Abstract
Objective: To analyze and summarize the clinical and imaging characteristics of patients with cytomegalovirus retinitis (CMVR) relapse after hematopoietic stem cell transplantation (HSCT). Methods: This retrospective case series study recruited patients with CMVR after HSCT. The study compared the patients with stable lesions and CMV-negative aqueous humor after treatment with those with relapse lesions and a CMV DNA load in aqueous humor which had increased again after treatment. The observation indexes were basic clinical information, best-corrected visual acuity, wide-angle fundus photography, optical coherence tomography (OCT), blood CD4+ T lymphocyte count, and aqueous humor CMV load of the patients. We summarized the data and statistically analyzed the differences between the relapse and non-relapse groups, as well as the correlations of the observed indicators. Results: The study recruited 52 patients with CMVR (82 eyes) after HSCT, of whom 11 patients (15 eyes) had recurrence after treatment (21.2%). The recurrence interval was 6.4 ± 4.9 months. The final best-corrected visual acuity of recurrent patients was 0.3 ± 0.3. The number of CD4+ T lymphocytes in recurrence patients at the time of onset was 126.7 ± 80.2/mm3. The median CMV DNA load detected in aqueous humor at the time of recurrence was 8.63 × 103 copies/mL. There was a significant difference in the CD4+ T lymphocyte count between the recurrence and the non-recurrence groups at onset. The onset of visual acuity in recurrence patients was significantly correlated with final visual acuity and recurrence lesion area. The fundus of recurred CMVR showed increased marginal activity of the original stable lesion. Concurrently, yellow-white new lesions appeared around the stable, atrophic, and necrotic lesions. OCT showed new diffuse hyperreflexic lesions in the retinal neuroepithelial layer near the old lesions. Inflammatory punctate hyperreflexes were observed in the vitreous, with vitreous liquefaction and contraction. Conclusion: This study suggests that the clinical features, fundus manifestations, and imaging features of CMVR recurrence after HSCT are different from those at the initial onset. Patients should be closely followed up after their condition is stable to be alert for CMVR recurrence.
1. Introduction
Cytomegalovirus (CMV) infection remains an important cause of morbidity and mortality in patients who undergo hematopoietic stem cell transplantation HSCT, because the estimated seroprevalence for blood and organ donors is 90% in China [1,2,3,4]. Cytomegalovirus retinitis (CMVR) is the most common fundus disease in people with low immune function [5,6,7,8]. Typical fundus manifestations of the disease are yellow-white fusion lesions that progress along the retinal vessels, accompanied by retinal hemorrhage and edema, showing a typical “cheese and tomato sauce” appearance. Systemic and intravitreal injections of ganciclovir have become a recognized method of treatment of CMVR [9].
However, in clinical practice, we found that patients with different immune reconstitution statuses after HSCT had different disease outcomes. Not all CMVR lesions are stable and can be maintained after aqueous humor polymerase chain reaction (PCR) detection turns negative. In recent years, domestic and foreign literature has gradually paid increased attention to the diagnosis and treatment of CMVR after HSCT; however, there are no reports on the clinical characteristics and imaging manifestations of patients with recurrence after CMVR treatment and HSCT. Therefore, this study analyzed and summarized the clinical characteristics and imaging changes in these patients, aiming to provide a basis for optimization of the diagnosis and treatment of CMVR after HSCT.
2. Materials and Methods
2.1. Data Collection
This retrospective case series study included patients who were diagnosed with CMVR after HSCT and recurrence after treatment at the Ophthalmology Center of Peking University Third Hospital from January 2018 to October 2022. Inclusion criteria: (1) History of HSCT; (2) CMVR diagnosis according to typical fundus manifestations and positive CMV DNA in aqueous humor with deoxyribonucleic acid (DNA) PCR testing; (3) Patients whose previous CMVR lesions were stable and aqueous humor CMV DNA was negative, who showed a recurrent CMVR lesion and recurrence of aqueous humor CMV DNA positivity. Exclusion criteria: Patients with a history of other systemic and eye diseases, who had undergone other eye surgery or laser treatment in the past, and who could not cooperate with regular follow-up and treatment, were excluded from the study. This study was approved by the Ethics Committee of Peking University Third Hospital. Ethics approval number: 2015; Medical Ethics Review number: 197. The study followed the principles of the Declaration of Helsinki.
The medical history and basic information of the enrolled patients were collected, including gender, age, primary disease, affected eyes, date of hematopoietic stem cell transplantation, date of ocular onset, date of ocular recurrence, peripheral blood CD4+ T lymphocyte count, and the results of ocular aqueous humor virus examination at the time of recurrence. The CMV DNA in aqueous humor was detected by PCR, and the CD4+ T lymphocyte count in the patients was detected by flow cytometry.
Follow-up observation was carried out on the CMVR lesions of the patients. CMVR lesion locations were divided into 3 zones: zone 1 consisted of the area within 3000 mm of the center of the macula (macular involvement) or within 1500 mm of the margin of the optic disc (disc involvement), zone 2 consisted of the area anterior to zone 1 to the equator, and zone 3 consisted of the area anterior to zone 2 to the ora serrata [5]. Image J software was used to measure the lesion and optic disc areas of the patients with recurrence, and the ratio of the lesion area to optic disc area was recorded.
2.2. Follow-Up and Treatment Plan
After the initial diagnosis of CMVR, all patients underwent regular intravitreal antiviral therapy until the lesion was completely stable and CMV DNA tests of the aqueous humor were negative. Thereafter, the patients were followed up once a month for the required eye examinations, including best-corrected visual acuity (BCVA), intraocular pressure, slit lamp examination, ophthalmoscopy after mydriasis, wide-angle fundus photography (Optos PLC, Dunfermline, UK), and optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany). According to the fundus examination, if the lesions were suspected to have recurred, PCR testing of the aqueous humor was performed to confirm CMVR recurrence, and the antiviral treatment was reinitiated.
All patients were administered an intravitreal injection of ganciclovir immediately after the CMVR diagnosis was confirmed. The dosage of ganciclovir was 3 mg/0.1 mL by intravitreal injection. During the induction period, the intravitreal injection was administered twice a week for 2 weeks. The maintenance period was once a week until the lesion became stable and the viral load of the aqueous humor turned negative. For systemic antiviral treatment, the hematologist in charge adjusted the drug regimen according to the systemic CMV infection, and regularly monitored the liver and kidney functions. For the patients with CMVR recurrence, intravitreal injections of ganciclovir were reinitiated. For the patients with poor response of fundus lesions after treatment, intravitreal injections of ganciclovir were combined with foscarnet sodium.
2.3. Statistical Analysis
SPSS software (version 24.0, Chicago, IL, USA) was used for statistical analysis. Descriptive data were recorded as mean ± standard deviation. t-tests and chi-squared tests were used to analyze the differences between groups, and Pearson’s correlation analysis was used to analyze the correlations. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Basic Data
During the follow-up period, the research team collected data on 52 cases (82 eyes) of CMVR after HSCT; these included 32 male (61.5%) and 20 female patients (38.5%). Of the 52 cases, 21 were monocular and 31 were binocular. The average age of patients was 27.0 ± 15.4 years (4–71 years). The best-corrected visual acuity of all patients at onset was 0.50 ± 0.39 (HM–1.0, LogMAR visual acuity: 0.71), and the best-corrected visual acuity of all patients at the treatment end point was 0.46 ± 0.36 (HM–1.0, LogMAR visual acuity: 0.69).
During the follow-up, 11 patients had recurrence during treatment, for a total of 15 eyes (recurrence rate: 21.2%). The recurrent patients included five males (45.5%) and six females (54.5%), with an average age of 30.3 ± 21.8 years (7–65 years), of which seven cases were monocular and four cases were binocular. The interval between recurrence and first onset was 6.4 ± 4.9 months (1–18 months). The initial best-corrected visual acuity of the patients with recurrence was 0.5 ± 0.4 (0.05–1.0, LogMAR visual acuity: 0.3). The best-corrected visual acuity record of the recurrent patients after treatment was 0.3 ± 0.3 (HM–1.0, LogMAR visual acuity: 0.5).
There was a significant difference between the recurrence and non-recurrence groups in the blood CD4 lymphocyte count at the onset. Other indicators, including gender ratio, unilateral and bilateral incidence ratio, onset age, initial visual acuity, final visual acuity, and viral load showed no statistical difference between groups (Table 1). The initial visual acuity of the recurrence patients was significantly correlated with recurrent visual acuity (p < 0.05, correlation coefficient 0.844). The patient’s visual acuity at the time of recurrence decreased significantly (p = 0.001). The correlation analysis of the visual acuity of the recurrence patients at onset and the lesion area at the time of recurrence had p = 0.064, and a correlation coefficient of −0.49 (Table 2 and Table 3).

Table 1.
Comparison of clinical data between recurrent and non-recurrent CMVR patients after HSCT.

Table 2.
Clinical information of patients with recurrent CMVR after HSCT.

Table 3.
Statistical table of clinical data of patients with recurrent CMVR after HSCT.
3.2. CD4+ T Lymphocyte Count
The peripheral blood CD4+ T lymphocyte count of all CMVR patients at the time of onset was 133 ± 67.5/mm3 (40–320/mm3). The average CD4+ T lymphocyte count of the recurrent CMVR patients was 129.7 ± 80.7/mm3 (30–288/mm3). The number of CD4+ T lymphocytes in blood was less than 50/mm3 in one case of recurrence, less than 200/mm3 in eight cases, and more than 200/mm3 in two cases. The CD4+ T lymphocyte count had no correlation with age of onset, recurrence interval, initial visual acuity, visual acuity at recurrence, viral load in aqueous humor at recurrence, and lesion area at recurrence.
3.3. Detection of Viral Load in Aqueous Humor
The CMV load at the onset of all patients was 0 to 6.64 × 107 copies/mL, median 7.18 × 103 copies/mL. The viral load of the recurrent patients was 2.23 × 102 to 3.07 × 107 copies/mL, median 8.63 × 103 copies/mL. Among them, four eyes had <1 × 103 copies/mL, nine had >1 × 103 and <1 × 105 copies/mL, and the CMV DNA load of two eyes was >1 × 105 copies/mL. There was no significant correlation between the viral load in aqueous humor of the recurrent patients and age of onset, recurrence interval, initial visual acuity, visual acuity at recurrence, CD4+ T lymphocyte count at recurrence, and lesion area at recurrence.
3.4. Characteristics of Fundus Lesions
After recurrence, nine eyes had fundus lesions in zone 1, eight had simultaneous optic disc and macula involvement (average visual acuity: 0.25), and one had mainly optic disc involvement. Two eyes had lesions in zone 2, one eye on the nasal side of the optic disc, and one on the temporal side of the vascular arch. One eye had lesions in zone 3. ImageJ software was used to measure the lesion and optic disc area of the recurrent patients, and the ratio of the lesion to optic disc area was recorded. The average lesion size was 62.6 ± 60.7 optic disc areas of the recurrent patients, including three eyes with >100 optic disc areas, ten eyes with >10 optic disc areas <100 optic disc areas, and two eyes with <10 optic disc areas. Recurrent fundus mainly manifested as a new yellowish fusion focus surrounding the original atrophic area, or a new small cluster of yellowish-white lesions, which may be accompanied by retinal vasculitis (Figure 1). Eight eyes had lesions involving the optic disc and macula simultaneously, one eye had retinal neovascularization, two eyes had retinal and inferior fiber proliferation, and three eyes had obvious vitreous inflammatory opacity.
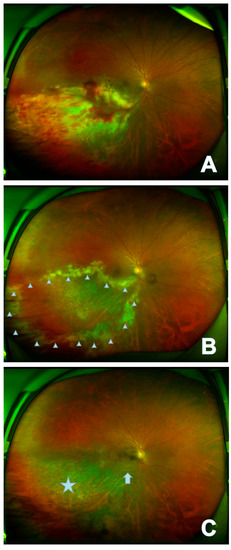
Figure 1.
In the right eye of case 6, the lesion appears as fulminant/edematous type. The onset focus of the fundus showed a yellow-white patchy fusion lesion that had formed in the infratemporal branch of the retina with development of retinal hemorrhage (A); recurrent fundus mainly manifests as new yellow-white fusion around the original atrophic area (△) (B); the stable focus showed gray atrophy of retina (☆) with local retinal artery occlusion (↑) (C).
The p-value of the correlation analysis between the lesion area and the initial visual acuity was 0.064, and the correlation coefficient was −0.49. The lesion area at recurrence was not related to the age of onset, recurrence interval, visual acuity at recurrence, CD4+ T lymphocyte count at recurrence, or viral load in aqueous humor at recurrence.
3.5. OCT Features
The OCT image of the lesion area was analyzed and described, with the scanning scope covering the original necrotic focus and the marginal recurrent focus. OCT images of recurrent lesions mainly showed diffuse hyperreflexes in the retinal neuroepithelial layer, blurred retinal layers, microcavity formation, cystoid edema within retinal layers, and exudative detachment of the retinal neuroepithelial layer, with punctate hyperreflexes in the vitreous body. During the observation and follow-up of these patients, OCT also detected liquefaction and contraction of the vitreous body due to inflammatory stimulation, as well as separation of the retinal inner limiting membrane and the vitreous posterior limiting membrane (Figure 2). Eight eyes of the enrolled patients showed punctate hyperreflexes of the vitreous body accompanied by traction of the posterior limiting membrane, and one eye showed retinal breaks on scanning. Among them, one eye underwent vitrectomy combined with silicone oil tamponade due to retinal detachment, and three eyes underwent vitrectomy to remove traction due to the aggravation of retinal proliferation.
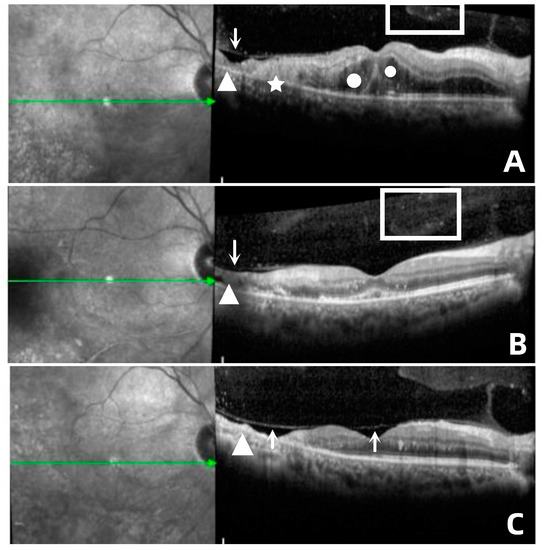
Figure 2.
(A) In case 6, the recurrent focus was located at the edge of the atrophic focus (△), showing diffuse hyperreflexes (☆) across the whole layer of the retina, accompanied by multiple punctate hyperreflexes in the vitreous body (□) and macular edema (○), as well as disorder of the outer layer of the retina. (B) Retinal edema gradually subsided; small cavities (↓) were formed in the necrotic area, and the outer structure of retina was partially repaired after antiviral treatment. (C) Stable lesions showed retinal atrophy with choroidal atrophy (△), vitreous liquefaction and contraction, and separation of the inner limiting membrane and vitreous posterior limiting membrane (↑).
4. Discussion
Cytomegalovirus retinitis is the most common opportunistic ocular infection in people with low immune function. The fundus mainly shows yellow-white granular fusion lesions with hemorrhage, accompanied by vitreous inflammation and retinal vasculitis, showing typical “cheese and tomato sauce” changes. Those most commonly affected are patients with AIDS and HSCT [10,11,12]. In recent years, many reports have been published on CMVR in patients with AIDS, but reports on CMVR after HSCT are limited, and the clinical characteristics of patients with CMVR recurrence after HSCT have not been reported.
There are many reasons for CMVR recurrence after HSCT, among which two key factors are the CD4+ T lymphocyte count and the CMV load in aqueous humor.
With regard to the CD4+ T lymphocyte count, the literature showed that the T lymphocyte subsets were significantly lower in CMVR cases within six months after HSCT (all p < 0.05) [13]. CMVR often occurs in patients whose CD4+ T lymphocyte count is less than 50/mm3 [6,14]. CMVR occurred more often among the recipients from alternative donors. The risk of viral infections is higher in HSCT from alternative donors including haploidentical donors and unrelated donors than human leukocyte antigen-matched sibling donors due to their severely depressed T cell-mediated immune response and insufficient resistance to the virus [15,16,17,18]. We found that the average CD4+ T lymphocyte count in the patients with recurrent CMVR after HSCT was significantly higher than the reference values provided in previous studies. The CD4+ T lymphocyte count in the recurrent group was significantly lower than that in the non-recurrent group. (1) These data show that even if the immune status of HSCT patients is not very poor, it is still possible to have a recurrence of ocular CMVR. Even if the number of CD4+ T lymphocytes has exceeded 200/mm3, stricter, more regular follow-ups should be carried out and the antiviral treatment should be supplemented in time according to the actual conditions of the patient. (2) The expert consensus on AIDS combined with CMVR treatment suggests that, if the CD4+ T lymphocyte count is 50–100/mm3, the fundus examination frequency should be once every three months and once every 6–12 months for those with a CD4+ T lymphocyte count greater than 100/mm3 [19]. This follow-up density is slightly insufficient for observing CMVR recurrence after HSCT. This study advocates that within six months after the first stabilization of CMVR in HSCT patients, the CMVR should be rechecked every 1–3 months in combination with individual immune status, and every three months from 6–12 months after the stabilization of CMVR. (3) This article summarizes the possible causes of immunosuppression in patients with CMVR recurrence after HSCT, such as immunological hemolysis and continuous multiple organ rejection. Six patients with CMVR recurrence after HSCT had a liver rejection. The existing literature also suggests that patients with liver rejection after HSCT are more likely to develop acute graft versus host reaction and have greater immune damage [19]. Acute and chronic graft versus host disease (GVHD) has been identified as an independent risk factor for the development of CMVR after HSCT for primary immunodeficiency and hematological disorders [20]. Patients with CMVR recurrence after HSCT experience more specificity in systemic and local inflammatory reactions and immune status [21]. We cannot simply follow the clinical experience of HSCT patients with CMVR at the first onset [22,23]. We should pay close attention to the changes in the disease and treat it with caution.
The CMV load in aqueous humor is also an important reason for CMVR recurrence in HSCT patients, and there is a linear relationship between the CMV load and the active lesion area of CMVR (the CMV load in aqueous humor = 3.38 + 0.01 × active lesion area) [6]. For patients with CMVR recurrence after HSCT, the CMV DNA load in aqueous humor varies greatly (102 to 107 copies/mL). The viral load in aqueous humor in three patients was less than 103 copies/mL when they relapsed. Therefore, the definition of 103 copies/mL as the standard for stopping treatment in some literature was also slightly inappropriate [16,17,24]. According to our results, even if the lesions of the CMVR patients after HSCT are completely stable and the CMV DNA in the eyes is completely negative, a considerable number of patients will still have CMVR lesion recurrence. From systemic virus infection, research indicated that even after the status of CMV antigenemia-negative had been achieved, active CMV infection remained in the retina with no symptoms, and as the CMV-infected lesions expanded, it was later recognized as CMVR [25,26,27]. Case 8 had the highest aqueous humor viral load, reaching 3.07 × 107 copies/mL, the blood CD4+ lymphocyte count of the patient before relapse decreased from 253/mm3 to 195/mm3 within 2 months, and the patient received an intraocular dexamethasone intravitreal implant injection. These two factors suggest that while immune reconstitution is complete, intraocular hormone injection should be administered with caution under the protection of antiviral drugs, otherwise it could cause serious recurrence.
This article summarizes the characteristics of recurrent fundus lesions. The p-value of the correlation analysis between the initial visual acuity and lesion area at the time of recurrence was 0.064, suggesting that the wider the range of the recurrent lesions, the worse the visual prognosis was, especially when the lesions involved the optic disc and macula concurrently. In addition, CMVR recurrent lesions presented as yellow foci developing around the edge of the original focus, suggesting that the edge of the CMVR focus was the main site of inflammatory response between virus and tissue. Holland et al. also believed that the degree of opacity of the lesion margin was related to the immune status and prognosis of CMVR patients [28]. The higher the edge opacity of the lesion and the closer the lesion to the posterior pole, the greater the threat to vision, and the more active intraocular antiviral treatment was required [29].
In this study, the OCT images of the lesion area were analyzed and described. OCT can also show the changes in vitreous in the process of CMVR. The liquefaction and contraction of the vitreous body stimulated by the inflammation will stretch the necrotic lesions, forming tiny holes, which would eventually lead to retinal detachment. This study also observed one eye with tractional retinal detachment secondary to severe vitreoretinal proliferation, which differs slightly from a previous report that suggests that patients with CMVR have rhegmatogenous retinal detachment [30]. Risk factors for retinal detachment in CMVR include large areas of retinitis, bilateral involvement, and large areas of active lesions near the vitreous base [11]. Therefore, the application of OCT in clinical follow-ups is also of great significance for observing CMVR lesions. It can detect subtle changes in the vitreoretinal interface early and remind doctors to effectively intervene in high-risk patients to avoid serious complications.
By collecting and summarizing CMVR recurrence patient data after HSCT, this paper discusses the specificity of CD4+ T cell count, viral load, fundus photography, and OCT characteristics from different perspectives. This study had a few limitations. The number of samples collected from patients is small because of population specificity, and the medication and treatment of this systemic disease cannot be unified. This caused difficulties in case collection, creating inevitable research bias, a problem that should be considered in similar studies going forward.
5. Conclusions
This study suggests that the clinical features, fundus manifestations, and imaging features of CMVR recurrence after HSCT are different from those at the initial onset. Patients should be closely followed up after their condition is stable to be alert to CMVR recurrence.
Author Contributions
Methodology, Y.L. and X.W.; software, X.W.; resources, J.H. and Z.M.; data curation, Y.L. and H.L.; writing—original draft preparation, X.W.; writing—review and editing, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation: The process and mechanism of corneal endothelitis caused by cytomegalovirus infection (81970768).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Peking University Third Hospital. Ethics approval number: 2015; Medical Ethics Review number: 197. The study followed the principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The clinical data in this study involve patient privacy and cannot be disclosed temporarily.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuhair, M.; Smit, G.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Green, M.; Leisenring, W.; Xie, H.; Mast, T.; Cui, Y.; Sandmaier, B.; Sorror, M.; Goyal, S.; Özkök, S.; Yi, J.; et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: A retrospective cohort study. Lancet Haematol. 2016, 3, e119–e127. [Google Scholar] [CrossRef]
- Inamoto, Y.; Petriček, I.; Burns, L.; Chhabra, S.; DeFilipp, Z.; Hematti, P.; Rovó, A.; Schears, R.; Shah, A.; Agrawal, V.; et al. Non-GVHD ocular complications after hematopoietic cell transplantation: Expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Transplant Complications Working Party of the EBMT. Bone Marrow Transpl. 2019, 54, 648–661. [Google Scholar] [CrossRef]
- Vassallo, F.; Nuzzi, R.; Cattani, I.; Dellacasa, C.; Giaccone, L.; De Rosa, F.G.; Cavallo, R.; Iovino, G.; Brunello, L.; Bruno, B.; et al. CMV retinitis in a stem cell transplant recipient treated with foscarnet intravitreal injection and CMV specific immunoglobulins. Ther. Adv. Hematol. 2020, 11, 2040620720975651. [Google Scholar] [CrossRef]
- Carmichael, A. Cytomegalovirus and the eye. Eye 2012, 26, 237–240. [Google Scholar] [CrossRef]
- Mao, F.; Sun, H.; Li, D.; Wang, S.; Lu, D. Diagnostic analysis of Optos panoramic laser scanning ophthalmoscope and aqueous humor detection in patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. Chin. J. Ocul. Fundus Dis. 2021, 37, 509–512. [Google Scholar]
- Pellegrini, M.; Bernabei, F.; Barbato, F.; Arpinati, M.; Giannaccare, G.; Versura, P.; Bonifazi, F. Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Am. J. Ophthalmol. 2021, 227, 25–34. [Google Scholar] [CrossRef]
- Long, Z.; Hou, J.; Miao, H. Neovascular complications from cytomegalovirus necrotizing retinopathy in patients after haploidentical hematopoietic stem cell transplantation. Retina 2021, 41, 1526–1532. [Google Scholar] [CrossRef]
- Meng, X.; Fu, H.; Zhu, X.; Wang, J.; Liu, X.; Yan, C.; Zhang, Y.; Mo, X.; Wang, Y.; Han, W.; et al. Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Ann. Hematol. 2020, 99, 2659–2670. [Google Scholar] [CrossRef]
- Holland, G.N.; Vaudaux, J.D.; Jeng, S.M.; Yu, F.; Goldenberg, D.T.; Folz, I.C.; Cumberland, W.G.; McCannel, C.A.; Helm, C.J.; Hardy, W.D. UCLA CMV Retinitis Study Group. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994). Am. J. Ophthalmol. 2008, 145, 5–11. [Google Scholar] [CrossRef]
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus retinitis: A review. J. Ocul. Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef]
- Lu, Y.; Hong, J.; Li, H.; Wang, X.; Ma, Z.; Wang, C. Relationship between opacity of cytomegalovirus retinitis lesion borders and aqueous viral load among patients after allogeneic hematopoietic stem cell transplantation. Chin. J. Ophthalmol. 2022, 58, 1033–1038. [Google Scholar]
- Mo, W.; Chen, X.; Zhang, X.; Wang, S.; Li, L.; Zhang, Y. The Potential Association of Delayed T Lymphocyte Reconstitution Within Six Months Post-Transplantation With the Risk of Cytomegalovirus Retinitis in Severe Aplastic Anemia Recipients. Front. Cell. Infect. Microbiol. 2022, 25, 900154. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, Y.; Zhang, W.; Lu, P. Detection of Intraocular Fluid and Infectious Diseases Study Clinical features of cytomegalovirus retinitis-associated uveitis in patients undergoing hematopoietic stem cell transplantation. Chin. J. Ocul. Fundus Dis. 2021, 37, 518–522. [Google Scholar]
- AIDS Group, Society of Tropical Diseases and Parasitology of Zhejiang Medical Association. Zhejiang expert consensus on diagnosis and treatment of cytomegalovirus retinitis in AIDS patients. Chin. J. Clin. Infect. Dis. 2019, 12, 331–338. [Google Scholar]
- Zhang, J.; Kamoi, K.; Zong, Y.; Yang, M.; Ohno-Matsui, K. Cytomegalovirus Anterior Uveitis: Clinical Manifestations, Diagnosis, Treatment, and Immunological Mechanisms. Viruses 2023, 15, 185. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Zhao, M.W.; Miao, H. Regime for cytomegalovirus retinitis based on aqueous virology and inflammatory cytokine determination. Chin. J. Ocul. Fundus Dis. 2020, 36, 1–4. [Google Scholar]
- Atay, D.; Akcay, A.; Erbey, F.; Ozturk, G. The impact of alternative donor types on viral infections in pediatric hematopoietic stem cell transplantation. Pediatr. Transpl. 2018, 22, e13109. [Google Scholar] [CrossRef]
- Chan, S.T.; Logan, A.C. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: Why the quest for meaningful prophylaxis still matters. Blood Rev. 2017, 31, 173–183. [Google Scholar] [CrossRef]
- Bhatt, S.; Bednarski, J. Immune Reconstitution in Pediatric Patients Following Hematopoietic Cell Transplant for Non-malignant Disorders. Front. Immunol. 2020, 18, 1988. [Google Scholar] [CrossRef]
- Iu, L.P.; Fan, M.C.; Lau, J.K.; Chan, T.S.; Kwong, Y.L.; Wong, I.Y. Long-term follow-up of cytomegalovirus retinitis in non-HIV immunocompromised patients: Clinical features and visual prognosis. Am. J. Ophthalmol. 2016, 165, 145–153. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.; Park, W.; Kim, R.; Kim, M.; Park, Y.; Kim, H.; Lee, S.; Lee, D.; Park, Y. Prognostic factors of cytomegalovirus retinitis after hematopoietic stem cell transplantation. PLoS ONE 2020, 15, e0238257. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, W.; Lee, Y.; Lee, D.; Lee, J. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology 2012, 119, 1892–1898. [Google Scholar] [CrossRef]
- Chen, W.; Long, Z.; Hou, J.; Miao, H.; Zhao, M. Continuous High-Dose (6 mg) vs. Low-Dose (3 mg) Intravitreal Ganciclovir for Cytomegalovirus Retinitis After Haploidentical Hematopoietic Stem Cell Transplantation: A Randomized Controlled Study. Front. Med. 2021, 24, 750760. [Google Scholar] [CrossRef]
- Suzuki, K.; Namba, K.; Mizuuchi, K.; Iwata, D.; Ito, T.; Hase, K.; Kitaichi, N.; Ishida, S. Development of cytomegalovirus retinitis after negative conversion of cytomegalovirus antigenemia due to systemic antiviral therapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 971–978. [Google Scholar] [CrossRef]
- Shapira, Y.; Mimouni, M.; Vishnevskia-Dai, V. Cytomegalovirus retinitis in HIV-negative patients-associated conditions, clinical presentation, diagnostic methods and treatment strategy. Acta Ophthalmol. 2018, 96, e761–e767. [Google Scholar] [CrossRef]
- Hong, S.; Kim, T.; Park, S.; Jung, J.; Lee, J.; Chong, Y.; Sung, H.; Lee, S.; Choi, S.; Kim, Y.; et al. Sensitivity of the Cytomegalovirus Antigenemia Assay to Diagnose Cytomegalovirus Retinitis. Infect. Chemother. 2016, 48, 302–308. [Google Scholar] [CrossRef]
- Holland, G.N.; Van Natta, M.L.; Goldenberg, D.T.; Ritts, R., Jr.; Danis, R.P.; Jabs, D.A.; Studies of Ocular Complications of AIDS Research Group. Relationship between opacity of cytomegalovirus retinitis lesion borders and severity of immunodeficiency among people with AIDS. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1853–1862. [Google Scholar] [CrossRef]
- Heng, M.; Jing, H. Influent factors for treating procedure of cytomegalovirus retinitis after allogeneic bone marrow hematopoietic stem cell transplantation. Chin. J. Ophthalmol. 2017, 53, 740–745. [Google Scholar]
- Fan, J.J.; Tao, Y.; Hwang, D.K. Comparison of intravitreal ganciclovir monotherapy and combination with foscarnet as initial therapy for cytomegalovirus retinitis. Int. J. Ophthalmol. 2018, 11, 1638–1642. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).