Abstract
Allergen-immunotherapy (AIT) is an efficacious and disease-modifying treatment option for IgE-mediated diseases. Among these allergic rhinitis, insect venom allergy, food allergy, and allergic asthma are the most common candidates for AIT. AIT gives rise to clinical immunotolerance which may last for years after the treatment cessation. Mechanisms of AIT include suppression of allergic inflammation in target tissues and stimulation of the production of blocking antibodies, especially IgG4 and IgA. These mechanisms are followed by a reduction of underlying allergen-specific Th2 cell-driven responses to the allergens. Tolerance induction takes place through the desensitization of effector cells and stimulation of regulatory T cells that show their effects by mechanisms involving cell-cell cross-talk, but also other mechanisms, e.g., by the production of immunomodulatory cytokines such as, e.g., IL-10 and TGF-beta. From a personalized medical perspective, there is a need for clinical biomarkers of value in selecting responders and optimizing patient care during AIT. Also, a deeper understanding of underlying mechanistic processes will improve AIT’s future outcomes. In this paper, the current knowledge of mechanisms in AIT is reviewed with a special focus on biomarkers of this therapy.
1. Introduction
Allergen-immunotherapy (AIT) is a way to treat IgE-mediated diseases, such as insect venom allergy, food allergy, allergic rhinitis, and allergic asthma, which cause desensitization against allergens. The classical AIT methods are subcutaneous (SCIT) and sublingual allergen immunotherapies (SLIT) [1].
Food immunotherapy is recently approved by FDA (for peanut oral immunotherapy [2]) and it has oral, sublingual, and epicutaneous application routes. Respiratory allergens and Hymenoptera venom allergy were introduced many years ago. For venom immunotherapy (VIT) currently, the only way of application is SCIT but for other forms of AIT, SLIT or SCIT may be preferred [3,4,5]. The primary aim of VIT is to prevent fatal or life-threatening reactions to stings. AIT aims to reduce or abolish allergy signs and symptoms by inducing tolerance. The effectiveness of AIT is not predictable in individual patients. [6].
Blocking IgG4 antibodies exerts their effect by inhibiting IgE-dependent reactions on e.g., mast cells, basophils and B cells. Antigen-specific T-regulatory cells produce interleukin-10 (IL-10) and suppress Th2 immunity, and the immune balance shifts from a Th2-type to a Th1-type immune response [7]. B-regulatory cells are a newly identified cell type and are involved in enhancing immune tolerance by suppressing effector T cells via IL-10, blocking dendritic cell maturation, and producing blocking antibodies. The success of AIT is also related to the reduction of effector cell numbers in target tissues [8].
Although AIT is an effective, safe and disease-modifying treatment, not all patients respond to the therapy significantly. The definition of appropriate biomarkers may help e.g., determine when to discontinue treatment in patients who respond well, predict relapse, and when to apply booster therapy. Currently, no clinical biomarker has been identified and validated to predict clinical response. Candidate biomarkers include e.g., allergen-specific IgE (sIgE), IgE/Total IgE ratio, sIgE/IgG4 ratio, basophil activation tests, some cytokines, serum inhibitory activity for IgE, cellular markers, and provocation tests. However, lack of standardization, reproducibility of the results, the definition of responders and non-responders, and technical difficulties in laboratory methods are the main problems related to the candidate biomarkers [8].
2. Mechanisms of AIT
2.1. Antibody Responses
The main mechanisms of allergen immunotherapy are summarised in Figure 1. During AIT, an early temporary increase in allergen-specific IgE (sIgE) in peripheral blood is observed, followed by a decrease in sIgE over time [9,10]. Allergen-specific IgA, IgG4 antibodies, i.e., blocking antibodies, increase throughout the AIT [11,12,13].
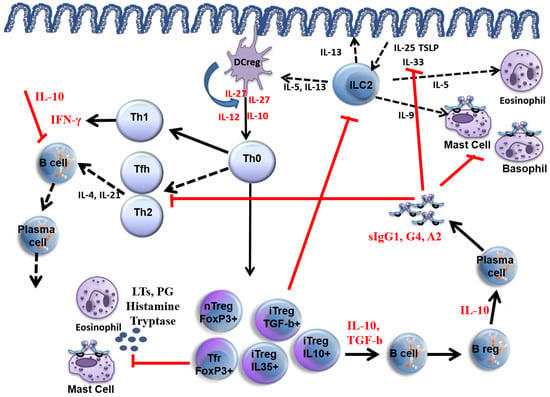
Figure 1.
Mechanisms of allergen immunotherapy. Red lines show how allergen immunotherapy inhibits allergic inflammation. Red cytokine levels increase as a result of AIT. Black lines are pathways of allergic inflammation, and black cytokines are those that increase during allergic inflammation. DC reg: Regulatory dendritic cell, ILC2: Innate lymphoid type 2 cell, LTs leukotrienes, PG: prostaglandin, Tfh: T follicular helper cell, nTreg: natural T regulatory cell, iTreg: inducible T regulatory cell, Tfr: follicular T regulatory cell. TSLP: Thymic stromal lymphopoietin.
An increase in saliva specific IgA was observed in children undergoing SLIT [14]. Additionally, in a recent study comparing local nasal and systemic IgA1 and IgA2 concentrations after SCIT and SLIT, an increase in IgA was observed in SLIT during and after immunotherapy, but not in the SCIT group [15]. This supports the idea that increased IgA in SLIT may be associated with clinical improvements observed during treatment and may play a role in blocking the binding of allergens to IgE receptors [16].
Blocking antibodies, especially IgG4, block the allergen-sIgE interaction by competing with sIgE. This blockage prevents e.g., cross-linking of allergen-sIgE complexes on basophils and mast cells. As a result, these effector cells’ activation is inhibited. This allergen-specific IgG elevation is seen not only in serum levels but also locally in nasal secretions [17]. Blocking antibodies also prevents the IgE-facilitated allergen presentation to T cells through the FcɣRIIb receptors on B cells. IL-10, a crucial cytokine produced by Tregs and Bregs, is involved in inhibiting allergen-specific effector cells. In addition, IL-10 induces IgG4 production while reducing total IgE and sIgE levels.
In allergen immunotherapy, blocking antibody levels decrease significantly within one year after AIT is discontinued [18,19,20]. However, IgG-related serum IgE inhibitory activity continues for years and is related to clinical response [21]. On the other hand, during venom immunotherapy (VIT), desensitization is related to high IgG4 levels and IgE-Inhibitory activity. When the VIT stops, sIgG4 and IgE-Facilitated allergen binding (IgE-FAB) inhibitory activity return to baseline within months of stopping AIT, and further follow-up showed a more persistent decrease in venom-sIgE levels. This observation suggests an alternative mechanism of prolonged protection other than blocking antibodies [22].
Food-specific IgE levels rise transiently in the early stages of treatment with food AIT, but then decline [23,24,25]. Although low baseline food-specific IgE may be considered a biomarker of tolerance development, lowering food-specific IgE below a certain threshold does not imply the development of tolerance. Food -specific IgG4 levels increase with food AIT [23,24,26]. However, changes in IgG4 levels or the ratio of food sIgE/IgG4 ratio seems not related to the tolerance development. One explanation may be that IgG4 is not associated with tolerance, as it reflects previous allergen exposure and thus reflects less severe allergy at baseline [27].
2.2. Cellular Responses
AIT acts by desensitizing effector cells [28], including mast cells and basophils. Not only the number of these cells is influenced, but also the cytokine release thresholds rise over time. At the beginning of rush VIT, basophil numbers start to decrease in the peripheral blood, surface antigens of basophils down-regulate and basophil-derived cytokines IL-13 and IL-4 decrease. Mast cell functions and serum tryptase levels decrease [29,30].
In every form of food immunotherapy i.e., oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT) a decrease in basophil activation may be seen [31,32,33]. Studies by peanut OIT and milk OIT/SLIT showed early decreases in basophil activation and this is related to the tolerance development at the end of the studies [33,34]. However, this decreased activation is transient and it rebounds after the cessation of immunotherapy in many studies [28,35,36].
AIT with aeroallergens inhibits both early and late-phase allergic responses at allergic target organs, by suppressing several cytokines and decreasing e.g., mast cell and basophil numbers. This observation suggests that AIT is effective at target organs as well as at the systemic level. AIT with other allergens is expected to have similar mechanisms [8,22,37].
T and B cell responses change during AIT as the immune tolerance develops. A change in immune responses from Th2 to Th1 cell type occurs, which is characterized by an increase in Th1-related interferon-gamma (IFN-γ) and a decrease in Th2-related IL-4 and IL-13 cytokine levels. As Th2 activity reduces, the function and numbers of Treg cell cells increase. Treg cells are critical in peripheral immune tolerance development with distinct subgroups; Natural T regulatory (nTreg) cells, which are transcription factor forkhead box P3 (FOXP3) positive, and inducible T regulatory (iTreg) cells such as IL-10 producing Tr1 cells and TGF-β producing Th3 cells. The major cytokine produced by Tregs is IL-10, which plays an inhibitory role on B cells by blocking the B7/CD28 pathway. This blockage leads to the suppression of dendritic cell maturation, MHC class II expression, and costimulatory ligand activation. Meanwhile, TGF-β suppresses the FcεRI on Langerhans cells, upregulates FOXP3, and RUNX, and helps CTLA-4 expression on T cells.
IL-10 plays a significant role in clinical response and immune tolerance during AIT. IL-10 blockade in peripheral blood reverses the effects of AIT, and allergen-specific proliferative and cytokine responses augment [38]. Following the AIT, CD4+CD25+FOXP3+ Treg cells increase in the inflammation site. This finding supports the idea that Treg cells play an essential role in allergen-specific immune tolerance. All types of allergens used in AIT cause a shift towards a regulatory/suppressor T cell response [8].
In a VIT study, dermal biopsies were taken before and three months after immunotherapy to evaluate allergen-driven alterations in cytokine mRNA expression and cellular differences. The results showed a prominent decrease in IL-4 mRNA expression as well as an increase in IL-10+ cell numbers and a trend of increase for IL-10 mRNA expression [39]. In another study, CD4+CD8+ T lymphocyte changes were analyzed before VIT, at the end of 5 days semi-rush, and at the 6th month of VIT. A significant decrease in IL-4+CD4+ and CD8+ T cell numbers was observed by the end of 5 days of semi-rush VIT. After six months of VIT, an increase in IL-2+IFN-γ+CD4+CD8+ T lymphocytes has been shown, confirming a shift from Th2 to Th1 type immune deviation. IL-10 levels in peripheral blood increased just by the second day of AIT, and by the end of 4th week of treatment, allergen-specific T cells were influenced by the suppressive effect of IL-10 [22].
From the perspective of food IT, one study using a tetramer-based approach found that Ara h 2-specific circulating memory B cells are induced early and transiently in peanut oral immunotherapy and clonal antigen-specific responses to immunotherapy has been shown [40]. In another study, sorting of Ara h 1 or 2 reactive B cells followed by deep sequencing showed that immunotherapy stimulated somatic mutations in IgG4 [41]. These findings support the idea that B cells may play important roles during AIT.
T follicular helper cells (Tfh) are characterized by CXCR5+ surface receptors and they are involved in B-cell maturation and Ig-class switching. A specific type of Tregs, i.e., CXCR5+ FOXP3+ Treg cells, are named follicular regulatory T (Tfr) cells. They move to the germinal centers in the lymph nodes and decrease T and B cell activity. An AIT study showed a significant suppression in Tfh cells following AIT. There is a transformation potential between Tfh and Tfr cells i.e., plasticity and Tfr cells produce more IL-10 compared to Tfh cells. This supports the idea that Tfr cells may have important roles during AIT and immune tolerance development which results in a significant decrease in Th2 responses.
Allergen-specific Breg cells that secrete IL-10 have been identified in beekeepers tolerant to bee stings and patients treated with VIT. Breg cells are CD73-CD25+CD71+ B cells that can suppress allergen-specific CD4+ T cells and stimulate allergen-specific IgG4 production during AIT. Additionally, Bregs produce IL-35 and TGF-beta.
Apart from well-known cell types, there are also some other cells, such as natural killer regulatory cells, which have the capacity for IL-10 production. These cells decrease allergen-induced T cell proliferation through the IL-10 production during AIT, and like other regulatory cell types, they may be involved in tolerance development.
2.3. Innate Lymphoid Cells and AIT
Innate lymphoid cell type-2 (ILC2) appears to take part in allergic reactions. The effect of AIT on ILC type 2 was studied in AIT. AIT blocked seasonal increases in ILC2s numbers in effector sites. A decrease in ILC2 number was parallel to the improvement and clinical scores of the patients receiving AIT. Otherwise, ILC1 is the major producer of IFNɣ and TNFα. In fact, the ratio of ILC2/ILC1 is high in perennial AR patients sensitized. This high ratio decreases and turns to normal levels during AIT. This effect is not seen in non-responders to AIT, and they present a similar ILC2/ILC1 ratio to untreated patients. Under the action of retinoic acid, ILC2 cells transform into IL-10-producing regulatory ILCs (ILCregs). These cells can reduce Th2 cells and ILC2 activation. Retinoic acid also stimulates peripheral Treg cell differentiation. By combining these together, ILCregs can participate in tolerance development in AIT mechanisms [8,42]. A recent finding supports this idea. Golebski et al. identified a distinct subset of ILC-2 that produces IL-10 and has regulatory properties that increase after AIT [43].
A distinct innate lymphoid cell type, ILC type 3, may have an important role during immune tolerance development in SLIT. They express CD40L and are located side by side with B cells in the tonsils. ILC3s stimulate IL-15 production in B cells, and IL-15 stimulates the CD40L expression in ILC3s. CD40L+ ILC3s induce IL-10-secreting Breg cells via the CD40L and BAFF-receptor-dependent pathway. ILC3-associated Breg cells are identified by CD27-IgD+IgM+CD24highCD38highCD1d+, and called immature transitional (itBreg) phenotype. This interaction seems to be essential for maintaining immune tolerance for self and harmless antigens and is inappropriate in allergic diseases. ILC3s, Breg cells and Treg cells interact closely in the interfollicular regions of the palatine tonsils. CD40L+ ILC3s may be required in maintaining immune tolerance in the tonsils through the induction of functional itBreg cells. These cells may act not only by cell-to-cell contact via programmed cell death ligand 1, but also by secreting IL-10 for immune tolerance development [42].
2.4. Histamine and Histamine Receptors
Desensitization during AIT starts as early as after the first few injections in SCIT. Histamine is an important mediator released from mast cells and exerts its effect through its receptors. Histamine receptor 2 (HR2) stimulation results in desensitization of basophils. Decreased basophil activity parallels clinical scores in AIT. H2R attenuates allergen-specific FcεRI-mediated basophil stimulation. HR2 has important roles in immune tolerance mechanisms. HR2 expression in Th2 cells reduces allergen-induced T cell responses and induces peripheral tolerance by increasing IL-10 production [11,12,13]. Histamine acts via HR2 and increases IL-10 production not only in dendritic cells but also in Th2 cells. It stimulates the suppressive effect of TGF-β on T cells and reduces the production of Th2 cytokines (IL-4 and IL-13 are the main Th2 type cytokines) [8,44].
3. Biomarkers for AIT
AIT is an effective method for the treatment of IgE-mediated allergic diseases. However, not all patients respond to the therapy. A validated biomarker is significant from the perspective of personalized medicine, including to obtain an adequate cost/benefit ratio. Surrogate biomarkers may help e.g., to identify good responders, when to stop treatment, predict relapse and when to apply a booster treatment. Currently, there is no defined and validated clinical biomarker to predict clinical response. Proposed biomarkers to assess the efficacy of allergen immunotherapy have been summarized in Table 1.
Despite the existence of several candidate biomarkers, there are challenges regarding standardization, reproducibility of results and identification of responders versus non-responders, and complexity of laboratory methods. Some of these biomarkers are discussed briefly.
Total IgE (tIgE) often increases in allergic patients. During AIT, total IgE levels first increase and then decrease over time. However, its value in diagnosing allergic disease and predicting AIT efficacy is conflicting, and different studies have yielded opposite results [21,45,46,47,48]. Allergen sIgE is the diagnostic method for allergic diseases together with skin prick testing. They also represent the standard tests as an inclusion criterion for AIT. During AIT, sIgE levels increase in the early stages and then decrease gradually throughout treatment. Early rise in sIgE does not associate with the clinical response, and the changes in sIgE levels cannot distinguish responders from non-responders. In the AIT studies, the serum sIgE/tIgE ratio (sIgE/tIgE) associated with rhinitis signs and symptoms scores, and even some cut-off values were defined to predict clinical response. However, conflicting data with other studies bring the need for better-defined studies to use the sIgE/tIgE ratio as a reliable tool [8,22,47,49].
Allergen-specific IgG4 and IgG1 increase during AIT in target organs and peripheral blood [11,12,13]. They have the blocking capacity for effector cells and are involved in developing immune tolerance. However, these antibodies are not considered reliable markers for the AIT response. In some SCIT studies, the increase in sIgG4 was not associated with clinical reactivity. Still, the clinical response was found to be associated with serum inhibitory activity for IgE, even after AIT cessation. This suggests that the concept of the functional significance of sIgG subgroups rather than serum levels is essential for sustained clinical tolerance [50].
A recent systematic review evaluated the clinical utility of microarray B cell epitope mapping as a potential biomarker for food allergy diagnosis, clinical severity, and response to immunotherapy [51]. In one of these studies, peanut oral immunotherapy showed an increase in IgG4 levels, a decrease in IgE level and diversity of Ara h 1, 2, and 3 epitopes at the same time [52]. Additionally, another study evaluated the IgE and IgG4 binding to cows’ milk peptides to assess the responders and non-responders to cows’ milk OIT [53]. In another work, authors offered two sets of IgE binding peptides to predict the ones with the slow response to desensitization and the ones with more adverse reactions during cows’ milk OIT [54].
Inhibition of allergen binding to IgE is evaluated by a validated flow cytometry-based test called Ig-E FAB [55]. It shows IgE-allergen complexes on FcεRII receptors (CD23) in B Cells. IgE-FAB is a promising biomarker, and its effectiveness must be tested in clinical trials. However, it is quite complex and is only available in a limited number of centers [50]. An alternative and simpler method has been developed: the enzyme-linked immunosorbent facilitated antigen binding assay (ELIFAB). In this method, soluble CD23 monomers are attached to a solid surface instead of B cell lines [56]. IgE-BF defines the competition between serum components and sIgE to bind to the allergen and is measured by a solid phase test [57]. IgE-BF increases during AIT and associates with clinical response [58,59]
AIT may prevent basophil activation through the allergen sIgG antibodies. Basophil activation may be detected either by histamine release or by identifying basophil surface markers CD63 and CD203c by flow cytometry assays [60,61,62]. A new flow cytometric method called the diamine oxidase (DAO) test is another alternative to see basophil activity during AIT. DAO uses histamine as its substrate and binds to intracellular histamine. Basophil activation leads to histamine release from cells [63,64] with a change in intracellular DAO levels indicating the activation level of the basophils. There is inconsistency in results between studies concerning basophil activation. At least in part, the conflicting results may be associated with the type of AIT. SCIT studies give more promising results compared to SLIT, as SLIT appears less effective in basophil suppression.
A shift from Th2 inflammation to Th1 inflammation occurs during allergen immunotherapy. During this transition, cytokines and chemokines play an important role. Not only Th1-related cytokines (e.g., IFN-ɣ, IL-12) [65,66], but also Treg-related cytokines (e.g., IL-10 and TGF-β) are increased [67,68]. There is also a decrease in Th2 response-related cytokines (e.g., IL-4, IL-13, IL-9, IL-17, TNF-α) [66]. However, there is inconsistency between studies and some of them do not find a strong relationship between clinical response and expected cytokine profile [69,70].
Chemokines are small proteins that have the ability to induce the migration of cells (chemoattractive functions) that play an important role in the pathophysiology of allergic disorders. Chemokines may increase (eotaxin [71], complement C4a [72], leptin [73], thymus and activation regulated protein (TARC) [71], transthyretin [72]); decrease (complement C3a, C5a [74] eotaxin [75]) or may be unchanged (adiponectin [76], tryptase [75], eosinophilic cationic protein (ECP) [75], soluble HLA molecules [77]) in different studies during AIT. Currently, there is no confirmed consensus on potential cytokine, chemokine or molecule biomarkers to predict clinical response to AIT.
Candidate cellular biomarkers related to the AIT response include regulatory T cells (Tregs), regulatory B cells (Bregs), native lymphoid cells (ILCs), dendritic cells, T helper cells (Th1, Th2), T follicular helper (Tfh) and T follicular regulator (Tfr) cells [7,8].
Breg cells appear to be important for the development of allergen tolerance and their function is mainly associated with the following possible mechanisms: IL-10-mediated suppression of effector T cells (including Th2 responses), stimulation of Treg cells, IL-10-mediated blockade of dendritic cells maturation, modulation of Tfh responses, and anti-inflammatory production of IgG4 antibodies [78].
All of these cells are modified during AIT. Some changes in these cells have been reported to distinguish between treatment groups and are generally associated with treatment outcomes. However, there is insufficient data to correlate any of these cells with clinical efficacy, and they are technically challenging to identify. They are currently unlikely to represent biomarkers.
Some markers related to polarized dendritic cells have been investigated to test the efficacy of SLIT as well. Component 1 (C1Q), receptor stabilin1 and STAB1 were found to be elevated in the blood samples of clinically responding patients, contrary to non-responding or control group patients. However, these findings need to be validated in further studies and practice [8,79].
In-vivo biomarkers evaluate the response to AIT directly in the involved organ by provocation with the relevant allergen. They include skin prick tests (SPT), intradermal tests, and conjunctival, nasal and bronchial provocation tests. In general, these tests are used in clinics for diagnostic purposes. Additionally, in-vivo biomarkers may also be used for the evaluation of the clinical efficacy of AIT. There are, however, several protocols that have been published on different challenge models. Therefore, there is a need for standardization and validation of these different provocation models.
Recently, the use of environmental challenge chambers (ECC) has become an alternative method to expose the patient to a predefined allergy dose for evaluation of provocation tests. An ECC represents a provocation test that may closely simulate natural exposure. An EEC may be used to expose the patient to a stable and reproducible allergen amount under the same environmental conditions. Additionally, as seen in some studies, ECC may determine the starting time for AIT studies. Comparable clinical scores were achieved in a study conducted by ECC with an allergen dose similar to environmental exposure. For AIT, further validation of the treatment effect size as assessed in the EEC challenges is needed to relate it to effect sizes found under natural exposure in field trials [80,81,82].
There is a clear need to find reliable biomarkers for food AIT to determine which patients will respond better and to see the efficacy of immunotherapy after treatment is discontinued. Currently, the standard approach to measuring efficacy is via pre-treatment and post-treatment double-blind, placebo-controlled food challenges. This approach can sometimes be time-consuming, resource-intensive, and carries the risk of serious allergic reactions, including anaphylaxis [79].
From a VIT perspective, sting challenges are a reliable method and gold standard for determining the efficacy of VIT [4,83]. In addition, field stings can provide information about the effectiveness of the treatment. Early sting challenges are fairly reliable for determining the efficacy of VIT [84], but repeated sting challenge trials three to five years after treatment can poorly identify patients with relapse [85,86]. Sting challenges are important not only for demonstrating treatment efficacy but also for health-related quality of life, and a passed challenge improves patients’ confidence as well [87].
Recently, epigenetic studies and omics technology also offer some new potential biomarkers for AIT. A dual SLIT study with grass and house dust mite allergens resulted in the induction of memory Treg cells via reduced DNA methylation of the CpG regions of the FOXP3+ locus [88]. A recent study of SCIT measured serum sample metabolites from patients who did and did not respond well to AIT and found that L-Tyrosine was an indicator of response and decreased in those who responded well. In addition, AIT was found to be associated with NO and nitric oxide synthase metabolism [89]. Eicosanoids, such as prostaglandins and leukotrienes, are important molecules for innate immune responses, and especially 12(S)-hydroxyeicosatetraenoic acid (HETE) and 15(S)-HETE is reduced after treatment with SCIT and they may be promising candidate molecules [90].

Table 1.
Biomarkers to assess the efficacy of allergen immunotherapy. (Adapted and modified from [7,91]).
Table 1.
Biomarkers to assess the efficacy of allergen immunotherapy. (Adapted and modified from [7,91]).
| Possible Biomarkers and Examples | Advantage | Limitation | References |
|---|---|---|---|
Specific IgE sIgE/tIgE | -Easy test to run, serum-based laboratory test -sIgE/tIgE seems to be reliable and promising marker to show clinical response | -sIgE rising at the beginning of AIT and not associated with the clinical response -sIgE/tIgE not validated yet. -Need for equivalence studies between tIgE units and sIgE units | [12,21,45,46,48,49,92,93,94,95,96] |
sIgG4 sIgE/sIgG4 | -Easy test to run, serum-based laboratory test -sIgG4 informative for allergen exposure -Currently, there is a commercial kit for sIgG4 | -It is not clear whether the sIgG4 levels and symptom and medication scores associate well. Need for further studies -Insufficient data on other IgG subsets -Limited data on local antibody levels -Low sIgG4 levels is a difficulty for measurement | [12,13,18,19,20,21,47,48,60,68,97,98,99,100] |
IgE-BF ELIFAB | -Serum-based laboratory test -High reproducibility is an advantage for IgE-FAB -IgE-FAB and IgE-BF associated with the clinical responses and reported in previous studies | -IgE-BF is not commercially available -It is not clear if IgE-FAB discriminates good responders -Despite the association between clinic response and IgE-FAB the number of studies is limited | [11,12,13,16,21,55,56,57,59,60,61,101,102,103,104] |
CD203c CD107 Diamine Oxidase | -Shows basophil activation with FcεRI mediated in-vivo response -Small amount of blood needed | -Test variability between different centers -Difficult technique -Lack of dose-response curves -Unresponsive basophils in some people | [11,60,63,64,105,106,107,108,109,110,111,112] |
IL-2R/IL-2 IL-4, IL-5, IL-9, IL-13, IL-17 IFN-γ, IL-12 TGF-β, IL-10 CCR3 TARC Tryptase | -Useful to understand the mechanisms of AIT -Local cytokine production may relate more to clinical manifestations | -There is no cytokine, chemokine or molecules identified to predict clinical response until now -Results may differ between centers and studies. -Specific T cell originated cytokine levels may be very low to be determined | [71,72,73,74,76,113] |
B regs Dendritic cells ILCs | -Tregs are very important in tolerance development -Bregs take very important roles in the mechanism of AIT -Treg and Breg cells may be more useful for drug development | -Technical difficulties -No data to show the association between Tregs and clinical response -Tregs appear very early in AIT so difficult to be a biomarker -Very low frequency of Tregs and Bregs | [7,78,79] |
Intradermal tests Conjunctival provocation tests Nasal Provocation tests Bronchial provocation tests Environmental Challenge Chambers (ECC) Food challenges Sting Challenges Allergen Challenges | -Standardised environmental factors -Seasonal pollen variations may be avoided -Surrogate markers of clinical response to AIT -ECC decrease variability in clinical studies, and they allow dose-response studies. -Food challenges help to find threshold levels for safe consumption -Early sting challenges are fairly reliable | -Mimics natural exposure but not the same -Lack of standardization for some allergen challenges -Cost of ECC is high, some reproducibility problems exist -Food challenges sometimes may be time-consuming, resource-intensive, and carries the risk of serious allergic reactions, including anaphylaxis -As time passes after treatment cessation sting challenges are poor to demonstrate relapse | [4,79,81,82,83,85,86,87,88,89,90] |
AIT: Allergen immunotherapy, CCR3: C-C chemokine receptor type 3, ECP: Eosinophilic cationic protein, ELIFAB: Enzyme-linked immunosorbent-facilitated antigen-binding assay, DAO: Diamine oxidase, IgE-BF: IgE-blocking factor, FAB: facilitated antigen binding, IFN-γ: Interferon-gamma, ILCs: Innate lymphoid cells, SPT: Skin prick tests, TARC: thymus and activation regulated protein, TGF-β: Transforming growth factor-beta.
4. Conclusions
As the only disease-modifying treatment option for individuals with IgE-mediated allergy, allergen immunotherapy has been shown to be both clinically effective and safe. In general, AIT is an effective treatment option. However, some patients do not respond to AIT well. There is an increasing understanding of underlying mechanisms in AIT laying the path for biomarkers for indication, monitoring, and optimizing the course of treatment. Through the modulation of innate and adaptive immune responses. To date, there has been no clear relationship between the immunological changes observed and responders and non-responders to AIT. However, IgE-FAB represents a promising biomarker. Antibody and cellular responses, molecules enable us many insights into the mechanisms of AIT but cannot be applied as biomarkers in a clinical setting yet. Finally, the use of the challenge test is still limited.
Further research is needed to confirm and interpret the possible association with biomarkers and clinical response to AIT.
Author Contributions
U.M.S. wrote the first draft of the paper. All authors contributed from the draft stage by critical commenting and revising and gave approval for submission of its final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Mattia Giovannini, None. Umit Sahiner, lecture fees from Mustela, Roxall. Maria M Escribese Lecture fees from Diater, Stallergene Geer and GSK. Giovanni Paoletti declares no conflict of interest. Enrico Heffler reports research grants from AstraZeneca, Boehringer Ingelheim, Circassia, GlaxoSmithKline, Nestlé Purina, Novartis, Sanofi, Teva and Valeas, outside of the submitted work. Montserrat Alvaro Lozano, consultancy fees from Sanofi Genentech. Domingo Barber grants to his Institution and personal fees from ALK-Abello, lectures fees from DIATER. Giorgio Walter Canonica reports having received research grants as well as being a lecturer or having received advisory board fees from A. Menarini, Alk-Abello, Allergy Therapeutics, AstraZeneca, Chiesi Farmaceutici, Firma, Genentech, Guidotti-Malesci, GlaxoSmithKline, Hal Allergy, Mylan, Novartis, Regeneron, Roche, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer, Valeas, Om-Pharma, outside of the submitted work. Oliver Pfaar reports reports grants and/or personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, GlaxoSmithKline, ROXALL Medizin, Novartis, Sanofi-Aventis and Sanofi-Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl-Boskamp, Inmunotek S.L., John Wiley and Sons, AS, Paul-Martini-Stiftung (PMS), Regeneron Pharmaceuticals Inc., RG Aerztefortbildung, Institut für Disease Management, Springer GmbH, AstraZeneca, IQVIA Commercial, Ingress Health, Wort&Bild Verlag, Verlag ME, Altamira Medica AG, Meinhardt Congress GmbH, Deutsche Forschungsgemeinschaft, Thieme, Deutsche AllergieLiga e.V., AeDA, Alfried-Krupp Krankenhaus, Procter and Gamble, Red Maple Trials Inc., Technical University Dresden, ECM Expo& Conference Management, all outside the submitted work; and he is member of EAACI Excom, member of ext. board of directors DGAKI; coordinator, main- or co-author of different position papers and guidelines in rhinology, allergology and allergen-immunotherapy.
References
- Pfaar, O.; Bousquet, J.; Durham, S.R.; Kleine-Tebbe, J.; Larche, M.; Roberts, G.; Shamji, M.H.; Gerth van Wijk, R. One hundred and ten years of Allergen Immunotherapy: A journey from empiric observation to evidence. Allergy 2022, 77, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Hise, K.; Rabin, R.L. Oral Immunotherapy for Food Allergy-a US Regulatory Perspective. Curr. Allergy Asthma Rep. 2020, 20, 77. [Google Scholar] [CrossRef]
- Alvaro-Lozano, M.; Akdis, C.A.; Akdis, M.; Alviani, C.; Angier, E.; Arasi, S.; Arzt-Gradwohl, L.; Barber, D.; Bazire, R.; Cavkaytar, O.; et al. Allergen Immunotherapy User’s Guide. Pediatr. Allergy Immunol. 2020, 31 (Suppl. 25), 1–101. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Varga, E.M.; Roberts, G.; Mosbech, H.; Bilo, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Dhami, S.; Nurmatov, U.; Arasi, S.; Khan, T.; Asaria, M.; Zaman, H.; Agarwal, A.; Netuveli, G.; Roberts, G.; Pfaar, O.; et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta-analysis. Allergy 2017, 72, 1597–1631. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, C.; Ridolo, E.; Mauro, M.; Pucciarini, F.; Heffler, E.; Canonica, G.W. Venom Immunotherapy and Aeroallergen Immunotherapy: How Do Their Outcomes Differ? Front. Allergy 2022, 3, 854080. [Google Scholar] [CrossRef]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Shamji, M.H.; Durham, S.R. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017, 140, 1485–1498. [Google Scholar] [CrossRef]
- Burks, A.W.; Calderon, M.A.; Casale, T.; Cox, L.; Demoly, P.; Jutel, M.; Nelson, H.; Akdis, C.A. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J. Allergy Clin. Immunol. 2013, 131, 1288–1296.e3. [Google Scholar]
- Cox, L.; Nelson, H.; Lockey, R.; Calabria, C.; Chacko, T.; Finegold, I.; Nelson, M.; Weber, R.; Bernstein, D.I.; Blessing-Moore, J.; et al. Allergen immunotherapy: A practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, S1–S55. [Google Scholar] [CrossRef]
- Shamji, M.H.; Layhadi, J.A.; Scadding, G.W.; Cheung, D.K.; Calderon, M.A.; Turka, L.A.; Phippard, D.; Durham, S.R. Basophil expression of diamine oxidase: A novel biomarker of allergen immunotherapy response. J. Allergy Clin. Immunol. 2015, 135, 913–921.e9. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.; Malling, H.J.; Worm, M.; Horak, F.; Jager, S.; Montagut, A.; Andre, C.; de Beaumont, O.; Melac, M. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2007, 120, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Kapp, A.; Colombo, G.; de Monchy, J.G.; Rak, S.; Emminger, W.; Riis, B.; Grønager, P.M.; Durham, S.R. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J. Allergy Clin. Immunol. 2008, 121, 512–518.e2. [Google Scholar] [CrossRef] [PubMed]
- Huoman, J.; Papapavlou, G.; Pap, A.; Alm, J.; Nilsson, L.J.; Jenmalm, M.C. Sublingual immunotherapy alters salivary IgA and systemic immune mediators in timothy allergic children. Pediatr. Allergy Immunol. 2019, 30, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Larson, D.; Eifan, A.; Scadding, G.W.; Qin, T.; Lawson, K.; Sever, M.L.; Macfarlane, E.; Layhadi, J.A.; Würtzen, P.A.; et al. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J. Allergy Clin. Immunol. 2021, 148, 1061–1071.e11. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.; von Maur, R.K.; Ishizaka, K.; Norman, P.S.; Lichtenstein, L.M. IgA and IgG anti-ragweed antibodies in nasal secretions. Quantitative measurements of antibodies and correlation with inhibition of histamine release. J. Clin. Investig. 1976, 57, 1041–1050. [Google Scholar] [CrossRef]
- Reisinger, J.; Horak, F.; Pauli, G.; van Hage, M.; Cromwell, O.; Konig, F.; Valenta, R.; Niederberger, V. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J. Allergy Clin. Immunol. 2005, 116, 347–354. [Google Scholar] [CrossRef]
- La Rosa, M.; Ranno, C.; Andre, C.; Carat, F.; Tosca, M.A.; Canonica, G.W. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 1999, 104, 425–432. [Google Scholar] [CrossRef]
- Troise, C.; Voltolini, S.; Canessa, A.; Pecora, S.; Negrini, A.C. Sublingual immunotherapy in Parietaria pollen-induced rhinitis: A double-blind study. J. Investig. Allergol. Clin. Immunol. 1995, 5, 25–30. [Google Scholar]
- Bahceciler, N.N.; Arikan, C.; Taylor, A.; Akdis, M.; Blaser, K.; Barlan, I.B.; Akdis, C.A. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int. Arch. Allergy Immunol. 2005, 136, 287–294. [Google Scholar] [CrossRef]
- James, L.K.; Shamji, M.H.; Walker, S.M.; Wilson, D.R.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Kimber, I.; Till, S.J.; Durham, S.R. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J. Allergy Clin. Immunol. 2011, 127, 509–516.e5. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Durham, S.R. Hymenoptera Venom Allergy: How Does Venom Immunotherapy Prevent Anaphylaxis From Bee and Wasp Stings? Front. Immunol. 2019, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Bird, J.A.; Kulis, M.; Laubach, S.; Pons, L.; Shreffler, W.; Steele, P.; Kamilaris, J.; Vickery, B.; Burks, A.W. Sublingual immunotherapy for peanut allergy: Clinical and immunologic evidence of desensitization. J. Allergy Clin. Immunol. 2011, 127, 640–646.e1. [Google Scholar] [CrossRef] [PubMed]
- Vickery, B.P.; Scurlock, A.M.; Kulis, M.; Steele, P.H.; Kamilaris, J.; Berglund, J.P.; Burk, C.; Hiegel, A.; Carlisle, S.; Christie, L.; et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy Clin. Immunol. 2014, 133, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Burks, A.W.; Keet, C.; Vickery, B.P.; Scurlock, A.M.; Wood, R.A.; Liu, A.H.; Sicherer, S.H.; Henning, A.K.; Lindblad, R.W.; et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J. Allergy Clin. Immunol. 2016, 137, 1117–1127.e10. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Jones, S.M.; Scurlock, A.M.; Perry, T.T.; Kemper, A.; Steele, P.; Hiegel, A.; Kamilaris, J.; Carlisle, S.; Yue, X.; et al. A randomized controlled study of peanut oral immunotherapy: Clinical desensitization and modulation of the allergic response. J. Allergy Clin. Immunol. 2011, 127, 654–660. [Google Scholar] [CrossRef]
- Baloh, C.H.; Huffaker, M.F.; Laidlaw, T. Biomarkers and mechanisms of tolerance induction in food allergic patients drive new therapeutic approaches. Front. Immunol. 2022, 13, 972103. [Google Scholar] [CrossRef]
- Tsai, M.; Mukai, K.; Chinthrajah, R.S.; Nadeau, K.C.; Galli, S.J. Sustained successful peanut oral immunotherapy associated with low basophil activation and peanut-specific IgE. J. Allergy Clin. Immunol. 2020, 145, 885–896.e6. [Google Scholar] [CrossRef]
- Plewako, H.; Wosinska, K.; Arvidsson, M.; Bjorkander, J.; Skov, P.S.; Hakansson, L.; Rak, S. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int. Arch. Allergy Immunol. 2006, 141, 346–353. [Google Scholar] [CrossRef]
- Dugas-Breit, S.; Przybilla, B.; Dugas, M.; Arnold, A.; Pfundstein, G.; Kuchenhoff, H.; Ruëff, F. Serum concentration of baseline mast cell tryptase: Evidence for a decline during long-term immunotherapy for Hymenoptera venom allergy. Clin. Exp. Allergy 2010, 40, 643–649. [Google Scholar] [CrossRef]
- Kulis, M.; Yue, X.; Guo, R.; Zhang, H.; Orgel, K.; Ye, P.; Li, Q.; Liu, Y.; Kim, E.; Burks, A.W.; et al. High- and low-dose oral immunotherapy similarly suppress pro-allergic cytokines and basophil activation in young children. Clin. Exp. Allergy 2019, 49, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Sicherer, S.H.; Burks, A.W.; Leung, D.Y.; Lindblad, R.W.; Dawson, P.; Henning, A.K.; Berin, M.C.; Chiang, D.; Vickery, B.P.; et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J. Allergy Clin. Immunol. 2017, 139, 1242–1252.e9. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Frischmeyer-Guerrerio, P.A.; Thyagarajan, A.; Schroeder, J.T.; Hamilton, R.G.; Boden, S.; Steele, P.; Driggers, S.; Burks, A.W.; Wood, R.A. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J. Allergy Clin. Immunol. 2012, 129, 448–455.e5. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.U.; Steinbrecher, J.; Calatroni, A.; Smith, N.; Ma, A.; Ruiter, B.; Virkud, Y.; Schneider, M.; Shreffler, W.G. Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy. J. Allergy Clin. Immunol. 2019, 144, 1310–1319.e4. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, M.; Narisety, S.D.; Guerrerio, A.L.; Chichester, K.L.; Keet, C.A.; Bieneman, A.P.; Hamilton, R.G.; Wood, R.A.; Schroeder, J.T.; Frischmeyer-Guerrerio, P.A. Suppression of the immunologic response to peanut during immunotherapy is often transient. J. Allergy Clin. Immunol. 2015, 135, 1283–1292. [Google Scholar] [CrossRef]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar] [CrossRef]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International Consensus on Allergen Immunotherapy II: Mechanisms, standardization, and pharmacoeconomics. J. Allergy Clin. Immunol. 2016, 137, 358–368. [Google Scholar] [CrossRef]
- Akdis, C.A.; Blesken, T.; Akdis, M.; Wuthrich, B.; Blaser, K. Role of interleukin 10 in specific immunotherapy. J. Clin. Investig. 1998, 102, 98–106. [Google Scholar] [CrossRef]
- Nasser, S.M.; Ying, S.; Meng, Q.; Kay, A.B.; Ewan, P.W. Interleukin-10 levels increase in cutaneous biopsies of patients undergoing wasp venom immunotherapy. Eur. J. Immunol. 2001, 31, 3704–3713. [Google Scholar] [CrossRef]
- Patil, S.U.; Ogunniyi, A.O.; Calatroni, A.; Tadigotla, V.R.; Ruiter, B.; Ma, A.; Moon, J.; Love, J.C.; Shreffler, W.G. Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin. Immunol. 2015, 136, 125–134.e12. [Google Scholar] [CrossRef]
- Hoh, R.A.; Joshi, S.A.; Liu, Y.; Wang, C.; Roskin, K.M.; Lee, J.Y.; Pham, T.; Looney, T.J.; Jackson, K.J.; Dixit, V.P.; et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. J. Allergy Clin. Immunol. 2016, 137, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Komlosi, Z.I.; Kovacs, N.; Sokolowska, M.; van de Veen, W.; Akdis, M.; Akdis, C.A. Mechanisms of Subcutaneous and Sublingual Aeroallergen Immunotherapy: What is New? Immunol. Allergy Clin. N Am. 2020, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef] [PubMed]
- Globinska, A.; Boonpiyathad, T.; Satitsuksanoa, P.; Kleuskens, M.; van de Veen, W.; Sokolowska, M.; Akdis, M. Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Ann. Allergy Asthma Immunol. 2018, 121, 306–312. [Google Scholar] [CrossRef]
- Keskin, O.; Tuncer, A.; Adalioglu, G.; Sekerel, B.E.; Sackesen, C.; Kalayci, O. The effects of grass pollen allergoid immunotherapy on clinical and immunological parameters in children with allergic rhinitis. Pediatr. Allergy Immunol. 2006, 17, 396–407. [Google Scholar] [CrossRef]
- Durham, S.R.; Yang, W.H.; Pedersen, M.R.; Johansen, N.; Rak, S. Sublingual immunotherapy with once-daily grass allergen tablets: A randomized controlled trial in seasonal allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2006, 117, 802–809. [Google Scholar] [CrossRef]
- Gehlhar, K.; Schlaak, M.; Becker, W.; Bufe, A. Monitoring allergen immunotherapy of pollen-allergic patients: The ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin. Exp. Allergy 1999, 29, 497–506. [Google Scholar] [CrossRef]
- Rolinck-Werninghaus, C.; Kopp, M.; Liebke, C.; Lange, J.; Wahn, U.; Niggemann, B. Lack of detectable alterations in immune responses during sublingual immunotherapy in children with seasonal allergic rhinoconjunctivitis to grass pollen. Int. Arch. Allergy Immunol. 2005, 136, 134–141. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Mansueto, P.; Pacor, M.L.; Rizzo, M.; Castello, F.; Martinelli, N.; Ditta, V.; Bianco, C.L.; Leto-Barone, M.S.; D’Alcamo, A.; et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2009, 123, 1103–1110.e4. [Google Scholar] [CrossRef]
- Shamji, M.H.; Ljorring, C.; Francis, J.N.; Calderon, M.A.; Larche, M.; Kimber, I.; Frew, A.J.; Ipsen, H.; Lund, K.; Würtzen, P.A.; et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012, 67, 217–226. [Google Scholar] [CrossRef]
- Sanchez-Ruano, L.; de la Hoz, B.; Martinez-Botas, J. Clinical utility of microarray B-cell epitope mapping in food allergies: A systematic review. Pediatr. Allergy Immunol. 2020, 31, 175–185. [Google Scholar] [CrossRef]
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134.e3. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Kuitunen, M.; Valori, M.; Rantanen, V.; Bardina, L.; Gimenez, G.; Makela, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow’s milk allergy. Pediatr. Allergy Immunol. 2014, 25, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Botas, J.; Rodriguez-Alvarez, M.; Cerecedo, I.; Vlaicu, C.; Dieguez, M.C.; Gomez-Coronado, D.; Fernandez-Rivas, M.; de la Hoz, B. Identification of novel peptide biomarkers to predict safety and efficacy of cow’s milk oral immunotherapy by peptide microarray. Clin. Exp. Allergy 2015, 45, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Wilcock, L.K.; Wachholz, P.A.; Dearman, R.J.; Kimber, I.; Wurtzen, P.A.; Larché, M.; Durham, S.R.; Francis, J.N. The IgE-facilitated allergen binding (FAB) assay: Validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J. Immunol. Methods 2006, 317, 71–79. [Google Scholar] [CrossRef]
- Shamji, M.H.; Francis, J.N.; Wurtzen, P.A.; Lund, K.; Durham, S.R.; Till, S.J. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: Inhibition by blocking antibody responses after immunotherapy. J. Allergy Clin. Immunol. 2013, 132, 1003–1005.e4. [Google Scholar] [CrossRef]
- Petersen, A.B.; Gudmann, P.; Milvang-Gronager, P.; Morkeberg, R.; Bogestrand, S.; Linneberg, A.; Johansen, N. Performance evaluation of a specific IgE assay developed for the ADVIA centaur immunoassay system. Clin. Biochem. 2004, 37, 882–892. [Google Scholar] [CrossRef]
- Blaiss, M.; Maloney, J.; Nolte, H.; Gawchik, S.; Yao, R.; Skoner, D.P. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J. Allergy Clin. Immunol. 2011, 127, 64–71.e4. [Google Scholar] [CrossRef]
- Corzo, J.L.; Carrillo, T.; Pedemonte, C.; Plaza Martin, A.M.; Martin Hurtado, S.; Dige, E.; Calderon, M.A. Tolerability during double-blind randomized phase I trials with the house dust mite allergy immunotherapy tablet in adults and children. J. Investig. Allergol. Clin. Immunol. 2014, 24, 154–161. [Google Scholar]
- Wachholz, P.A.; Soni, N.K.; Till, S.J.; Durham, S.R. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 112, 915–922. [Google Scholar] [CrossRef]
- van Neerven, R.J.; Wikborg, T.; Lund, G.; Jacobsen, B.; Brinch-Nielsen, A.; Arnved, J.; Ipsen, H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999, 163, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Kepley, C.L.; Cambier, J.C.; Morel, P.A.; Lujan, D.; Ortega, E.; Wilson, B.S.; Oliver, J.M. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J. Allergy Clin. Immunol. 2000, 106, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.H.; Mertens, C.H.; Hagendorens, M.M.; Stevens, W.J.; De Clerck, L.S. Analyzing histamine release by flow cytometry (HistaFlow): A novel instrument to study the degranulation patterns of basophils. J. Immunol. Methods 2012, 375, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nullens, S.; Sabato, V.; Faber, M.; Leysen, J.; Bridts, C.H.; De Clerck, L.S.; Falcone, F.H.; Maurer, M.; Ebo, D.G. Basophilic histamine content and release during venom immunotherapy: Insights by flow cytometry. Cytom. B Clin. Cytom. 2013, 84, 173–178. [Google Scholar] [CrossRef]
- Cosmi, L.; Santarlasci, V.; Angeli, R.; Liotta, F.; Maggi, L.; Frosali, F.; Rossi, O.; Falagiani, P.; Riva, G.; Romagnani, S.; et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clin. Exp. Allergy 2006, 36, 261–272. [Google Scholar] [CrossRef]
- Fanta, C.; Bohle, B.; Hirt, W.; Siemann, U.; Horak, F.; Kraft, D.; Ebner, H.; Ebner, C. Systemic immunological changes induced by administration of grass pollen allergens via the oral mucosa during sublingual immunotherapy. Int. Arch. Allergy Immunol. 1999, 120, 218–224. [Google Scholar] [CrossRef]
- Jutel, M.; Akdis, M.; Budak, F.; Aebischer-Casaulta, C.; Wrzyszcz, M.; Blaser, K.; Akdis, C.A. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003, 33, 1205–1214. [Google Scholar] [CrossRef]
- Bohle, B.; Kinaciyan, T.; Gerstmayr, M.; Radakovics, A.; Jahn-Schmid, B.; Ebner, C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007, 120, 707–713. [Google Scholar] [CrossRef]
- Francis, J.N.; Till, S.J.; Durham, S.R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 111, 1255–1261. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Nouri-Aria, K.T.; Wilson, D.R.; Walker, S.M.; Verhoef, A.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology 2002, 105, 56–62. [Google Scholar] [CrossRef]
- Plewako, H.; Holmberg, K.; Oancea, I.; Gotlib, T.; Samolinski, B.; Rak, S. A follow-up study of immunotherapy-treated birch-allergic patients: Effect on the expression of chemokines in the nasal mucosa. Clin. Exp. Allergy. 2008, 38, 1124–1131. [Google Scholar] [CrossRef]
- Makino, Y.; Noguchi, E.; Takahashi, N.; Matsumoto, Y.; Kubo, S.; Yamada, T.; Imoto, Y.; Ito, Y.; Osawa, Y.; Shibasaki, M.; et al. Apolipoprotein A-IV is a candidate target molecule for the treatment of seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2010, 126, 1163–1169.e5. [Google Scholar] [CrossRef]
- Salmivesi, S.; Paassilta, M.; Huhtala, H.; Nieminen, R.; Moilanen, E.; Korppi, M. Changes in biomarkers during a six-month oral immunotherapy intervention for cow’s milk allergy. Acta Paediatr. 2016, 105, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, E.; He, M. Cytokine Responses to Specific Immunotherapy in House Dust Mite-Induced Allergic Rhinitis Patients. Inflammation 2015, 38, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.W.; Eifan, A.O.; Lao-Araya, M.; Penagos, M.; Poon, S.Y.; Steveling, E.; Yan, R.; Switzer, A.; Phippard, D.; Togias, A.; et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy 2015, 70, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; De Amici, M.; Murdaca, G.; Filaci, G.; Fenoglio, D.; Marseglia, G.L. Adipokines and sublingual immunotherapy: Preliminary report. Hum. Immunol. 2009, 70, 73–78. [Google Scholar] [CrossRef]
- Ciprandi, G.; Continia, P.; Fenoglio, D.; Sormani, M.P.; Negrini, S.; Puppo, F.; Indiveri, F. Relationship between soluble HLA-G and HLA-A,-B,-C serum levels, and interferon-gamma production after sublingual immunotherapy in patients with allergic rhinitis. Hum. Immunol. 2008, 69, 409–413. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W. The role of regulatory B cells in allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 447–452. [Google Scholar] [CrossRef]
- Sindher, S.B.; Long, A.; Acharya, S.; Sampath, V.; Nadeau, K.C. The Use of Biomarkers to Predict Aero-Allergen and Food Immunotherapy Responses. Clin. Rev. Allergy Immunol. 2018, 55, 190–204. [Google Scholar] [CrossRef]
- Rosner-Friese, K.; Kaul, S.; Vieths, S.; Pfaar, O. Environmental exposure chambers in allergen immunotherapy trials: Current status and clinical validation needs. J. Allergy Clin. Immunol. 2015, 135, 636–643. [Google Scholar] [CrossRef]
- Zieglmayer, P.U.; Pfaar, O. Update on the use of allergen challenge chambers in immunotherapy: Clinical implications. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Bergmann, K.C.; Bonini, S.; Compalati, E.; Domis, N.; de Blay, F.; de Kam, P.; Devillier, P.; Durham, S.R.; Ellis, A.K.; et al. Technical standards in allergen exposure chambers worldwide—An EAACI Task Force Report. Allergy 2021, 76, 3589–3612. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Leon, B.; Martinez San Ireneo, M.; de la Roca, F.; Arenas, L.; Alfaya Arias, T.; Cordobes, C.; Marqués, L.; Vega, A.; Moreno-Aguila, C. The Lights and the Shadows of Controlled Sting Challenge With Hymenoptera. J. Investig. Allergol. Clin. Immunol. 2022, 32, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, R.; Kemeny, D.M.; Richards, D. Sub-class of IgG anti-bee venom antibody produced during bee venom immunotherapy and its relationship to long-term protection from bee stings and following termination of venom immunotherapy. Clin. Allergy 1986, 16, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.; Kwiterovich, K.A.; Kagey-Sobotka, A.; Valentine, M.D.; Lichtenstein, L.M. Discontinuing venom immunotherapy: Outcome after five years. J. Allergy Clin. Immunol. 1996, 97, 579–587. [Google Scholar] [CrossRef] [PubMed]
- van Halteren, H.K.; van der Linden, P.W.; Burgers, J.A.; Bartelink, A.K. Discontinuation of yellow jacket venom immunotherapy: Follow-up of 75 patients by means of deliberate sting challenge. J. Allergy Clin. Immunol. 1997, 100, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Teufel, M.; Feidt, A.; Giel, K.E.; Zipfel, S.; Biedermann, T. Tolerated wasp sting challenge improves health-related quality of life in patients allergic to wasp venom. J. Allergy Clin. Immunol. 2013, 132, 489–490. [Google Scholar] [CrossRef]
- Swamy, R.S.; Reshamwala, N.; Hunter, T.; Vissamsetti, S.; Santos, C.B.; Baroody, F.M.; Hwang, P.H.; Hoyte, E.G.; Garcia, M.A.; Nadeau, K.C. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J. Allergy Clin. Immunol. 2012, 130, 215–224.e7. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Pan, C.; Ma, T.T.; Chen, Y.L.; Yan, W.J.; Liu, J.G.; Cao, M.-D.; Huang, H.-D.; Wang, D.-Y.; Wang, X.-Y.; et al. Clinical Efficacy Evaluation of 1-Year Subcutaneous Immunotherapy for Artemisia sieversiana Pollen Allergic Rhinitis by Serum Metabolomics. Front. Pharmacol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Zheng, P.; Bian, X.; Zhai, Y.; Li, C.; Li, N.; Hao, C.; Huang, H.; Luo, W.; Huang, Z.; Liao, C.; et al. Metabolomics reveals a correlation between hydroxyeicosatetraenoic acids and allergic asthma: Evidence from three years’ immunotherapy. Pediatr. Allergy Immunol. 2021, 32, 1654–1662. [Google Scholar] [CrossRef]
- Pfaar, O.; Bonini, S.; Cardona, V.; Demoly, P.; Jakob, T.; Jutel, M.; Kleine-Tebbe, J.; Klimek, L.; Klysner, S.; Kopp, M.V.; et al. Perspectives in allergen immunotherapy: 2017 and beyond. Allergy 2018, 73 (Suppl. 104), 5–23. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Walker, S.M.; Varga, E.M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Pilette, C.; Nouri-Aria, K.T.; Jacobson, M.R.; Wilcock, L.K.; Detry, B.; Walker, S.M.; Francis, J.N.; Durham, S.R. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J. Immunol. 2007, 178, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Yonekura, S.; Horiguchi, S.; Taniguchi, Y.; Saito, A.; Yasueda, H.; Inamine, A.; Nakayama, T.; Takemori, T.; Taniguchi, M.; et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin. Immunol. 2011, 139, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Eifan, A.O.; Akkoc, T.; Yildiz, A.; Keles, S.; Ozdemir, C.; Bahceciler, N.N.; Barlan, I.B. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: An open randomized controlled trial. Clin. Exp. Allergy 2010, 40, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, M.; Yue, W.; Zhou, J.; Li, R.; Lin, J.; Li, Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int. Arch. Allergy Immunol. 2014, 164, 210–217. [Google Scholar] [CrossRef]
- Gomez, E.; Fernandez, T.D.; Dona, I.; Rondon, C.; Campo, P.; Gomez, F.; Salas, M.; Gonzalez, M.; Perkins, J.R.; Palomares, F.; et al. Initial immunological changes as predictors for house dust mite immunotherapy response. Clin. Exp. Allergy 2015, 45, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Moverare, R.; Elfman, L.; Vesterinen, E.; Metso, T.; Haahtela, T. Development of new IgE specificities to allergenic components in birch pollen extract during specific immunotherapy studied with immunoblotting and Pharmacia CAP System. Allergy 2002, 57, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.S.; Nolte, H.; Creticos, P.; Maloney, J.; Wu, J.; Bernstein, D.I. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J. Allergy Clin. Immunol. 2011, 127, 72–80.e2. [Google Scholar] [CrossRef]
- Wollmann, E.; Lupinek, C.; Kundi, M.; Selb, R.; Niederberger, V.; Valenta, R. Reduction in allergen-specific IgE binding as measured by microarray: A possible surrogate marker for effects of specific immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 806–809.e7. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004, 172, 3252–3559. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Emminger, W.; Kapp, A.; de Monchy, J.G.; Rak, S.; Scadding, G.K.; Wurtzen, P.A.; Andersen, J.S.; Tholstrup, B.; Riis, B.; et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J. Allergy Clin. Immunol. 2012, 129, 717–725.e5. [Google Scholar] [CrossRef] [PubMed]
- Wurtzen, P.A.; Lund, G.; Lund, K.; Arvidsson, M.; Rak, S.; Ipsen, H. A double-blind placebo-controlled birch allergy vaccination study II: Correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin. Exp. Allergy 2008, 38, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Gessner, C.; Kroker, A.; Schwab, J.A.; Pohl, W.; Villesen, H.; Wüstenberg, E.; Emminger, W. Immunologic effects and tolerability profile of in-season initiation of a standardized-quality grass allergy immunotherapy tablet: A phase III, multicenter, randomized, double-blind, placebo-controlled trial in adults with grass pollen-induced rhinoconjunctivitis. Clin. Ther. 2011, 33, 828–840. [Google Scholar]
- Van Overtvelt, L.; Baron-Bodo, V.; Horiot, S.; Moussu, H.; Ricarte, C.; Horak, F.; Zieglmayer, P.; Zieglmayer, R.; Montagut, A.; Galvain, S.; et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy 2011, 66, 1530–1537. [Google Scholar] [CrossRef]
- Buhring, H.J.; Streble, A.; Valent, P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int. Arch. Allergy Immunol. 2004, 133, 317–329. [Google Scholar] [CrossRef]
- Knol, E.F.; Mul, F.P.; Jansen, H.; Calafat, J.; Roos, D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J. Allergy Clin. Immunol. 1991, 88, 328–338. [Google Scholar] [CrossRef]
- Hennersdorf, F.; Florian, S.; Jakob, A.; Baumgartner, K.; Sonneck, K.; Nordheim, A.; Biedermann, T.; Valent, P.; Bühring, H.-J. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005, 15, 325–335. [Google Scholar] [CrossRef]
- Kepil Ozdemir, S.; Sin, B.A.; Guloglu, D.; Ikinciogullari, A.; Gencturk, Z.; Misirligil, Z. Short-term preseasonal immunotherapy: Is early clinical efficacy related to the basophil response? Int. Arch. Allergy Immunol. 2014, 164, 237–245. [Google Scholar] [CrossRef]
- Aasbjerg, K.; Backer, V.; Lund, G.; Holm, J.; Nielsen, N.C.; Holse, M.; Wagtmann, V.R.; Würtzen, P.A. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin. Exp. Allergy 2014, 44, 417–428. [Google Scholar] [CrossRef]
- Ceuppens, J.L.; Bullens, D.; Kleinjans, H.; van der Werf, J.; The PURETHAL Birch Efficacy Study Group. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: Clinical and immunological effects. Clin. Exp. Allergy 2009, 39, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, C.; Bouley, J.; Moussu, H.; Luce, S.; Duchateau, M.; Chamot-Rooke, J.; Pallardy, M.; Lombardi, V.; Nony, E.; Baron-Bodo, V.; et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J. Allergy Clin. Immunol. 2016, 137, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.W.; Calderon, M.A.; Shamji, M.H.; Eifan, A.O.; Penagos, M.; Dumitru, F.; Sever, M.L.; Bahnson, H.T.; Lawson, K.; Harris, K.M.; et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. JAMA 2017, 317, 615–625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).