Deciphering Differential Behavior of Immune Responses as the Foundation for Precision Dosing in Allergen Immunotherapy
Abstract
1. Introduction
2. Examples of Dose Adaptation in Inflammatory and Allergic Drugs
2.1. Dosage Adaptation of Methotrexate in Psoriasis
2.2. Incremental Posology in Oral Immunotherapy for Food Allergy
2.3. Adaptation of Treatment Modalities for Treating Airway Allergy and Asthma Symptoms
3. Dose Adaptation in Respiratory Sublingual Immunotherapy
3.1. Intrinsic and Extrinsic Factors Leading to Individual Inter-Variability of the Immune Response to Sublingual Immunotherapy
3.1.1. Intrinsic Characteristics
- Genetics and gender
- Age
3.1.2. Extrinsic Characteristics
- Infections
- Conditions of exposure upon AIT administration
- Microbiota composition
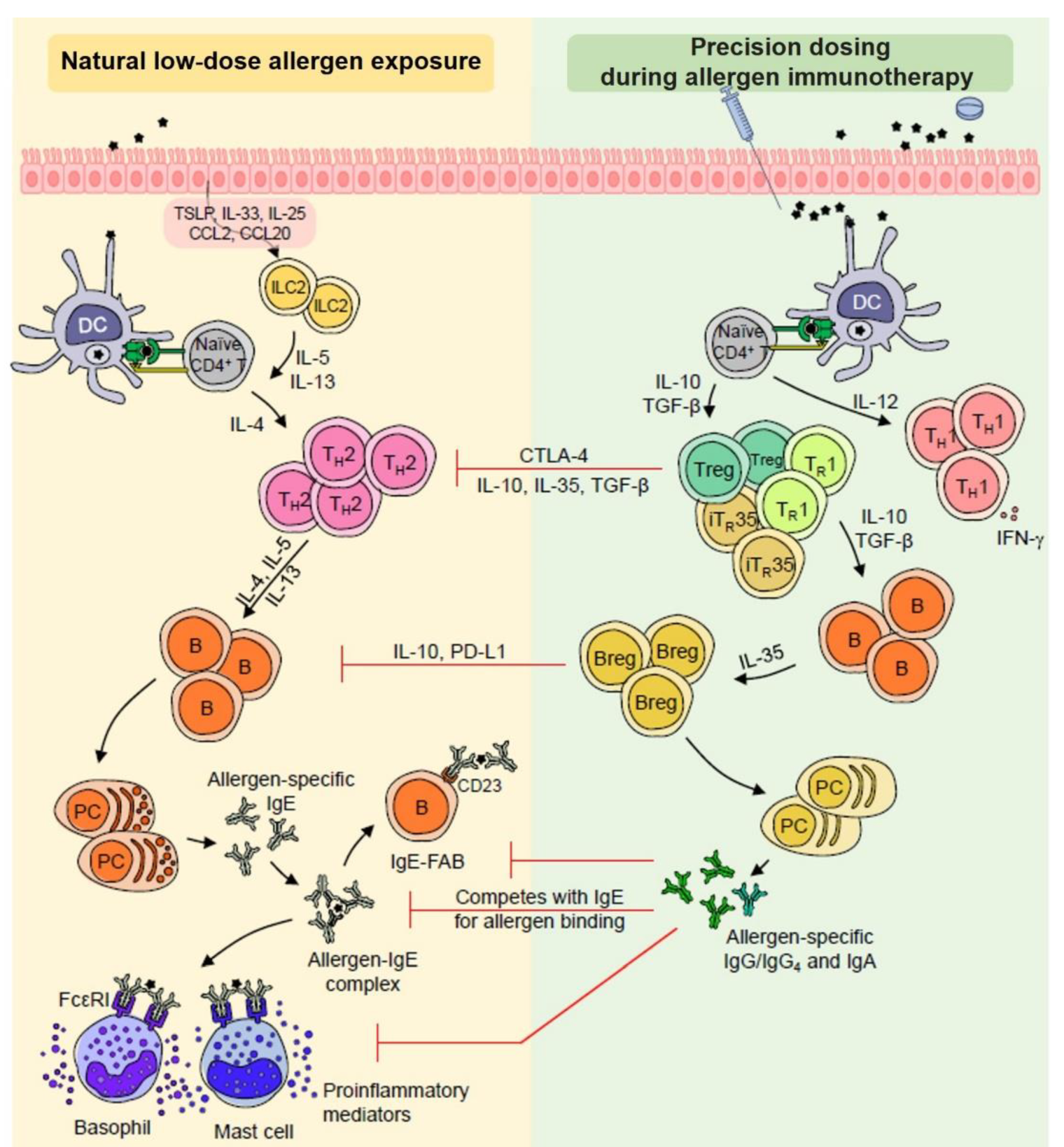
3.2. Differential Behavior of Immune Responses According to Clinical Responses during AIT
3.2.1. Allergen Presentation Capacity of DCs and DC Polarization in the Context of AIT
3.2.2. Innate Lymphoid Cells (ILCs) in the AIT Mechanism
3.2.3. T and B Regulatory (reg) Subsets and AIT
3.2.4. The Role of Humoral Responses for Reducing Basophils and Mast Cell Threshold Sensitivity during AIT
3.2.5. Coordinated Immune Responses as a Novel Finding for Patient Stratification
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwich, A.S.; Polasek, T.M.; Aronson, J.K.; Ogungbenro, K.; Wright, D.F.; Achour, B.; Reny, J.-L.; Daali, Y.; Eiermann, B.; Cook, J.; et al. Model-Informed Precision Dosing: Background, Requirements, Validation, Implementation, and Forward Trajectory of Individualizing Drug Therapy. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Layhadi, J.A.; Sharif, H.; Penagos, M.; Durham, S.R. Immunological Responses and Biomarkers for Allergen-Specific Immunotherapy Against Inhaled Allergens. J. Allergy Clin. Immunol. Pract. 2021, 9, 1769–1778. [Google Scholar] [CrossRef]
- Demoly, P.; Calderón, M.A. Dosing and efficacy in specific immunotherapy. Allergy 2011, 66, 38–40. [Google Scholar] [CrossRef]
- Herland, K.; Akselsen, J.-P.; Skjønsberg, O.H.; Bjermer, L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir. Med. 2005, 99, 11–19. [Google Scholar] [CrossRef]
- Thétis-Soulié, M.; Hosotte, M.; Grozelier, I.; Baillez, C.; Scurati, S.; Mercier, V. The MaDo real-life study of dose adjustment of allergen immunotherapy liquid formulations in an indication of respiratory allergic disease: Reasons, practices, and outcomes. Front. Allergy 2022, 3, 971155. [Google Scholar] [CrossRef]
- Vidal. Quelles Sont les Causes du Psoriasis? Available online: https://www.vidal.fr/maladies/peau-cheveux-ongles/psoriasis/causes.html (accessed on 31 January 2023).
- Grpso, F.P. Lettre d’information Patient pour le Traitement du Psoriasis par Méthotrexate. Available online: http://grpso.org/upload/fiche/5540-Lettre-dinformation-patient-pour-le-traitement-du-psoriasis-par-methotrexate.pdf (accessed on 31 January 2023).
- Schmiegelow, K. Advances in individual prediction of methotrexate toxicity: A review. Br. J. Haematol. 2009, 146, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Menting, S.; Dekker, P.; Limpens, J.; Hooft, L.; Spuls, P. Methotrexate Dosing Regimen for Plaque-type Psoriasis: A Systematic Review of the Use of Test-dose, Start-dose, Dosing Scheme, Dose Adjustments, Maximum Dose and Folic Acid Supplementation. Acta Dermato-Venereol. 2016, 96, 23–28. [Google Scholar] [CrossRef]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of Methotrexate Toxicity: A Comprehensive Literature Review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.; Van Ede, A.E.; Haagsma, C.J.; Laar, M.A.F.J.V.D.; Huizinga, T.W.J.; Kruijsen, M.W.M.; Laan, R.F.J.M. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 423–426. [Google Scholar] [CrossRef]
- Corriger, J.; Bittencourt, M.D.C. Médecine personnalisée et allergies alimentaires. Rev. Française D’allergologie 2020, 60, 8S10–8S14. [Google Scholar] [CrossRef]
- Houben, G.F.; Baumert, J.L.; Blom, W.M.; Kruizinga, A.G.; Meima, M.Y.; Remington, B.C.; Wheeler, M.W.; Westerhout, J.; Taylor, S.L. Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization. Food Chem. Toxicol. 2020, 146, 111831. [Google Scholar] [CrossRef] [PubMed]
- Remington, B.C.; Westerhout, J.; Meima, M.Y.; Blom, W.M.; Kruizinga, A.G.; Wheeler, M.W.; Taylor, S.L.; Houben, G.F.; Baumert, J.L. Updated population minimal eliciting dose distributions for use in risk assessment of 14 priority food allergens. Food Chem. Toxicol. 2020, 139, 111259. [Google Scholar] [CrossRef]
- Kim, E.H.; Burks, A.W. Food allergy immunotherapy: Oral immunotherapy and epicutaneous immunotherapy. Allergy 2020, 75, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Barshow, S.M.; Kulis, M.D.; Burks, A.W.; Kim, E.H. Mechanisms of oral immunotherapy. Clin. Exp. Allergy 2021, 51, 527–535. [Google Scholar] [CrossRef]
- Masoli, M.; Weatherall, M.; Holt, S.; Beasley, R. Clinical dose-response relationship of fluticasone propionate in adults with asthma. Thorax 2004, 59, 16–20. [Google Scholar]
- Lowe, P.J.; Renard, D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br. J. Clin. Pharmacol. 2011, 72, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.J.; Tannenbaum, S.; Gautier, A.; Jimenez, P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br. J. Clin. Pharmacol. 2009, 68, 61–76. [Google Scholar] [CrossRef]
- Grabenhenrich, L.B.; Gough, H.; Reich, A.; Eckers, N.; Zepp, F.; Nitsche, O.; Forster, J.; Schuster, A.; Schramm, D.; Bauer, C.-P.; et al. Early-life determinants of asthma from birth to age 20 years: A German birth cohort study. J. Allergy Clin. Immunol. 2014, 133, 979–988. [Google Scholar] [CrossRef]
- Meng, J.-F.; Rosenwasser, L.J. Unraveling the Genetic Basis of Asthma and Allergic Diseases. Allergy Asthma Immunol. Res. 2010, 2, 215–227. [Google Scholar] [CrossRef]
- Halonen, M.; Lohman, I.C.; Stern, D.A.; Spangenberg, A.; Anderson, D.; Mobley, S.; Ciano, K.; Peck, M.; Wright, A.L. Th1/Th2 Patterns and Balance in Cytokine Production in the Parents and Infants of a Large Birth Cohort. J. Immunol. 2009, 182, 3285–3293. [Google Scholar] [CrossRef]
- Lai, X.; Li, J.; Xiao, X.; Liu, E.; Zhang, C.; Wang, H.; Gjesing, B.; Zhong, N.; Spangfort, M.D. Specific IgG4 Production during House Dust Mite Immunotherapy among Age, Gender and Allergic Disease Populations. Int. Arch. Allergy Immunol. 2013, 160, 37–46. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.A.; Simons, F.E.R.; Malling, H.-J.; Lockey, R.F.; Moingeon, P.; Demoly, P. Sublingual allergen immunotherapy: Mode of action and its relationship with the safety profile. Allergy 2012, 67, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Lancaster, J.N.; Singarapu, N.; Hale, L.P.; Ehrlich, L.I.R.; Richie, E.R. Age-Related Changes in Thymic Central Tolerance. Front. Immunol. 2021, 12, 676236. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, S.; Hester, A.K.; Yangming, X.; Kraig, E.B.; Griffith, A.V. Age-associated changes in central T cell tolerance induction. J. Immunol. 2019, 202 (Suppl. 1), 115.25. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Bozek, A.; Cudak, A.; Canonica, G.W. Long-term efficacy of injected allergen immunotherapy for treatment of grass pollen allergy in elderly patients with allergic rhinitis. Allergy Asthma Proc. Off. J. Reg. State Allergy Soc. 2020, 41, 271–277. [Google Scholar] [CrossRef]
- Bozek, A.; Ignasiak, B.; Filipowska, B.; Jarzab, J. House dust mite sublingual immunotherapy: A double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin. Exp. Allergy 2013, 43, 242–248. [Google Scholar] [CrossRef]
- Bozek, A.; Kolodziejczyk, K.; Warkocka-Szoltysek, B.; Jarzab, J. Grass Pollen Sublingual Immunotherapy: A Double-Blind, Placebo-Controlled Study in Elderly Patients with Seasonal Allergic Rhinitis. Am. J. Rhinol. Allergy 2014, 28, 423–427. [Google Scholar] [CrossRef]
- Bozek, A.; Starczewska-Dymek, L.; Jarzab, J. Prolonged effect of allergen sublingual immunotherapy for house dust mites in elderly patients. Ann. Allergy Asthma Immunol. 2017, 119, 77–82. [Google Scholar] [CrossRef]
- Klimek, L.; Jutel, M.; Akdis, C.; Bousquet, J.; Akdis, M.; Bachert, C.; Agache, I.; Ansotegui, I.; Bedbrook, A.; Bosnic-Anticevich, S.; et al. Handling of allergen immunotherapy in the COVID-19 pandemic: An ARIA-EAACI statement. Allergy 2020, 75, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Message, S.D.; Laza-Stanca, V.; Edwards, M.R.; Wark, P.; Bartlett, N.; Kebadze, T.; Mallia, P.; Stanciu, L.A.; Parker, H.L.; et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat. Med. 2006, 12, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Pitsios, C.; Demoly, P.; Bilò, M.B.; van Wijk, R.G.; Pfaar, O.; Sturm, G.J.; del Rio, P.R.; Tsoumani, M.; Gawlik, R.; Paraskevopoulos, G.; et al. Clinical contraindications to allergen immunotherapy: An EAACI position paper. Allergy 2015, 70, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Lemmermann, N.A.W.; Maxeiner, J.; Podlech, J.; Beckert, H.; Freitag, K.; Teschner, D.; Ries, F.; Taube, C.; Buhl, R.; et al. Coincident airway exposure to low-potency allergen and cytomegalovirus sensitizes for allergic airway disease by viral activation of migratory dendritic cells. PLoS Pathog. 2019, 15, e1007595. [Google Scholar] [CrossRef]
- Forsyth, K.S.; Eisenlohr, L.C. Giving CD4+ T cells the slip: Viral interference with MHC class II-restricted antigen processing and presentation. Curr. Opin. Immunol. 2016, 40, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Larenas-Linnemann, D.E.; Epstein, T.; Ponda, P.; Bernstein, D.; Williams, P.; Creticos, P. Gaps in allergen immunotherapy administration and subcutaneous allergen immunotherapy dose adjustment schedules: Need for prospective data. Ann. Allergy, Asthma Immunol. 2020, 125, 505–506. [Google Scholar] [CrossRef]
- Leung, T.F. In-season Dosage Adjustment for Pollen Subcutaneous Immunotherapy: The Controversy Continues. J. Allergy Clin. Immunol. Pract. 2017, 5, 1440–1441. [Google Scholar] [CrossRef]
- Cecchi, L.; D’Amato, G.; Annesi-Maesano, I. External exposome and allergic respiratory and skin diseases. J. Allergy Clin. Immunol. 2018, 141, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.; McDonald-Hyman, C.; Noth, E.M.; Pratt, B.; Hammond, S.K.; Balmes, J.; Tager, I. Ambient air pollution impairs regulatory T-cell function in asthma. J. Allergy Clin. Immunol. 2010, 126, 845–852. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, K.; Kim, S.W. Effects of Antibiotics on the Development of Asthma and Other Allergic Diseases in Children and Adolescents. Allergy, Asthma Immunol. Res. 2018, 10, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, W.-T.; Pang, M.; Fan, Q.-L.; Hua, J.-L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Bax, H.J.; Bergmann, C.; Bianchini, R.; Cozen, W.; Gould, H.J.; Hartmann, K.; Josephs, D.H.; Levi-Schaffer, F.; Penichet, M.L.; et al. AllergoOncology: Microbiota in allergy and cancer—A European Academy for Allergy and Clinical Immunology position paper. Allergy 2019, 74, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Groeger, D.; O’Mahony, L. Biology of the Microbiome 1. Gastroenterol. Clin. N. Am. 2017, 46, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Jerzynska, J.; Stelmach, W.; Balcerak, J.; Woicka-Kolejwa, K.; Rychlik, B.; Blauz, A.; Wachulec, M.; Stelmach, P.; Majak, P.; Stelmach, I. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016, 37, 324–334. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, M.; Innocentin, S.; Dorella, F.; Rocha, C.; Mariat, D.; Pontes, D.; Miyoshi, A.; Azevedo, V.; Langella, P.; Chatel, J.-M. Immunotherapy of allergic diseases using probiotics or recombinant probiotics. J. Appl. Microbiol. 2013, 115, 319–333. [Google Scholar] [CrossRef]
- Kawahara, T. Inhibitory effect of heat-killed Lactobacillus strain on immunoglobulin E-mediated degranulation and late-phase immune reactions of mouse bone marrow-derived mast cells. Anim. Sci. J. 2010, 81, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Allam, J.-P.; Duan, Y.; Winter, J.; Stojanovski, G.; Fronhoffs, F.; Wenghoefer, M.; Bieber, T.; Peng, W.-M.; Novak, N. Tolerogenic T cells, Th1/Th17 cytokines and TLR2/TLR4 expressing dendritic cells predominate the microenvironment within distinct oral mucosal sites. Allergy 2011, 66, 532–539. [Google Scholar] [CrossRef]
- Allam, J.-P.; Novak, N.; Fuchs, C.; Asen, S.; Bergé, S.; Appel, T.; Geiger, E.; Kochan, J.P.; Bieber, T. Characterization of dendritic cells from human oral mucosa: A new Langerhans’ cell type with high constitutive FcϵRI expression. J. Allergy Clin. Immunol. 2003, 112, 141–148. [Google Scholar] [CrossRef]
- Allam, J.-P.; Würtzen, P.A.; Reinartz, M.; Winter, J.; Vrtala, S.; Chen, K.-W.; Valenta, R.; Wenghoefer, M.; Appel, T.; Gros, E.; et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-β1 and IL-10–producing properties. J. Allergy Clin. Immunol. 2010, 126, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Mascarell, L.; Lombardi, V.; Louise, A.; Saint-Lu, N.; Chabre, H.; Moussu, H.; Betbeder, D.; Balazuc, A.-M.; Van Overtvelt, L.; Moingeon, P. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J. Allergy Clin. Immunol. 2008, 122, 603–609. [Google Scholar] [CrossRef]
- Mascarell, L.; Saint-Lu, N.; Moussu, H.; Zimmer, A.; Louise, A.; Lone, Y.; Ladant, D.; Leclerc, C.; Tourdot, S.; Van Overtvelt, L.; et al. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunol. 2011, 4, 638–647. [Google Scholar] [CrossRef]
- Mascarell, L.; Lombardi, V.; Zimmer, A.; Louise, A.; Tourdot, S.; Van Overtvelt, L.; Moingeon, P. Mapping of the lingual immune system reveals the presence of both regulatory and effector CD4+T cells. Clin. Exp. Allergy 2009, 39, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Bahceciler, N.N. Immune mechanisms induced by sublingual immunotherapy in allergic respiratory diseases. Clin. Exp. Immunol. 2022, 209, 262–269. [Google Scholar] [CrossRef]
- Hagner, S.; Rask, C.; Brimnes, J.; Andersen, P.S.; Raifer, H.; Renz, H.; Garn, H. House Dust Mite-Specific Sublingual Immunotherapy Prevents the Development of Allergic Inflammation in a Mouse Model of Experimental Asthma. Int. Arch. Allergy Immunol. 2016, 170, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Kaminuma, O.; Suzuki, K.; Mori, A. Effect of Sublingual Immunotherapy on Antigen-Induced Bronchial and Nasal Inflammation in Mice. Int. Arch. Allergy Immunol. 2010, 152, 75–78. [Google Scholar] [CrossRef]
- Tourdot, S.; Airouche, S.; Berjont, N.; Moussu, H.; Betbeder, D.; Nony, E.; Floch, V.B.-L.; Baron-Bodo, V.; Mascarell, L.; Moingeon, P. Efficacy of sublingual vectorized recombinant Bet v 1a in a mouse model of birch pollen allergic asthma. Vaccine 2013, 31, 2628–2637. [Google Scholar] [CrossRef] [PubMed]
- La Grutta, S.; Arena, A.; D’Anneo, W.R.; Gammeri, E.; Leonardi, S.; Trimarchi, A.; Platania, D.; La Rosa, M. Evaluation of the antiinflammatory and clinical effects of sublingual immunotherapy with carbamylated allergoid in allergic asthma with or without rhinitis. A 12-month perspective randomized, controlled, trial. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 40–44. [Google Scholar] [PubMed]
- Marogna, M.; Spadolini, I.; Massolo, A.; Canonica, G.W.; Passalacqua, G. Clinical, functional, and immunologic effects of sublingual immunotherapy in birch pollinosis: A 3-year randomized controlled study. J. Allergy Clin. Immunol. 2005, 115, 1184–1188. [Google Scholar] [CrossRef]
- Chougnet, C.A.; Thacker, R.I.; Shehata, H.M.; Hennies, C.M.; Lehn, M.A.; Lages, C.S.; Janssen, E.M. Loss of Phagocytic and Antigen Cross-Presenting Capacity in Aging Dendritic Cells Is Associated with Mitochondrial Dysfunction. J. Immunol. 2015, 195, 2624–2632. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.F.; de Souza, A.P.D.; Borges, T.J.; Bonorino, C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: Decreased stimulation by aged dendritic cells. Mech. Ageing Dev. 2011, 132, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Goldstein, D.R. Impact of aging on antigen presentation cell function of dendritic cells. Curr. Opin. Immunol. 2013, 25, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Allam, J.-P.; Stojanovski, G.; Friedrichs, N.; Peng, W.; Bieber, T.; Wenzel, J.; Novak, N. Distribution of Langerhans cells and mast cells within the human oral mucosa: New application sites of allergens in sublingual immunotherapy? Allergy 2008, 63, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Mascarell, L.; Rak, S.; Worm, M.; Mélac, M.; Soulie, S.; Lescaille, G.; Lemoine, F.; Jospin, F.; Paul, S.; Caplier, L.; et al. Characterization of oral immune cells in birch pollen-allergic patients: Impact of the oral allergy syndrome and sublingual allergen immunotherapy on antigen-presenting cells. Allergy 2015, 70, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, C.; Bouley, J.; Moussu, H.; Luce, S.; Duchateau, M.; Chamot-Rooke, J.; Pallardy, M.; Lombardi, V.; Nony, E.; Baron-Bodo, V.; et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J. Allergy Clin. Immunol. 2016, 137, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Bouley, J.; Le Mignon, M.; Pliquet, E.; Horiot, S.; Turfkruyer, M.; Baron-Bodo, V.; Horak, F.; Nony, E.; Louise, A.; et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 129, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Laoubi, L.; Lacoffrette, M.; Valsesia, S.; Lenief, V.; Guironnet-Paquet, A.; Mosnier, A.; Dubois, G.; Cartier, A.; Monti, L.; Marvel, J.; et al. Epicutaneous allergen immunotherapy induces a profound and selective modulation in skin dendritic-cell subsets. J. Allergy Clin. Immunol. 2022, 150, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M. Les cellules lymphoïdes innées. De nouveaux acteurs de la réponse immune mucosale. (Innate lymphoid cells: New players of the mucosal immune response). Médecine/Sciences 2014, 30, 280–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beuraud, C.; Lombardi, V.; Luce, S.; Horiot, S.; Naline, E.; Neukirch, C.; Airouche, S.; Perchet, T.; Golub, R.; Devillier, P.; et al. CCR 10 + ILC 2s with ILC 1-like properties exhibit a protective function in severe allergic asthma. Allergy 2019, 74, 933–943. [Google Scholar] [CrossRef]
- Lombardi, V.; Beuraud, C.; Neukirch, C.; Moussu, H.; Morizur, L.; Horiot, S.; Luce, S.; Wambre, E.; Linsley, P.; Chollet-Martin, S.; et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J. Allergy Clin. Immunol. 2016, 138, 305–308. [Google Scholar] [CrossRef]
- Lao-Araya, M.; Steveling, E.H.; Scadding, G.W.; Durham, S.R.; Shamji, M.H. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J. Allergy Clin. Immunol. 2014, 134, 1193–1195. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Mitthamsiri, W.; Pradubpongsa, P.; Sangasapaviliya, A.; Boonpiyathad, T. Decreased CRTH2 Expression and Response to Allergen Re-stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2018, 10, 662–674. [Google Scholar] [CrossRef]
- Eljaszewicz, A.; Ruchti, F.; Radzikowska, U.; Globinska, A.; Boonpiyathad, T.; Gschwend, A.; Morita, H.; Helbling, A.; Arasi, S.; Kahlert, H.; et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2021, 147, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Bohle, B.; Kinaciyan, T.; Gerstmayr, M.; Radakovics, A.; Jahn-Schmid, B.; Ebner, C. Sublingual immunotherapy induces IL-10–producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007, 120, 707–713. [Google Scholar] [CrossRef]
- Rolland, J.M.; Gardner, L.; O’Hehir, R. Functional regulatory T cells and allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.W.; Shamji, M.H.; Jacobson, M.R.; Lee, D.I.; Wilson, D.; Lima, M.T.; Pitkin, L.; Pilette, C.; Nouri-Aria, K.; Durham, S.R. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 598–606. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Stanic, B.; Wirz, O.F.; Jansen, K.; Globinska, A.; Akdis, M. Role of regulatory B cells in immune tolerance to allergens and beyond. J. Allergy Clin. Immunol. 2016, 138, 654–665. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef]
- Pfaar, O.; Agache, I.; de Blay, F.; Bonini, S.; Chaker, A.M.; Durham, S.R.; Gawlik, R.; Hellings, P.W.; Jutel, M.; Kleine-Tebbe, J.; et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy 2019, 74 (Suppl. 108), 3–25. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Layhadi, J.A.; Achkova, D.; Kouser, L.; Perera-Webb, A.; Couto-Francisco, N.C.; Parkin, R.V.; Matsuoka, T.; Scadding, G.; Ashton-Rickardt, P.G.; et al. Role of IL-35 in sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 1131–1142. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C.; Cheng, Z.; Yang, J. Aberrant Th2 Immune Responses Are Associated with a Reduced Frequency of IL-35-Induced Regulatory T Cells after Allergen Exposure in Patients with Allergic Asthma. Allergy Asthma Immunol. Res. 2020, 12, 1029–1045. [Google Scholar] [CrossRef]
- Zissler, U.M.; Jakwerth, C.A.; Guerth, F.M.; Pechtold, L.; Aguilar-Pimentel, J.A.; Dietz, K.; Suttner, K.; Piontek, G.; Haller, B.; Hajdu, Z.; et al. Early IL-10 producing B-cells and coinciding Th/Tr17 shifts during three year grass-pollen AIT. Ebiomedicine 2018, 36, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; van de Veen, W.; Wirz, O.; Sokolowska, M.; Rückert, B.; Tan, G.; Sangasapaviliya, A.; Pradubpongsa, P.; Fuengthong, R.; Thantiworasit, P.; et al. Role of Der p 1–specific B cells in immune tolerance during 2 years of house dust mite–specific immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Antunes, R.D.S.; Westernberg, L.; Pham, J.; Birrueta, G.; Peters, B.; Sette, A.; Schulten, V. Characterization and epitope identification of the T cell response in non-allergic individuals exposed to mouse allergen. World Allergy Organ. J. 2019, 12, 100026. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Kepley, C.L.; Cambier, J.; Morel, P.; Lujan, D.; Ortega, E.; Wilson, B.S.; Oliver, J.M. Negative regulation of FcϵRI signaling by FcγRII costimulation in human blood basophils. J. Allergy Clin. Immunol. 2000, 106, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lalek, N.; Kosnik, M.; Silar, M.; Korosec, P. Immunoglobulin G-dependent changes in basophil allergen threshold sensitivity during birch pollen immunotherapy. Clin. Exp. Allergy 2010, 40, 1186–1193. [Google Scholar] [CrossRef]
- Shamji, M.H.; Layhadi, J.A.; Scadding, G.W.; Cheung, D.K.; Calderon, M.A.; Turka, L.A.; Phippard, D.; Durham, S.R. Basophil expression of diamine oxidase: A novel biomarker of allergen immunotherapy response. J. Allergy Clin. Immunol. 2015, 135, 913–921. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Heeringa, J.J.; McKenzie, C.I.; Varese, N.; Hew, M.; Bakx, A.T.C.M.; Aui, P.M.; Rolland, J.M.; O’Hehir, R.E.; Zelm, M.C. Induction of IgG 2 and IgG 4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy 2020, 75, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Bordas-Le Floch, V.; Berjont, N.; Batard, T.; Varese, N.; O’Hehir, R.E.; Canonica, W.G.; Zelm, M.C.; Mascarell, L. Coordinated IgG2 and IgE responses as a marker of allergen immunotherapy efficacy. Allergy 2021, 77, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnan, A.; Nicolas, J.-F.; Caimmi, D.; Vocanson, M.; Haddad, T.; Colas, L.; Scurati, S.; Mascarell, L.; Shamji, M.H. Deciphering Differential Behavior of Immune Responses as the Foundation for Precision Dosing in Allergen Immunotherapy. J. Pers. Med. 2023, 13, 324. https://doi.org/10.3390/jpm13020324
Magnan A, Nicolas J-F, Caimmi D, Vocanson M, Haddad T, Colas L, Scurati S, Mascarell L, Shamji MH. Deciphering Differential Behavior of Immune Responses as the Foundation for Precision Dosing in Allergen Immunotherapy. Journal of Personalized Medicine. 2023; 13(2):324. https://doi.org/10.3390/jpm13020324
Chicago/Turabian StyleMagnan, Antoine, Jean-François Nicolas, Davide Caimmi, Marc Vocanson, Thierry Haddad, Luc Colas, Silvia Scurati, Laurent Mascarell, and Mohamed H. Shamji. 2023. "Deciphering Differential Behavior of Immune Responses as the Foundation for Precision Dosing in Allergen Immunotherapy" Journal of Personalized Medicine 13, no. 2: 324. https://doi.org/10.3390/jpm13020324
APA StyleMagnan, A., Nicolas, J.-F., Caimmi, D., Vocanson, M., Haddad, T., Colas, L., Scurati, S., Mascarell, L., & Shamji, M. H. (2023). Deciphering Differential Behavior of Immune Responses as the Foundation for Precision Dosing in Allergen Immunotherapy. Journal of Personalized Medicine, 13(2), 324. https://doi.org/10.3390/jpm13020324





