Peri-Interventional Triple Therapy with Dabigatran Modifies Vasomotion after Bare-Metal Stent Implantation in a Pig Coronary Artery Model
Abstract
1. Introduction
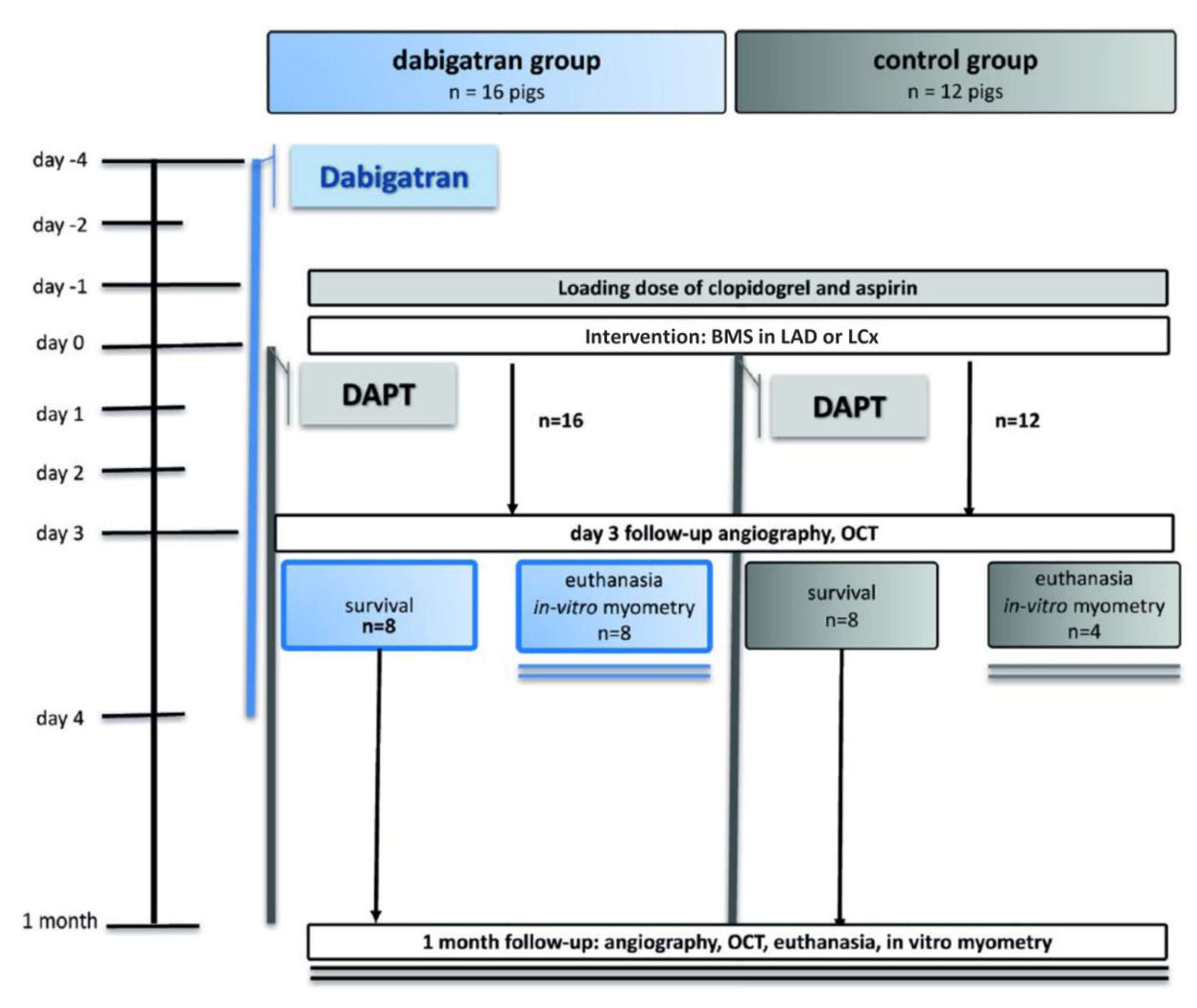
2. Materials and Methods
2.1. Drug Dosing
2.2. PCI
2.3. Follow-Up
2.4. Analyses
2.4.1. Quantitative Coronary Angiography
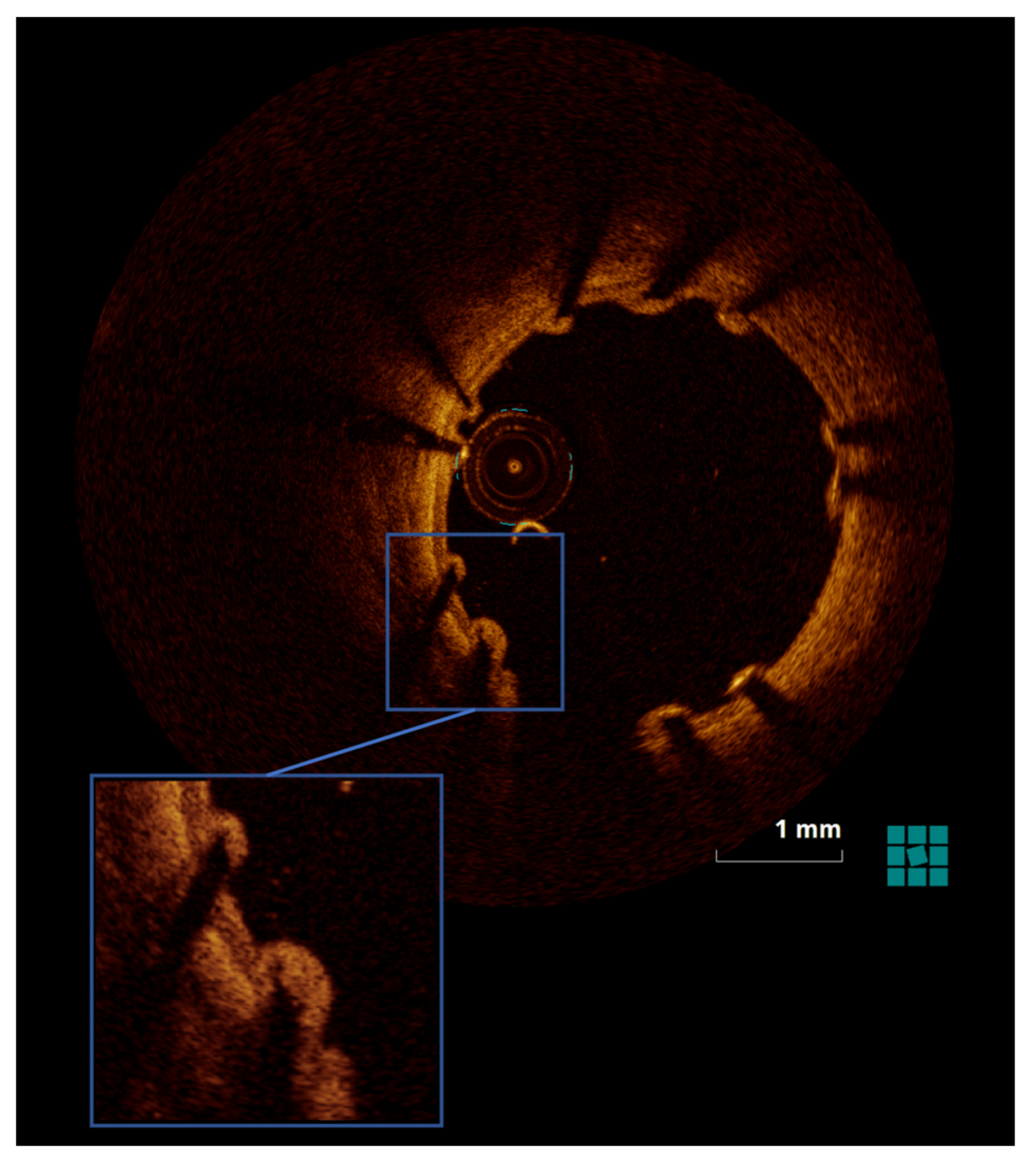
2.4.2. OCT
2.4.3. Histomorphometry and Histopathology
2.4.4. qPCR
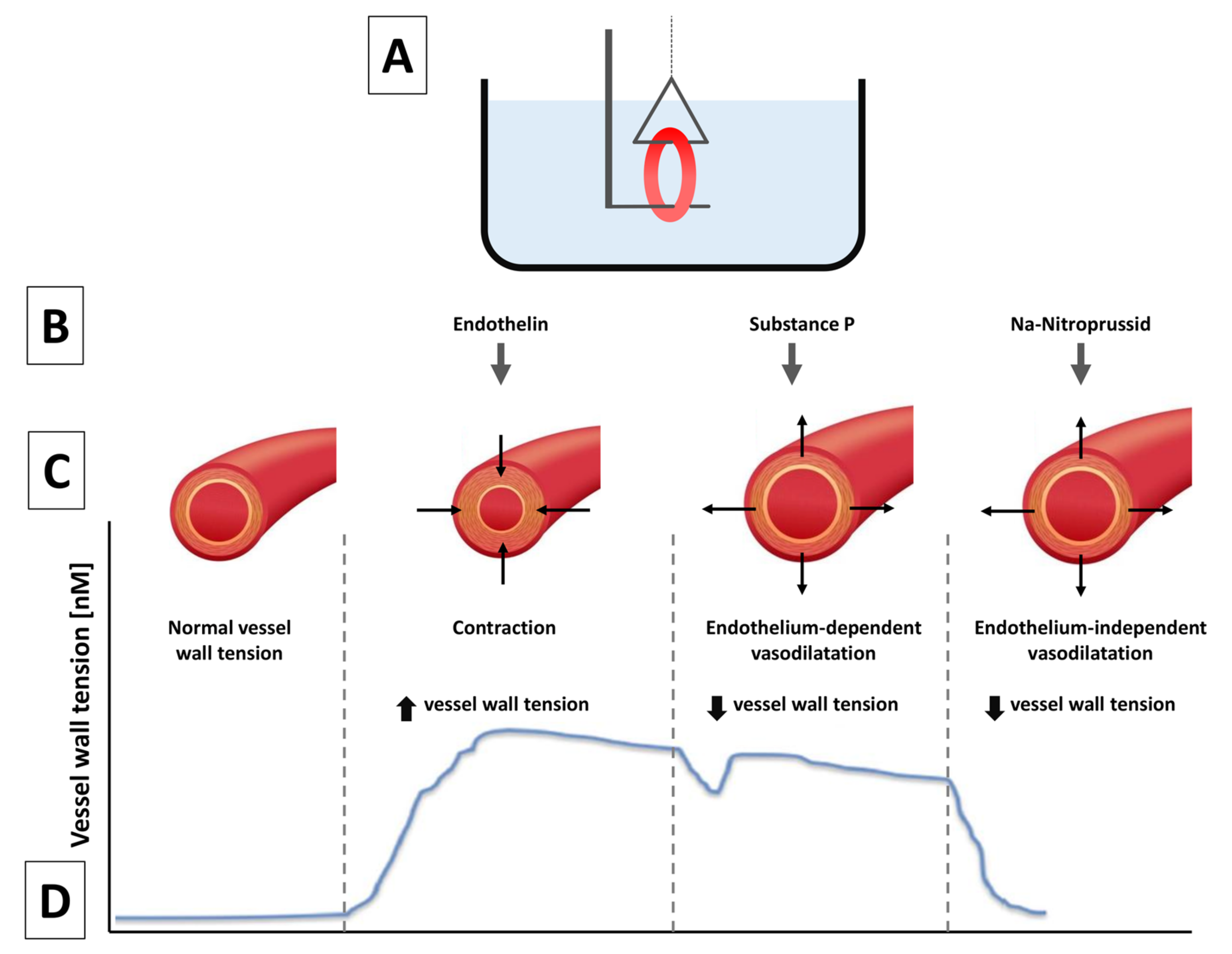
2.4.5. Vasomotor Responses
2.4.6. Statistics
3. Results
3.1. Measurements
3.1.1. Quantitative Coronary Angiography
3.1.2. OCT
3.1.3. Histomorphometry and Histopathology
3.1.4. Vasomotor Edge Response
3.1.5. qPCR Results for MCP-1, PAR-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [PubMed]
- Stefanini, G.G.; Byrne, R.A.; Windecker, S.; Kastrati, A. State of the art: Coronary artery stents-past, present and future. EuroIntervention 2017, 13, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.-P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.A.; et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 366, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hemetsberger, R.; Farhan, S.; Lukovic, D.; Zlabinger, K.; Hajagos-Toth, J.; Bota, J.; Garcia-Garcia, H.M.; Ay, C.; Samaha, E.; Gaspar, R.; et al. Peri-interventional Triple Therapy With Dabigatran Improves Vasomotion and Promotes Endothelialization in Porcine Coronary Stenting Model. Front. Cardiovasc. Med. 2021, 8, 690476. [Google Scholar] [CrossRef] [PubMed]
- McKellar, S.H. Effectiveness of dabigatran etexilate for thromboprophy-laxis of mechanical heart valves. J. Thorac. Cardiovasc. Surg. 2011, 141, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Schober, A. Chemokines in vascular remodeling. Thromb. Haemost. 2007, 97, 730–737. [Google Scholar] [CrossRef]
- Blanchard, D.; Danzi, G.; Urban, P.; Moseri, M.; Juergens, C.; Guyon, P.; Nowak, B.; Tresucosol, D.; Suttorp, M.; Farshid, A.; et al. A novel ultra-thin bare metal stent (BMS): Results from a worldwide registry. Eurointervention 2007, 3, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Carter, A. Drug-eluting stents in preclinical studies: Updated consensus recommendations for preclinical evaluation. Circ. Cardiovasc. Interv. 2008, 1, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Karim, M.A.; Edwards, W.D.; Schwartz, R.S. Relationship of vascular thrombosis and inflammatory leukocyte infil-tration to neointimal growth following porcine coronary artery stent placement. Atherosclerosis 1996, 124, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Taubman, M.B.; Rollins, B.J.; Poon, M.; Marmur, J.; Green, R.S.; Berk, B.C.; Nadal-Ginard, B.; Green, R.S.; Berk, B.C.; Nadal-Ginard, B. JE mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Circ. Res. 1992, 70, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Matsumori, A.; Ohashi, N.; Shioi, T.; Ono, K.; Harada, A.; Matsushima, K.; Sasayama, S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ. Res. 1999, 84, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L.; Schofield, L.M.; Weber, D.K.; Kolodgie, F.; Virmani, R.; Khaw, B.A. Uptake of 111In-Z2D3 on SPECT imaging in a swine model of coronary stent restenosis correlated with cell proliferation. J. Nucl. Med. 2004, 45. [Google Scholar]
- Nakazawa, G. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 2011, 57, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Pavo, N.; Syeda, B.; Bernhart, A.; Szentirmai, E.; Hemetsberger, R.; Samaha, E.; Plass, C.; Zlabinger, K.; Pavo, I. Preclinical randomised safety, efficacy and physiologic study of the silicon dioxide inert-coated Axetis and bare metal stent: Short-, mid- and long-term outcome. EuroIntervention 2015, 11, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Thanyasiri, P.; Kathir, K.; Celermajer, D.S.; Adams, M.R. Endothelial dysfunction and restenosis following percutaneous coronary intervention. Int. J. Cardiol. 2007, 119, 362–367. [Google Scholar] [PubMed]
- Plass, C.A.; Sabdyusheva-Litschauer, I.; Bernhart, A.; Samaha, E. Time Course of Endothelium-Dependent and -Independent Coronary Vasomotor Response to Coronary Balloons and Stents. JACC: Cardiovasc. Interv. 2012, 5, 741–751. [Google Scholar] [PubMed]
- Plass, C.A.; Schmid, W.; Holy, E.W.; Kreatschitsch, U.; Laggner, H.; Volf, I. Redox-sensitive impairment of porcine coronary artery vasodilation by hypochlorite-modified LDL. Atherosclerosis 2007, 190, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.; Hemetsberger, R.; Matiasek, J.; Strehblow, C.; Pavo, N.; Khorsand, A.; Petrási, Z.; Kaider, A.; Glogar, D. Implantation of paclitaxel-eluting stent impairs the vascular compliance of arteries in porcine coronary stenting model. Atherosclerosis 2009, 202, 144–151. [Google Scholar] [CrossRef] [PubMed]
| Immediately after PCI | Day 3 after PCI | |
|---|---|---|
| Dabigatran group | 3.17 ± 4.54 | 5.81 ± 3.27 |
| Control group | 11.60 ± 10.64 | 3.63 ± 1.41 |
| P | 0.044 | 0.120 |
| Inflammation | Fibrin Deposition | Endothelialization (%) | ||||
|---|---|---|---|---|---|---|
| Day 3 | 1 Month | Day 3 | 1 Month | Day 3 | 1 Month | |
| Dabigatran group | 0.55 ± 1.01 | 1.67 ± 1.86 | 1.44 ± 0.73 | 0.50 ± 0.84 | 38.89 ± 30.90 | 100 ± 0 |
| Control group | 1.00 ± 1.15 | 0.38 ± 0.52 | 1.75 ± 0.50 | 0.88 ± 0.64 | 25.00 ± 20.41 | 100 ± 0 |
| P | 0.48 | 0.16 | 0.47 | 0.26 | 0.50 | 1.0 |
| Day 3 Non-PCI Coronary | Day 3 BMS | 1 Month BMS | |
|---|---|---|---|
| Endothelin-induced constriction [mN] | |||
| Dabigatran group | 11.18 ± 3.95 | 10.97 ± 3.85 | 9.03 ± 4.94 |
| Control group | 9.01 ± 2.46 | 7.32 ± 5.41 | 8.10 ± 3.14 |
| P | 0.54 | 0.03 | 0.45 |
| Endothelium-dependent vasodilatation (substance P–induced vasodilatation [%]) | |||
| Dabigatran group | 78 ± 18 | 63 ± 28 | 59 ± 30 |
| Control group | 60 ± 34 | 72 ± 50 | 57 ± 29 |
| P | 0.07 | 0.53 | 0.82 |
| Endothelium-independent vasodilatation (Na-nitroprusside–induced vasodilatation [mN/s/mN]) | |||
| Dabigatran group | 0.15 ± 0.09 | 0.081 ± 0.047 | 0.15 ± 0.11 |
| Control group | 0.13 ± 0.06 | 0.097 ± 0.033 | 0.14 ± 0.06 |
| P | 0.42 | 0.33 | 0.47 |
| MCP-1 (Log Fold Change) | PAR-1 (Log Fold Change) | |
|---|---|---|
| Dabigatran group | 0.84 ± 0.23 | −0.24 ± 0.19 |
| Control group | 0.14 ± 0.50 | −0.27 ± 0.31 |
| P | 0.045 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemetsberger, R.; Farhan, S.; Lukovic, D.; Zlabinger, K.; Hajagos-Toth, J.; Bota, J.; Garcia-Garcia, H.M.; Ay, C.; Samaha, E.; Gaspar, R.; et al. Peri-Interventional Triple Therapy with Dabigatran Modifies Vasomotion after Bare-Metal Stent Implantation in a Pig Coronary Artery Model. J. Pers. Med. 2023, 13, 280. https://doi.org/10.3390/jpm13020280
Hemetsberger R, Farhan S, Lukovic D, Zlabinger K, Hajagos-Toth J, Bota J, Garcia-Garcia HM, Ay C, Samaha E, Gaspar R, et al. Peri-Interventional Triple Therapy with Dabigatran Modifies Vasomotion after Bare-Metal Stent Implantation in a Pig Coronary Artery Model. Journal of Personalized Medicine. 2023; 13(2):280. https://doi.org/10.3390/jpm13020280
Chicago/Turabian StyleHemetsberger, Rayyan, Serdar Farhan, Dominika Lukovic, Katrin Zlabinger, Judit Hajagos-Toth, Judit Bota, Hector M. Garcia-Garcia, Cihan Ay, Eslam Samaha, Robert Gaspar, and et al. 2023. "Peri-Interventional Triple Therapy with Dabigatran Modifies Vasomotion after Bare-Metal Stent Implantation in a Pig Coronary Artery Model" Journal of Personalized Medicine 13, no. 2: 280. https://doi.org/10.3390/jpm13020280
APA StyleHemetsberger, R., Farhan, S., Lukovic, D., Zlabinger, K., Hajagos-Toth, J., Bota, J., Garcia-Garcia, H. M., Ay, C., Samaha, E., Gaspar, R., Garamvölgyi, R., Huber, K., Spannbauer, A., & Gyöngyösi, M. (2023). Peri-Interventional Triple Therapy with Dabigatran Modifies Vasomotion after Bare-Metal Stent Implantation in a Pig Coronary Artery Model. Journal of Personalized Medicine, 13(2), 280. https://doi.org/10.3390/jpm13020280







