity, and management implications. J. Oncol. Pract. 2018, 14, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S.; Smalley, S.R.; Benedetti, J.; Hundahl, S.A.; Estes, N.C.; Stemmermann, G.N.; Haller, D.G.; Ajani, J.A.; Gunderson, L.L.; Jessup, J.M.; et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 2001, 345, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Park, J.Y.; Park, K.B.; Kwon, O.K.; Lee, S.S.; Chung, H.Y. Prognostic factors in stage IB gastric cancer after surgical resection. J. Gastric Cancer 2020, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Araki, I.; Hosoda, K.; Yamashita, K.; Katada, N.; Sakuramoto, S.; Moriya, H.; Mieno, H.; Ema, A.; Kikuchi, S.; Mikami, T.; et al. Prognostic impact of venous invasion in stage IB node-negative gastric cancer. Gastric Cancer 2015, 18, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Ohara, M.; Domen, H.; Shichinohe, T.; Hirano, S.; Ishizaka, M. Differences in risk factors between patterns of recurrence in patients after curative resection for advanced gastric carcinoma. World J. Surg. Oncol. 2013, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Ojima, T.; Katsuda, M.; Hayata, K.; Goda, T.; Kitadani, J.; Tominaga, S.; Fukuda, N.; Nakai, T.; Yamaue, H. Venous invasion is a risk factor for recurrence of pT1 gastric cancer with lymph node metastasis. J. Gastrointest. Surg. 2022, 26, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kodera, Y.; Kochi, M.; Ichikawa, W.; Kakeji, Y.; Sano, T.; Nagao, N.; Takahashi, M.; Takagane, A.; Watanabe, T.; et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patient with Stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol. 2019, 37, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [PubMed]
- Park, S.H.; Lim, D.H.; Sohn, T.S.; Lee, J.; Zang, D.Y.; Kim, S.T.; Kang, J.H.; Oh, S.Y.; Hwang, I.G.; Ji, J.H.; et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: The ARTIST 2 trial. Ann. Oncol. 2021, 32, 368–374. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Total (n = 94) | Recurrence | p Value | ||
|---|---|---|---|---|---|
| Yes (n = 4) | No (n = 90) | ||||
| Age | |||||
| Median (range) | 70 (42–92) | 77 (70–86) | 70 (42–92) | 0.130 | |
| Sex | |||||
| Male/Female | 65/29 | 4/0 | 61/29 | 0.226 | |
| Location | |||||
| Upper/Middle/Lower | 21/16/57 | 0/0/4 | 21/16/53 | 0.980 | |
| Surgery | |||||
| DG/TG/others * | 69/23/2 | 3/1/0 | 66/22/2 | 0.956 | |
| Lymph node dissection | |||||
| D1/D1+/D2 | 8/44/42 | 0/2/2 | 8/42/40 | 0.822 | |
| Number of dissected lymph nodes | |||||
| Mean ± SD | 26.6 ± 12.0 | 18.0 ± 8.0 | 27 ± 12.1 | 0.145 | |
| Synchronous multiple GCs | |||||
| Yes/No | 10/84 | 1/3 | 9/81 | 0.367 | |
| Histology | |||||
| wel/mod/muc/por | 33/23/0/38 | 2/0/0/2 | 31/23/0/36 | 0.501 | |
| Depth of tumor invasion | |||||
| pT1/pT2/pT3 | 69/9/16 | 0/1/3 | 69/8/13 | 0.002 | |
| Cancer stromal volume | |||||
| med/int/sci/not described * | 3/43/11/37 | 0/2/2/0 | 3/41/9/37 | 0.659 | |
| Tumor infiltration pattern | |||||
| INFa/INFb/INFc/not described * | 3/46/19/26 | 0/2/2/0 | 3/44/17/26 | 0.925 | |
| VI grade | |||||
| v0/v1/v2/v3 | 55/27/7/5 | 0/1/1/2 | 55/26/6/3 | <0.001 | |
| Resection margin status | |||||
| R0/R1 + R2 | 94/0 | 4/0 | 90/0 | N.C. | |
| pTNM | |||||
| I/IIA | 78/16 | 1/3 | 77/13 | 0.015 | |
| Neoadjuvant chemotherapy | |||||
| Yes/No/unknown * | 0/82/12 | 0/4/0 | 0/78/12 | N.C. | |
| Adjuvant chemotherapy | |||||
| Yes/No/unknown * | 9/73/12 | 2/2/0 | 7/71/12 | 0.058 | |
| Patients | No. 111 | No. 115 | No. 189 | No. 197 |
|---|---|---|---|---|
| Age | 71 | 70 | 83 | 86 |
| Sex | Male | Male | Male | Male |
| Location | Low | Low | Low | Low |
| Surgery | DG | TG | DG | DG |
| Lymph node dissection | D2 | D1+ | D2 | D1+ |
| Number of dissected lymph nodes | 14 | 30 | 15 | 13 |
| Synchronous multiple GCs (Number) | Yes (2) | No | No | No |
| Histology | por, non-solid | por, non-solid | wel | wel |
| Depth of tumor invasion | pT3 | pT3 | pT3 | pT2 |
| Cancer stromal volume | sci | sci | int | int |
| Tumor infiltration pattern | INFc | INFc | INFb | INFb |
| VI grade | v1 | v3 | v2 | v3 |
| Resection margin status | R0 | R0 | R0 | R0 |
| Neoadjuvant chemotherapy | No | No | No | No |
| Adjuvant chemotherapy | No | No | S-1 | S-1 |
| Site of recurrence | Residual stomach Peritoneum | Peritoneum | Liver | Liver |
| Parameters | HR (95% CI) | p Value | |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.087 (0.969–1.219) | 0.154 | |
| Sex (Male) | 35.79 (0.005–276 × 103) | 0.433 | |
| Surgery (TG) | 1.022 (0.106–9.829) | 0.985 | |
| Lymph node dissection (D2) | 1.245 (0.175–8.841) | 0.826 | |
| Number of dissected lymph nodes | 0.938 (0.859–1.024) | 0.152 | |
| Synchronous multiple GCs (Yes) | 3.055 (0.318–29.372) | 0.333 | |
| pT | 6.465 (1.283–32.591) | 0.024 | |
| Histology (por) | 1.507 (0.212–10.698) | 0.682 | |
| Tumor infiltration pattern (INFc) | 2.595 (0.366–18.426) | 0.340 | |
| Cancer stromal volume (sci) | 4.290 (0.604–30.466) | 0.145 | |
| VI grade | 4.936 (1.758–13.653) | 0.002 | |
| pTNM stage (IIA) | 15.033 (1.563–144.603) | 0.019 | |
| Adjuvant chemotherapy (Yes) | 8.972 (1.262–63.807) | 0.028 | |
| Multivariate analysis | |||
| pT | 3.472 (0.550–21.932) | 0.186 | |
| VI grade | 3.099 (1.004–9.571) | 0.049 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, Y.; Kurata, Y.; Ichinose, M. The Impact of Venous Invasion on the Postoperative Recurrence of pT1–3N0cM0 Gastric Cancer. J. Pers. Med. 2023, 13, 734. https://doi.org/10.3390/jpm13050734
Imai Y, Kurata Y, Ichinose M. The Impact of Venous Invasion on the Postoperative Recurrence of pT1–3N0cM0 Gastric Cancer. Journal of Personalized Medicine. 2023; 13(5):734. https://doi.org/10.3390/jpm13050734
Chicago/Turabian StyleImai, Yasuo, Yoshihiro Kurata, and Masanori Ichinose. 2023. "The Impact of Venous Invasion on the Postoperative Recurrence of pT1–3N0cM0 Gastric Cancer" Journal of Personalized Medicine 13, no. 5: 734. https://doi.org/10.3390/jpm13050734
APA StyleImai, Y., Kurata, Y., & Ichinose, M. (2023). The Impact of Venous Invasion on the Postoperative Recurrence of pT1–3N0cM0 Gastric Cancer. Journal of Personalized Medicine, 13(5), 734. https://doi.org/10.3390/jpm13050734







