In Vivo Validation of a Novel Computational Approach to Assess Microcirculatory Resistance Based on a Single Angiographic View
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. μQFR and AMR Computation
2.3. Wire-Derived IMR Measurement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
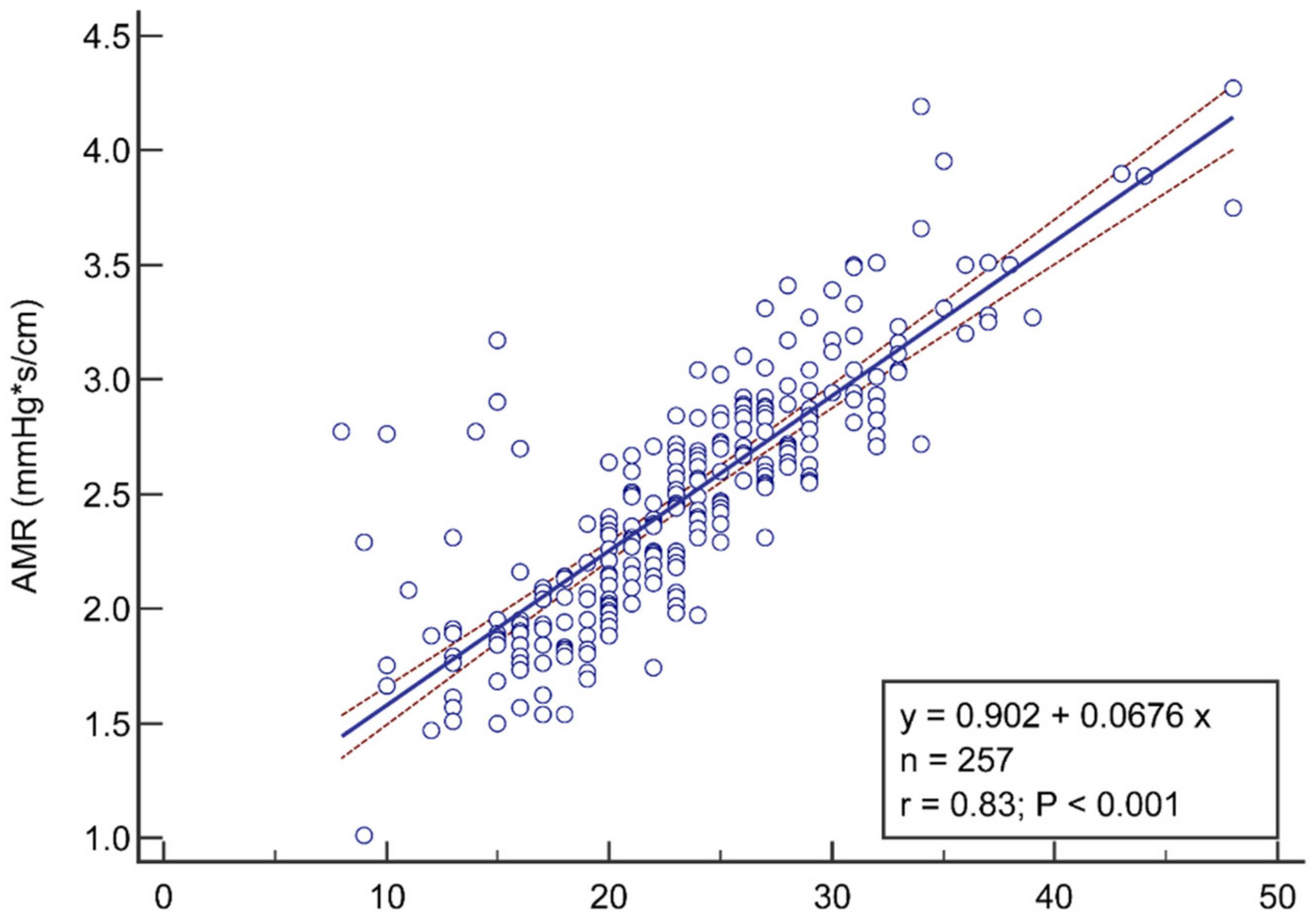
3.2. Correlation and Diagnostic Performance of AMR
3.3. The Influence of Vessel Type and Clinical Presentations on Sensitivity and Specificity of AMR
3.4. Reproducibility of AMR Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaski, J.C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of Ischemic Heart Disease. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef]
- Ford, T.J.; Ong, P.; Sechtem, U.; Beltrame, J.; Camici, P.G.; Crea, F.; Kaski, J.C.; Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; et al. Assessment of Vascular Dysfunction in Patients without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc. Interv. 2020, 13, 1847–1864. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- Scarsini, R.; Shanmuganathan, M.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Burrage, M.K.; Terentes-Printzios, D.; Langrish, J.; Lucking, A.; Fahrni, G.; et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and CMR After STEMI Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.J.; Whitbourn, R.J.; Burns, A.T.; Somaratne, J.; Leitl, G.; Macisaac, A.I.; Wilson, A. The index of microvascular resistance identifies patients with periprocedural myocardial infarction in elective percutaneous coronary intervention. Heart 2012, 98, 1492–1497. [Google Scholar] [CrossRef]
- Pighi, M.; Gratta, A.; Marin, F.; Bellamoli, M.; Lunardi, M.; Fezzi, S.; Zivelonghi, C.; Pesarini, G.; Tomai, F.; Ribichini, F. Cardiac allograft vasculopathy: Pathogenesis, diagnosis and therapy. Transplant. Rev. 2020, 34, 100569. [Google Scholar] [CrossRef] [PubMed]
- Tona, F.; Osto, E.; Famoso, G.; Previato, M.; Fedrigo, M.; Vecchiati, A.; Perazzolo Marra, M.; Tellatin, S.; Bellu, R.; Tarantini, G.; et al. Coronary microvascular dysfunction correlates with the new onset of cardiac allograft vasculopathy in heart transplant patients with normal coronary angiography. Am. J. Transplant. 2015, 15, 1400–1406. [Google Scholar] [CrossRef]
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Aarnoudse, W.; Pijls, N.H.; De Bruyne, B.; Balsam, L.B.; Cooke, D.T.; Robbins, R.C.; Fitzgerald, P.J.; Yeung, A.C.; Yock, P.G. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: Experimental validation. Circulation 2004, 109, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Ribichini, F.; Ferreira, V.M.; Channon, K.M.; et al. Angiography-derived index of microcirculatory resistance (IMR). Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Tebaldi, M.; Biscaglia, S.; Di Girolamo, D.; Erriquez, A.; Penzo, C.; Tumscitz, C.; Campo, G. Angio-Based Index of Microcirculatory Resistance for the Assessment of the Coronary Resistance: A Proof of Concept Study. J. Interv. Cardiol. 2020, 2020, 8887369. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Lee, J.M.; Choi, K.H.; Lee, S.H.; Wang, L.; Kakuta, T.; Koo, B.K.; Escaned, J. Coronary microcirculation assessment using functional angiography: Development of a wire-free method applicable to conventional coronary angiograms. Catheter. Cardiovasc. Interv. 2021, 98, 1027–1037. [Google Scholar] [CrossRef]
- Fernández-Peregrina, E.; Garcia-Garcia, H.M.; Sans-Rosello, J.; Sanz-Sanchez, J.; Kotronias, R.; Scarsini, R.; Echavarria-Pinto, M.; Tebaldi, M.; De Maria, G.L. Angiography-derived versus invasively-determined index of microcirculatory resistance in the assessment of coronary microcirculation: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2022, 99, 2018–2025. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; Pu, J.; et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef]
- Tu, S.; Echavarria-Pinto, M.; von Birgelen, C.; Holm, N.R.; Pyxaras, S.A.; Kumsars, I.; Lam, M.K.; Valkenburg, I.; Toth, G.G.; Li, Y.; et al. Fractional flow reserve and coronary bifurcation anatomy: A novel quantitative model to assess and report the stenosis severity of bifurcation lesions. JACC Cardiovasc. Interv. 2015, 8, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Ding, D.; Chang, Y.; Li, C.; Wijns, W.; Xu, B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: A novel method based on bifurcation fractal law. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97 (Suppl. S2), 1040–1047. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Westra, J.; Ding, D.; Zhao, Q.; Yang, J.; Sun, Z.; Huang, J.; Pu, J.; Xu, B. Automatic coronary blood flow computation: Validation in quantitative flow ratio from coronary angiography. Int. J. Cardiovasc. Imaging 2019, 35, 587–595. [Google Scholar] [CrossRef]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Okada, K.; Kobashigawa, J.A.; Kobayashi, Y.; Luikart, H.; Sana, S.; Daun, T.; Chmura, S.A.; Sinha, S.; Cohen, G.; et al. Angiotensin-Converting Enzyme Inhibition Early After Heart Transplantation. J. Am. Coll. Cardiol. 2017, 69, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Sans-Roselló, J.; Fernández-Peregrina, E.; Duran-Cambra, A.; Carreras-Mora, J.; Sionis, A.; Álvarez-García, J.; García-García, H.M. Prognostic Value of Microvascular Resistance at Rest in Patients With Takotsubo Syndrome. JACC Cardiovasc. Imaging 2022, 15, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, G.; Fezzi, S.; Widmann, M.; Lia, M.; Rizzetto, F.; Mammone, C.; Pazzi, S.; Piccolo, S.; Galli, V.; Pighi, M.; et al. Angiography-derived Index of Microvascular Resistance (IMR) in Takotsubo syndrome. Int. J. Cardiovasc. Imaging 2022, 43, ehac544-1133. [Google Scholar] [CrossRef]
- Demir, O.M.; Boerhout, C.K.M.; de Waard, G.A.; van de Hoef, T.P.; Patel, N.; Beijk, M.A.M.; Williams, R.; Rahman, H.; Everaars, H.; Kharbanda, R.K.; et al. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc. Interv. 2022, 15, 1060–1070. [Google Scholar] [CrossRef]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison with [15O] H2O PET. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.P.; de Waard, G.A.; De Silva, K.; Lumley, M.; Asrress, K.; Arri, S.; Ellis, H.; Mir, A.; Clapp, B.; Chiribiri, A.; et al. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction or Stable Angina Pectoris. Am. J. Cardiol. 2018, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Fezzi, S.; Pesarini, G.; Del Sole, P.A.; Venturi, G.; Mammone, C.; Marcoli, M.; Gambaro, A.; Tavella, D.; Pighi, M.; et al. Impact of physiologically diffuse versus focal pattern of coronary disease on quantitative flow reserve diagnostic accuracy. Catheter. Cardiovasc. Interv. 2021, 99, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Carrasco-Moraleja, M.; Aparisi, A.; Rodriguez-Gabella, T.; Campo, A.; Gutiérrez, H.; Julca, F.; Gómez, I.; San Román, J.A.; Amat-Santos, I.J. Quantitative flow ratio-Meta-analysis and systematic review. Catheter. Cardiovasc. Interv. 2021, 97, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, S.; Huang, J.; Lunardi, M.; Ding, D.; Ribichini, F.; Tu, S.; Wijns, W. Coronary physiology in the catheterisation laboratory: An A to Z practical guide. AsiaIntervention 2022, 8, 86–109. [Google Scholar] [CrossRef]
- Mejía-Rentería, H.; Lee, J.M.; Lauri, F.; van der Hoeven, N.W.; de Waard, G.A.; Macaya, F.; Pérez-Vizcayno, M.J.; Gonzalo, N.; Jiménez-Quevedo, P.; Nombela-Franco, L.; et al. Influence of Microcirculatory Dysfunction on Angiography-Based Functional Assessment of Coronary Stenoses. JACC Cardiovasc. Interv. 2018, 11, 741–753. [Google Scholar] [CrossRef]




| Patient Characteristics | n = 163 |
|---|---|
| Age, years | 62.8 ± 11.4 |
| Male | 105 (64.4%) |
| LVEF% | 58.0 ± 7.6 |
| Hypertension | 107 (65.6%) |
| Hyperlipidemia | 58 (35.6%) |
| Diabetes mellitus | 41 (25.2%) |
| Smoking history | 58 (35.6%) |
| Previous PCI | 10 (6.1%) |
| Previous CABG | 0 (0.0%) |
| Clinical presentation | n = 163 |
| ACS | 121 (74.2%) |
| Myocardial infarction | 80 (49.1%) |
| STEMI | 44 (27.0%) |
| MI ≤ 7d | 41 (25.2%) |
| MI > 7d | 3 (1.8%) |
| NSTEMI | 36 (22.1%) |
| MI ≤ 7d | 3 (1.8%) |
| MI > 7d | 33 (20.3%) |
| Unstable angina | 41 (25.2%) |
| CCS | 42 (25.8%) |
| Obstructive CAD | 28 (17.2%) |
| Non-obstructive CAD | 14 (8.6%) |
| Vessel | |
|---|---|
| LAD | 119 (46.3%) |
| LCX | 60 (23.3%) |
| RCA | 78 (30.3%) |
| FFR | 0.91 (0.87, 0.96) |
| QFR | 0.94 (0.91, 0.97) |
| Flow velocity, cm/s | 16.87 ± 5.42 |
| IMR | 23.6 ± 6.8 |
| AMR, mmHg*s/cm | 2.5 ± 0.5 |
| Reference vessel diameter, mm | 2.63 ± 0.68 |
| MLD, mm | 1.87 ± 0.55 |
| DS, % | 28.78 ± 9.24 |
| Length, mm | 14.53 ± 9.08 |
| Best Cutoff Value for AMR | AMR > 2.5 |
|---|---|
| Accuracy, % (95% CI) | 87.2 (83.0–91.3) |
| Sensitivity, % (95% CI) | 93.5 (87.0–97.3) |
| Specificity, % (95% CI) | 82.7 (75.6–88.4) |
| PPV, % (95% CI) | 79.4 (71.2–86.1) |
| NPV, % (95% CI) | 94.7 (89.3–97.8) |
| +LR, (95% CI) | 5.39 (3.80–7.70) |
| −LR, (95% CI) | 0.08 (0.04–0.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Fezzi, S.; Sun, P.; Ding, N.; Li, X.; Hu, X.; Wang, S.; Wijns, W.; Lu, Z.; Tu, S. In Vivo Validation of a Novel Computational Approach to Assess Microcirculatory Resistance Based on a Single Angiographic View. J. Pers. Med. 2022, 12, 1798. https://doi.org/10.3390/jpm12111798
Fan Y, Fezzi S, Sun P, Ding N, Li X, Hu X, Wang S, Wijns W, Lu Z, Tu S. In Vivo Validation of a Novel Computational Approach to Assess Microcirculatory Resistance Based on a Single Angiographic View. Journal of Personalized Medicine. 2022; 12(11):1798. https://doi.org/10.3390/jpm12111798
Chicago/Turabian StyleFan, Yongzhen, Simone Fezzi, Pengcheng Sun, Nan Ding, Xiaohui Li, Xiaorong Hu, Shuang Wang, William Wijns, Zhibing Lu, and Shengxian Tu. 2022. "In Vivo Validation of a Novel Computational Approach to Assess Microcirculatory Resistance Based on a Single Angiographic View" Journal of Personalized Medicine 12, no. 11: 1798. https://doi.org/10.3390/jpm12111798
APA StyleFan, Y., Fezzi, S., Sun, P., Ding, N., Li, X., Hu, X., Wang, S., Wijns, W., Lu, Z., & Tu, S. (2022). In Vivo Validation of a Novel Computational Approach to Assess Microcirculatory Resistance Based on a Single Angiographic View. Journal of Personalized Medicine, 12(11), 1798. https://doi.org/10.3390/jpm12111798







