Effect of Surgical Release of Entrapped Peripheral Nerves in Sensorimotor Diabetic Neuropathy on Pain and Sensory Dysfunction—Study Protocol of a Prospective, Controlled Clinical Trial
Abstract
1. Introduction
2. Study Objectives
3. Materials and Methods
3.1. Participants, Eligibility Criteria
- Age 18–90 years;
- Type 2 or type 1 diabetes mellitus diagnosed according to the guidelines of the German Diabetes Association [31].
- Diabetic sensorimotor polyneuropathy of both lower extremities [32]:
- -
- Neuropathy Deficit Score > 5 points;
- -
- Neuropathy Deficit Score 3–5 points + Neuropathy Symptom Score > 4 points.
- Clinical evidence of bilateral local nerve compression of the tibial nerve in the tarsal tunnel and/or the common peroneal nerve under the muscle fascia of the peroneus longus muscle:
- -
- Predominant focal (pain) symptoms in the innervation area of the nerve distal to the compression site;
- -
- Hoffman–Tinel’s sign over the compression site;
- -
- Characteristic electrodiagnostic findings of compression e.g., local conduction block;
- -
- Characteristic findings of compression in the MR-neurography, e.g., local T2 weighted hyperintensity of the nerve within the compression site.
- Bilateral, therapy-refractory pain in the area supplied by the potentially entrapped nerve under conventional, guideline-compliant analgesic therapy (VAS >5);
- Bilateral hypesthesia in the area supplied by the potentially entrapped nerve defined as a tactile perception threshold greater than 6 g but less than 60 g.
- Exclusion criteria are as follows:
- HbA1c in the blood plasma > 8.5% (i.e., poor disease control);
- Reduced ability to give consent and/or legal capacity (e.g., due to mental illness);
- Contraindications to lower extremity surgery;
- Contraindications for electrodiagnostics, such as implanted cardiac pacemaker/defibrillator;
- Diseases that potentially confound the outcomes of interest, in particular:
- -
- Other diseases of the peripheral or central nervous system not mentioned under “Inclusion criteria”;
- -
- Moderate or severe peripheral arterial disease (ankle–brachial index <0.75, or missing foot pulses from the dorsal pedis artery or posterior tibial artery on at least one lower extremity);
- -
- Dermatological conditions of the lower extremities.
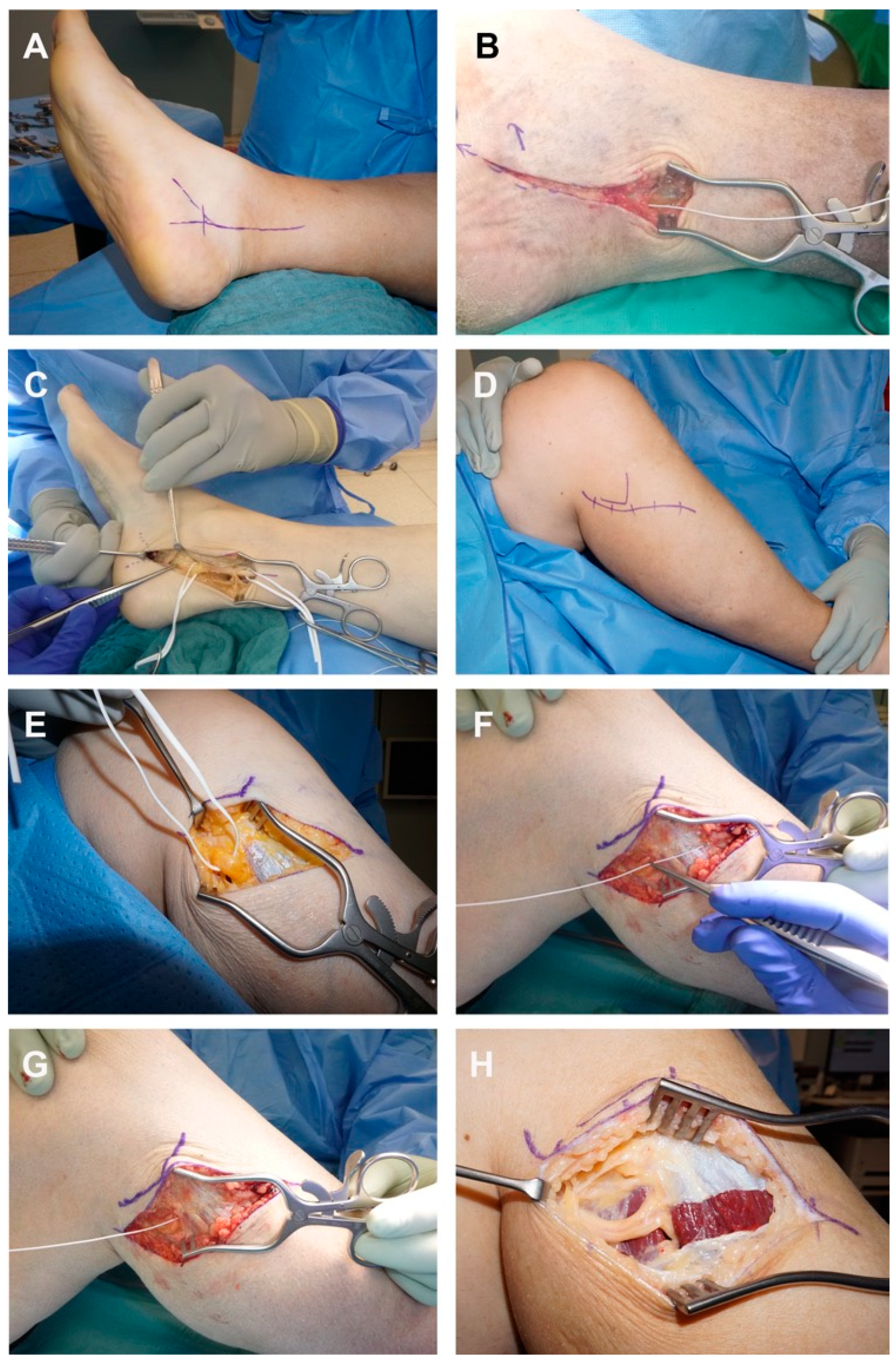
3.2. Intervention
3.3. Outcomes
3.3.1. Pain Intensity
3.3.2. Sensory Function
Tactile Sensory Threshold
Two-Point Discrimination
Quantitative Sensory Testing (QST)
3.3.3. Motor Function
3.3.4. Nerve Conduction Velocity
3.3.5. Tissue Biopsies
3.4. Participant Timeline
3.5. Sample Size
3.6. Recruitment
3.7. Allocation and Blinding
3.8. Data Management and Statistics
- A reduced pain intensity in the innervation area of the nerve distal to the decompression site 3, 6, and 12 months postoperatively, compared to the preoperative pain intensity and compared to an intraindividual non-decompressed control (contralateral lower extremity).
- A reduced analgesics consumption 3, 6, and 12 months postoperatively, compared to the preoperative baseline.
- Improved sensation (two-point discrimination, tactile detection threshold) in the innervation area of the nerve distal to the decompression site 3 and 12 months postoperatively compared to the preoperative situation and compared to an intra-individual, non-decompressed control (contralateral lower extremity).
- Increased isometric muscle strength in target muscles supplied by muscle branches arising from the decompressed nerve distal to the decompression site, 3 and 12 months postoperatively, compared to the preoperative situation and compared to an intra-individual, non-decompressed control (contralateral lower extremity)
- An increase in nerve conduction velocity across the decompression site 3 and 12 months postoperatively compared to the preoperative situation and compared to an intra-subject non-decompressed control (contralateral lower limb).
- A lower prevalence of neuropathic ulcerations in the innervated skin area of the nerve distal to the decompression site compared to an intra-individual, non-decompressed control (contralateral lower extremity) in the 12-month postoperative follow-up period.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Maser, R.E.; Steenkiste, A.R.; Dorman, J.S.; Nielsen, V.K.; Bass, E.B.; Manjoo, Q.; Drash, A.L.; Becker, D.J.; Kuller, L.H.; Greene, D.A.; et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989, 38, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L.; Smith, T.; Havsager, A.M.; Madsen, C.; Kjeldsen, M.J.; Dalsgaard, N.J.; Gaist, D.; Schroder, H.D.; Sindrup, S.H. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J. Clin. Neuromuscul. Dis. 2001, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Price, R.S.; Feldman, E.L. Distal Symmetric Polyneuropathy: A Review. JAMA 2015, 314, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Zhivov, A.; Allgeier, S.; Winter, K.; Ziegler, I.; Bruggemann, J.; Strom, A.; Peschel, S.; Kohler, B.; et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014, 63, 2454–2463. [Google Scholar] [CrossRef]
- Leckelt, J.; Guimaraes, P.; Kott, A.; Ruggeri, A.; Stachs, O.; Baltrusch, S. Early detection of diabetic neuropathy by investigating CNFL and IENFD in thy1-YFP mice. J. Endocrinol. 2016, 231, 147–157. [Google Scholar] [CrossRef]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Smith, D.R.; Fernyhough, P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol. Dis. 2013, 51, 56–65. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. New Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Ostergaard, L.; Finnerup, N.B.; Terkelsen, A.J.; Olesen, R.A.; Drasbek, K.R.; Knudsen, L.; Jespersen, S.N.; Frystyk, J.; Charles, M.; Thomsen, R.W.; et al. The effects of capillary dysfunction on oxygen and glucose extraction in diabetic neuropathy. Diabetologia 2015, 58, 666–677. [Google Scholar] [CrossRef]
- Mizisin, A.P. Mechanisms of diabetic neuropathy: Schwann cells. Handb. Clin. Neurol. 2014, 126, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, J.; Wei, M.; Bao, Y.; Hu, B. Preliminary evaluation of the sural nerve using 22-MHz ultrasound: A new approach for evaluation of diabetic cutaneous neuropathy. PloS ONE 2012, 7, e32730. [Google Scholar] [CrossRef]
- Macare van Maurik, J.F.; Schouten, M.E.; ten Katen, I.; van Hal, M.; Peters, E.J.; Kon, M. Ultrasound findings after surgical decompression of the tarsal tunnel in patients with painful diabetic polyneuropathy: A prospective randomized study. Diabetes Care 2014, 37, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, S.; Teng, H.; Wang, P.; Wu, M.; Zhou, X.; Xu, W.; Zhang, Q.; Ran, H. Diagnostic performance of two-dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy. Eur. Radiol. 2018, 29, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Kundalic, B.; Ugrenovic, S.; Jovanovic, I.; Stefanovic, N.; Petrovic, V.; Kundalic, J.; Stojanovic, V.; Zivkovic, V.; Antic, V. Morphometric analysis of connective tissue sheaths of sural nerve in diabetic and nondiabetic patients. BioMed Res. Int. 2014, 2014, 870930. [Google Scholar] [CrossRef]
- Hill, R. Extracellular matrix remodelling in human diabetic neuropathy. J. Anat. 2009, 214, 219–225. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.H.; Yang, S.N.; Yoon, J.S. Sonographic features of peripheral nerves at multiple sites in patients with diabetic polyneuropathy. J. Diabetes Its Complicat. 2016, 30, 518–523. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Z.; Guo, Z.; Zhao, F.; Wang, Y.; Cai, L.; Yang, J. Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochem. Biophys. 2014, 70, 1385–1391. [Google Scholar] [CrossRef]
- Zhao, H.; Song, X.; Li, Z.; Wang, X. Risk factors associated with nonalcohol fatty liver disease and fibrosis among patients with type 2 diabetes mellitus. Medicine 2018, 97, e123562018. [Google Scholar] [CrossRef]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, K.; Namer, B.; Schmidt, R.; Schmelz, M.; Hilliges, M.; Weidner, C.; Carr, R.W.; Handwerker, H.; Jørum, E.; Torebjörk, H.E. Abnormal function of C-fibers in patients with diabetic neuropathy. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 11287–11294. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sato, J.; Kawanishi, M.; Mizumura, K. Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin-diabetic rats but not normal rats in vitro. Pain 2002, 99, 475–484. [Google Scholar] [CrossRef]
- Hill, R.E.; Williams, P.E. Perineurial cell basement membrane thickening and myelinated nerve fibre loss in diabetic and nondiabetic peripheral nerve. J. Neurol. Sci. 2004, 217, 157–163. [Google Scholar] [CrossRef]
- Chen, R.J.; Lin, C.C.; Ju, M.S. In situ transverse elasticity and blood perfusion change of sciatic nerves in normal and diabetic rats. Clin. Biomech. 2010, 25, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A.J.M.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2016, 40, 136–154. [Google Scholar] [CrossRef]
- Wong, M.C.; Chung, J.W.; Wong, T.K. Effects of treatments for symptoms of painful diabetic neuropathy: Systematic review. BMJ Clin. Res. Ed. 2007, 335, 87. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Nationale VersorgungsLeitlinie—Neuropathie bei Diabetes im Erwachsenenalter; AWMF: Dusseldorf, Germany, 2011. [Google Scholar]
- Boyd, K.U.; Brown, J.M. Injury and Compression Neuropathy in the Lower Extremity. In Nerve Surgery; Mackinnon, S., Ed.; Thieme Medical Publishers: New York, NY, USA, 2015. [Google Scholar]
- Petersmann, A.; Nauck, M.; Müller-Wieland, D.; Kerner, W.; Müller, U.A.; Landgraf, R.; Freckmann, G.; Heinemann, L. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2018, 126, 406–410. [Google Scholar] [CrossRef]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef]
- Dellon, A.L. The Dellon approach to neurolysis in the neuropathy patient with chronic nerve compression. Handchir. Mikrochir. Plast. Chir. 2008, 40, 351–360. [Google Scholar] [CrossRef]
- Mucke, M.; Cuhls, H.; Radbruch, L.; Baron, R.; Maier, C.; Tolle, T.; Treede, R.D.; Rolke, R. Quantitative sensory testing (QST). English version. Schmerz 2021, 35, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dellon, A.L.; Mackinnon, S.E.; Crosby, P.M. Reliability of two-point discrimination measurements. J. Hand Surg. 1987, 12, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Spink, M.J.; Fotoohabadi, M.R.; Menz, H.B. Foot and ankle strength assessment using hand-held dynamometry: Reliability and age-related differences. Gerontology 2010, 56, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Losier, K.; Newsham-West, R.J.; Schneiders, A.G.; Sullivan, S.J. Raising the standards of the calf-raise test: A systematic review. J. Sci. Med. Sport 2009, 12, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Losier, K.; Wessman, C.; Alricsson, M.; Svantesson, U. Updated reliability and normative values for the standing heel-rise test in healthy adults. Physiotherapy 2017, 103, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Macare van Maurik, J.F.; van Hal, M.; van Eijk, R.P.; Kon, M.; Peters, E.J. Value of surgical decompression of compressed nerves in the lower extremity in patients with painful diabetic neuropathy: A randomized controlled trial. Plast. Reconstr. Surg. 2014, 134, 325–332. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, W.; Yang, M.; Ma, Q.; Li, G.; Zhong, W. Surgical decompression of painful diabetic peripheral neuropathy: The role of pain distribution. PloS ONE 2014, 9, e109827. [Google Scholar] [CrossRef]
- Karagoz, H.; Yuksel, F.; Ulkur, E.; Celikoz, B. Early and late results of nerve decompression procedures in diabetic neuropathy: A series from Turkiye. J. Reconstr. Microsurg. 2008, 24, 95–101. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Z.L.; Yu, Y.B.; Yang, W.Q.; Zhang, L. Two-Point Discrimination Predicts Pain Relief after Lower Limb Nerve Decompression for Painful Diabetic Peripheral Neuropathy. Plast. Reconstr. Surg. 2018, 141, 397e–403e. [Google Scholar] [CrossRef]
- Rinkel, W.D.; de Kleijn, J.L.; Macare van Maurik, J.F.M.; Coert, J.H. Optimization of Surgical Outcome in Lower Extremity Nerve Decompression Surgery. Plast. Reconstr. Surg. 2018, 141, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, W.; Yang, M.; Li, G.; Ma, Q.; Yang, X. Impact of diabetes mellitus duration on effect of lower extremity nerve decompression in 1,526 diabetic peripheral neuropathy patients. Acta Neurochir. 2014, 156, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Trignano, E.; Fallico, N.; Chen, H.C.; Faenza, M.; Bolognini, A.; Armenti, A.; Santanelli Di Pompeo, F.; Rubino, C.; Campus, G.V. Evaluation of peripheral microcirculation improvement of foot after tarsal tunnel release in diabetic patients by transcutaneous oximetry. Microsurgery 2016, 36, 37–41. [Google Scholar] [CrossRef]
- Wartolowska, K.A.; Feakins, B.G.; Collins, G.S.; Cook, J.; Judge, A.; Rombach, I.; Dean, B.J.; Smith, J.A.; Carr, A.J. The magnitude and temporal changes of response in the placebo arm of surgical randomized controlled trials: A systematic review and meta-analysis. Trials 2016, 17, 589. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.P.; Gu, C.N.; Ahmed, A.T.; Murad, M.H.; Wang, Z.; Kallmes, D.F.; Brinjikji, W. Sham surgical procedures for pain intervention result in significant improvements in pain: Systematic review and meta-analysis. J. Clin. Epidemiol. 2017, 83, 18–23. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daeschler, S.C.; Pennekamp, A.; Tsilingiris, D.; Bursacovschi, C.; Aman, M.; Eisa, A.; Boecker, A.; Klimitz, F.; Stolle, A.; Kopf, S.; et al. Effect of Surgical Release of Entrapped Peripheral Nerves in Sensorimotor Diabetic Neuropathy on Pain and Sensory Dysfunction—Study Protocol of a Prospective, Controlled Clinical Trial. J. Pers. Med. 2023, 13, 348. https://doi.org/10.3390/jpm13020348
Daeschler SC, Pennekamp A, Tsilingiris D, Bursacovschi C, Aman M, Eisa A, Boecker A, Klimitz F, Stolle A, Kopf S, et al. Effect of Surgical Release of Entrapped Peripheral Nerves in Sensorimotor Diabetic Neuropathy on Pain and Sensory Dysfunction—Study Protocol of a Prospective, Controlled Clinical Trial. Journal of Personalized Medicine. 2023; 13(2):348. https://doi.org/10.3390/jpm13020348
Chicago/Turabian StyleDaeschler, Simeon C., Anna Pennekamp, Dimitrios Tsilingiris, Catalina Bursacovschi, Martin Aman, Amr Eisa, Arne Boecker, Felix Klimitz, Annette Stolle, Stefan Kopf, and et al. 2023. "Effect of Surgical Release of Entrapped Peripheral Nerves in Sensorimotor Diabetic Neuropathy on Pain and Sensory Dysfunction—Study Protocol of a Prospective, Controlled Clinical Trial" Journal of Personalized Medicine 13, no. 2: 348. https://doi.org/10.3390/jpm13020348
APA StyleDaeschler, S. C., Pennekamp, A., Tsilingiris, D., Bursacovschi, C., Aman, M., Eisa, A., Boecker, A., Klimitz, F., Stolle, A., Kopf, S., Schwarz, D., Bendszus, M., Kneser, U., Kender, Z., Szendroedi, J., & Harhaus, L. (2023). Effect of Surgical Release of Entrapped Peripheral Nerves in Sensorimotor Diabetic Neuropathy on Pain and Sensory Dysfunction—Study Protocol of a Prospective, Controlled Clinical Trial. Journal of Personalized Medicine, 13(2), 348. https://doi.org/10.3390/jpm13020348







