Abstract
Due to the rich vascularization and lymphatic drainage of the pulmonary tissue, lung metastases (LM) are not uncommon in patients with cancer. Radiomics is an active research field aimed at the extraction of quantitative data from diagnostic images, which can serve as useful imaging biomarkers for a more effective, personalized patient care. Our purpose is to illustrate the current applications, strengths and weaknesses of radiomics for lesion characterization, treatment planning and prognostic assessment in patients with LM, based on a systematic review of the literature.
1. Introduction
The lung is the second commonest site for metastases from extra-thoracic cancers, occurring in 20–54% of metastatic patients. Pulmonary involvement may result from direct tumor invasion or from hematogenous or lymphatic spread of tumor cells. In the adult population, the most frequent primary tumors that disseminate to the lung include breast, colorectal, renal, uterine cancer, leiomyosarcoma and head and neck carcinoma. In limited cases, the primary tumor origin cannot be identified.
Treatment options for patients with lung metastases (LM) include surgery, radiotherapy, and local or systemic therapies. In recent years, the efficacy of systemic therapies has improved due to advancements in treatment strategies and the introduction of molecular targeted drugs. In parallel, the increasing availability of new therapeutic approaches has created the need to identify beforehand which patients are eligible to a given specific treatment or another, raising the requirements for proper disease staging to a more evolved and complex level [,,,,,,,,].
Differentiating pulmonary metastases from primary or benign lung lesions is of paramount importance. Among imaging modalities, multidetector computed tomography (MDCT) and 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) play a major role in the detection, characterization and follow-up of lung cancer (either primary or LM). While MDCT is the mainstay for morphological assessment (allowing to evaluate features such as lesion size, morphology and growth rate), 18F-FDG PET/CT can complement morphological imaging by providing functional information related to tumor metabolism.
Despite advancements in MDCT and 18F-FDG PET/CT technologies, the diagnostic accuracy of both modalities can be limited by false positive (e.g., increased contrast enhancement or 18F-FDG uptake in non-neoplastic conditions, such as infections or inflammatory lesions) and false negative findings (as can happen, e.g., with poorly vascularized malignancies and/or those with relatively low 18F-FDG metabolism). Lung biopsy is the diagnostic gold standard to distinguish between primitive lung tumors or LM, but it is an invasive technique that may not be performed in all cases, cannot be repeated indefinitely, and may be burdened by more or less serious complications. Another inherent downside to conventional biopsy is the fact that, even if guided by imaging, it can provide information solely related to the specific tumor specimen from which cells are sampled, only allowing a limited assessment of tumor heterogeneity with no ability to interrogate the whole tumor structure [,,,,].
Minimally invasive techniques for the quantitative evaluation of biomarkers [,,,,,,,,] and response to therapies [,,,,,,,,] have emerged, such as liquid biopsy [,,,,,]. Radiomics is an emerging methodology aiming to convert diagnostic images into information that reflect pathophysiological properties of the tumor []. By tapping the computational power of artificial intelligence (AI) systems, a large amount of information that go beyond visual image assessment can be collected through the extraction and analysis of radiomics features from medical images, obtained in a routine setting with conventional imaging protocols (e.g., for tumor staging and follow-up). This approach has the potential to enhance options to carry out patient-specific diagnostic and prognostic evaluations, allowing e.g., to predict early treatment response and avoid undue over- or undertreatment depending on the biological aggressiveness of each patient-specific disease, and paving the way to a more individualized patient management [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Other advantages of radiomics over conventional biopsy include the possibility to capture the tumor tissue in its entirety (providing information regarding its clonal heterogeneity and the tumor microenvironment along with its stromal component), to guide biopsy towards specific tumor areas, and to be repeatable at virtually any time during the disease course, allowing longitudinal monitoring with improved opportunities of optimizing treatment strategies [,,,].
Our aim is to explore the current literature regarding the potential of radiomics in the field of diagnosis, treatment planning and outcome prediction of LM.
2. Methods
We systematically searched the literature to assess the role of radiomics in the management of LM from diagnosis to prognosis. We searched the PubMed (https://pubmed.ncbi.nlm.nih.gov, assessed on 20 November 2022) and Scopus (https://www.scopus.com, assessed on 20 November 2022) databases using a combination of the following search terms: ((“radiomics” OR “machine learning”) AND (metastases OR metastasis) AND (“lung” OR “pulmonary”)). The search period ranged from January 2015 to November 2022, and the search was performed on 20 November 2022. Only articles written in English language were selected. The search was restricted to “article, abstract and keys” in the Scopus database.
To improve the quality of inclusion criteria, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines []. The radiomics quality score (RQS) was calculated to assess the characteristics and the quality of the methodology of the studies taken into consideration, as described by Lambin et al. [].
3. Results
All retrieved publications (n = 890) were separately uploaded to EndNote™, and all duplicate records were eliminated (n = 281).
Two reviewers (M.G., L.F.) manually screened all articles by title and abstract, and if eligible, their full text was retrieved. Exclusion criteria were as follows: (1) animal studies, (2) reviews, posters and conference papers, (3) entries related to predicting the onset of LM from primitive tumors, and (4) entries related to computer aided detection of LM.
544 studies were excluded due to having irrelevant titles and abstracts. Of the remaining articles, 43 were excluded due to being conference papers, posters and reviews, 2 due to being related to LM detection, 3 due to being animal studies, and 9 due to being related to predicting the onset of LM from primitive tumors (Figure 1).
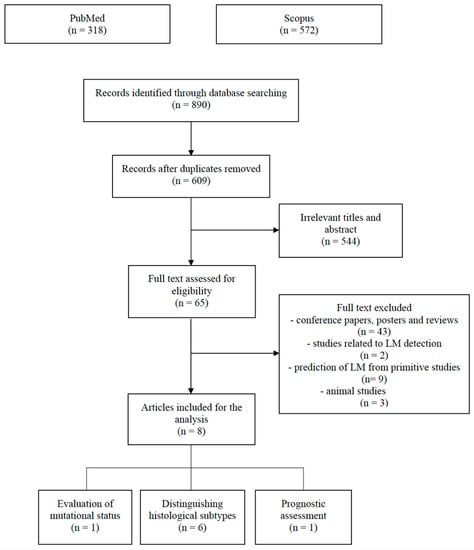
Figure 1.
PRISMA flow chart illustrating the inclusion and exclusion criteria for article search.
Finally, 8 articles were collected and divided into the following categories: (1) distinguishing histological subtypes (n = 6), (2) evaluation of mutational status (n = 1) and (3) prognostic assessment (n = 1) (Table 1).

Table 1.
Methodology and main findings of the 8 articles selected. ML = Machine Learning.
4. Discussion
Differentiating primary lung cancer from LM is of great clinical interest because of the profound differences in their prognostic and therapeutic implications [,]. Furthermore, a solitary pulmonary nodule may be more difficult to interpret in patients with a history of cancer, as it may be a primary lung tumor, a metastasis, or a benign lesion [,,]. From an imaging viewpoint, the ability to recognize various diseases based on qualitative criteria relies on observer expertise and experience, whereas quantitative parameters, such as pulmonary nodule diameter and/or growth rate, are surrogate biomarkers of malignancy [,,,].
In this context, 3 articles evaluated the role of radiomics in differentiating primary lung tumors from metastases, of which 2 analyzed PET/CT images and 1 MDCT images. Kirienko et al. [] evaluated 534 patients who underwent 18F-FDG PET/CT for characterization of lung nodule or for staging of a suspected lung tumor before biopsy, with the aim to evaluate the ability of radiomics to discriminate primitive lung tumor from LM and to predict the histological subtypes among primary lung cancers. They demonstrated that texture features obtained from PET images were able to differentiate between primary lung cancer and metastases (AUC > 0.90), whereas the analysis of co-registered CT data showed limited ability to distinguish between the two groups. Furthermore, the data obtained from PET images showed higher ability to discriminate between histological subtypes than those derived from CT images. More specifically, as to differentiation between primary subgroups based on CT features, the AUCs in the training and validation groups were 0.81 ± 0.02 and 0.69 ± 0.04 for adenocarcinoma versus squamous cell carcinoma or other histological subtypes, 0.85 ± 0.02 and 0.70 ± 0.05 for squamous cell carcinoma versus adenocarcinoma or other histological subtypes, and 0.77 ± 0.03 and 0.57 ± 0.05 for other histological subtypes versus squamous cell carcinoma or adenocarcinoma. The same analyses for the PET data revealed AUCs of 0.90 ± 0.10 and 0.80 ± 0.04, 0.80 ± 0.02 and 0.61 ± 0.06, and 0.97 ± 0.01 and 0.88 ± 0.04, respectively.
In a study performed on 769 patients, Zhou et al. [] showed that 18F-FDG PET/CT features allowed to distinguish primary lung tumors from LM (AUC = 0.98), and that the results derived from the CT dataset were generally poorer than those obtained from the PET dataset. Furthermore, they achieved a good performance in discriminating lung adenocarcinoma from squamous cell carcinoma (AUC = 0.89) by using a combination of gradient boosting decision tree (GBDT) feature selection method with GBDT classification in the PET dataset. The combination of the GBDT feature selection method with the random forest (RF) classification had the highest AUC of 0.83 in the CT dataset. Of note, most of the decision tree (DT)-based models were overfitted, revealing that the classification method was inappropriate for usage in clinical practice.
In the aforementioned papers, the poorer performance obtained with CT data could be due to the fact that the CT dataset used for anatomical localization of PET/CT scans had a lower tissue contrast resolution (resulting from the lack of intravenous contrast material administration) and a lower overall quality than regular MDCT images used for radiological diagnosis, possibly introducing a bias in the determination of radiomics features. However, these findings could underscore the ability of texture analysis of PET images to detect the expression of underlying biological processes as revealed by an increased 18F-FDG uptake.
Zhong et al. [] analyzed MDCT images of 97 s primary lung cancers and 155 LM, and constructed a nomogram model integrating clinical data, imaging characteristics (such as distribution of lesions, central or peripheral type, contours, and spiculation), and radiomics features. They achieved an excellent (0.94 and 0.90 in the training and validation cohorts, respectively) discriminative capability to distinguish LM from second primitive lung cancer in patients with a history of cancer. The radiomics model alone had good discriminative performance, with an AUC of 0.87 (95% confidence interval, 0.81–0.92) in the training set and 0.76 (95% confidence interval, 0.67–0.85) in the validation set. Moreover, the decision curve analysis (DCA) showed that the comprehensive model had a higher clinical value than that without the radiomics score.
At primary tumor staging or follow-up, patients frequently present with pulmonary nodules with indeterminate characteristics, such as <1 cm in diameter, single or double, no typical CT signs of malignancy, and no elevation of 18F-FDG standardized uptake value (SUV) at PET-CT imaging. These indeterminate lung nodules usually undergo long-term follow-up, bringing additional cost and patient anxiety and possibly delaying treatment. Therefore, a noninvasive diagnostic tool allowing a reliable and immediate characterization of such lesions would fulfil a highly unmet clinical need.
Hu et al. [] evaluated 194 patients with colorectal cancer and MDCT finding of at least one indeterminate pulmonary nodule sized 5–20 mm in diameter. Three models were generated (i.e., a clinical model with significant clinical risk factors, a radiomics model with radiomics features constructed by the least absolute shrinkage and selection operator (LASSO) algorithm, and a clinical-radiomics model with significant variables selected by the stepwise logistic regression) to quantitatively assess the risk of such patients to develop LM. A nomogram was built based on the best performing model, and DCA was applied to test the clinical usefulness. The rad-score DCA showed more benefit than clinical DCA (based on N stage, chronicity, and size of the nodule) in predicting the risk of LM. Moreover, the clinical-radiomics nomogram was successfully developed with a favorable discrimination in the training cohort (AUC = 0.92, 95% confidence interval: 0.88–0.97) and the validation cohort (AUC = 0.92, 95% confidence interval: 0.85–0.98) and good calibration, and it achieved the greatest clinical usefulness, being capable of discriminating LM from non-metastatic nodules (84.9% sensitivity and 91.1% specificity in the training cohort). A higher nodal stage was found to be a significant predictor of metastases in patients with colorectal cancer and indeterminate pulmonary nodules (warranting a closer monitoring for early detection and follow-up of LM in this patient subset), and metachronous nodules were closely correlated with LM occurrence and had an excess predictive impact compared with N stage.
Liu et al. [] analyzed radiomics features from contrast enhanced MDCT images to differentiate benign nodules from metastatic pulmonary nodules from colorectal cancer. A total of 320 nodules sized less than or 1 cm in diameter were evaluated, of which 200 metastatic nodules were included in the training cohort, 60 benign nodules in one verification cohort, and 60 metastatic nodules in another verification cohort. All nodules were divided into four groups according to their maximum diameter. Through cross-validation on 100 experiments, 11 features remained stable for more than 90 times in LM, but not in benign nodules. Such stability may be related to the essential characteristics of metastatic nodules, possibly representing a relevant factor to distinguish metastatic pulmonary nodules from benign ones. Of note, 8 of 11 features belong to ‘CoLIAGe’ (i.e., Co-occurrence of Local Anisotropic Gradient Orientations), a radiomics descriptor that identifies differences in the local entropy pattern and can differentiate subtle pathology differences from similar morphological manifestations, unlike morphological descriptors (such as shape and edges), which can be similar in small benign nodules and metastatic nodules.
Shang et al. [] explored the role of radiomics to differentiate lung metastatic nodules from breast, colorectal and renal cancer by means of MDCT radiomics features. They performed a retrospective analysis including 252 LM from 78 patients, which were randomly divided into a training cohort (n = 176) and a test cohort (n = 76). The metastases originated from colorectal cancer (n = 97), breast cancer (n = 87), and renal carcinoma (n = 68), and additional 77 LM were used for external validation. A three-class model was built using the LASSO method, showing a good discriminative performance for the various tumor histotypes (AUC: colorectal cancer LM vs. renal carcinoma LM 0.84, breast cancer LM vs. colorectal cancer LM 0.80, breast cancer LM vs. renal carcinoma LM 0.94), along with AUCs of 0.77, 0.78, and 0.84 in the external validation cohort. Interestingly, breast cancer LM showed a low value of normalized run variance, maximum probability, and dependence nonuniformity. This may indicate more homogeneity in texture patterns, possibly due to dense fibrous tissue hyperplasia in breast cancer lesions, as compared with renal carcinoma (which is prone to intratumor hemorrhage and necrosis/cystic degeneration) and colorectal cancer (which tends to have fewer stroma elements inside it).
Treatment options for LM may include local therapies, such as thermal ablation techniques and stereotactic ablative body radiation [,,,,,,,,], chemotherapy [,] and, in selected cases, surgery [,,]. The introduction of new systemic treatments, including immunotherapy and target therapies, has improved the prognosis of patients with metastatic tumors, but only a subset of mutated patients can benefit from such treatments []. Angus et al. [] attempted to detect radiomics features related to BRAF mutations in LM from melanoma in patients undergoing pre-treatment MDCT. 540 radiomics were extracted from 169 lung lesions detected in 103 patients (51 BRAF-mutant, 52 BRAF-wild type), and a combination of machine learning methods was used to build BRAF decision models based on radiomics features and Lung Image Database Consortium (LIDC) criteria. No radiomics features were found to be able to differentiate BRAF-mutant and BRAF-wild type LM, with models based on radiomics features and LIDC criteria performing as poorly as guessing. A potential explanation for this finding it that as the NRAS and BRAF genes are both involved in the MPAK pathway, activating mutations of the NRAS gene in BRAF-wild type melanoma or the BRAF gene in BRAF-mutant melanoma may lead to a similar phenotype.
To our knowledge, only Miao et al. [] evaluated the role of radiomics to predict prognosis in patients with LM. They investigated radiomics features from contrast-enhanced MDCT examinations of 51 patients to predict the effectiveness of epirubicin combined with ifosfamide as first-line treatment in LM from soft tissue sarcoma. Lung metastases were used as target lesions (total n = 86), and patients were split into a progression group (n = 29), a stable group (n = 34), and a partial response group (n = 23). In total, 851 radiomics features were extracted for each target lesion, and then narrowed down to 2 radiomics features (wavelet-HHH_First Order Mean and wavelet-LHL_GLRLM Long Run Low Grey Level Emphasis) by dimensionality reduction. Such features were used to build a decision tree classifier model with a good predictive value (AUC = 0.91, 95% confidence interval: 0.858–0.969 in the training group, and AUC = 0.85, 95% confidence interval: 0.72–0.96 in the testing group). This finding has a relevant practical value because, if there is a high likelihood of disease progression according to the predictive model, alternative treatment strategies can promptly be enacted, potentially improving disease-free survival and overall quality of life.
Despite its potential in providing an added value over conventional imaging approaches in the management of patients with LM and other cancers, radiomics is still far from being universally adopted in clinical practice. This is due to several factors, including a lack of harmonization of imaging protocols, clinical validation issues, and an overall poor scientific quality of the studies in the field [,,,]. In line with this scenario, a RQS of 27.8% (range 22.2–38.9%) was calculated for the 8 articles retrieved in this review (Table 2), consistent with other reviews focused on radiomics [,,,,,,,,,,,,,,,].

Table 2.
RQS calculation for the 8 articles selected, based on the criteria illustrated by Lambin et al. [].
Moreover, in all studies patient enrollment was retrospective in design, validation was performed on datasets from the same institutions, and data access was not granted to the public. Three studies (37.5%) [,,] evaluated the potential applicability of radiomics models in a clinical setting by means of DCA. In most studies (75%) [,,,,,] multiple segmentations were performed to evaluate the robustness of radiomics features in relation to segmentation variability.
This review has some limitations. Firstly, we selected only studies in which radiomics features had been obtained from LM, leaving out any studies related to LM prediction from primitive tumors. Secondly, the high variability of the studies under investigation (e.g., regarding methodology) makes it difficult to compare data across them.
5. Conclusions
Radiomics is a relatively recent field of research in rapid evolution, and the potential use of radiomics for LM is a niche subfield, as demonstrated by the scarcity of studies collected in this review. Their lack of clinical relevance assessment and their retrospective design in a single center setting without external validation are shortcomings that currently curtail the robustness and clinical applicability of the study findings. Furthermore, no solid data have been collected so far demonstrating a correspondence between specific textural features and biological processes and explaining it in terms of radiomics-pathology correlations. In this setting, specific initiatives (such as the Image Biomarker Standardization Initiative, IBSI) have been undertaken to promote the adoption of a standardized approach for the definition, nomenclature, and calculation of radiomics features, resulting in improved statistical reliability as long as calculation settings are harmonized too. A greater standardization of radiomics methods and their validation on larger patient samples obtained from multicenter studies (possibly based on standardized image acquisition protocols), as well as a better integration of radiomics software in real world clinical and radiological environments [,,,,,,], could be key to increase the role of radiomics in diagnosis, treatment planning and individual outcome prediction in patients with LM.
Author Contributions
Conceptualization, M.G. and V.G.; methodology, M.G.; software, R.F. and I.S.; validation, F.D.M., F.B., F.G. and M.S.; formal analysis, M.G. and R.F.; investigation, M.G., D.C. and G.G.; resources, A.B. (Alessandra Borgheresi) and A.B. (Antonio Barile); data curation, L.F.; writing—original draft preparation, M.G.; writing—review and editing, M.G. and L.F.; supervision, V.M., A.G. and N.G.; project administration, A.B. (Antonio Barile). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data can be made publicly available upon reasonable request.
Acknowledgments
The authors are grateful to Alessandra Trocino, librarian at the National Cancer Institute of Naples, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ierardi, A.M.; Carnevale, A.; Chiarello, S.; Cavazza, M.; Stellato, E.; Mendogni, P.; Palleschi, A.; Tosi, D.; Giganti, M.; Carrafiello, G. A Narrative Review on Pulmonary Metastases Management by Non-Surgical Local Techniques: Where Do We Stand? AME Surg. J. 2021, 1, 24. [Google Scholar] [CrossRef]
- Gerull, W.D.; Puri, V.; Kozower, B.D. The Epidemiology and Biology of Pulmonary Metastases. J. Thorac. Dis. 2021, 13, 2585–2589. [Google Scholar] [CrossRef]
- Stella, G.M.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-Seeking Metastases. Cancers 2019, 11, 1010. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Thomas, R.; Howard, S.A.; Laferriere, S.L.; Braschi-Amirfarzan, M. A Review of the Mechanisms and Clinical Implications of Precision Cancer Therapy-Related Toxicity: A Primer for the Radiologist. AJR Am. J. Roentgenol. 2020, 215, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Mangiameli, G.; Cioffi, U.; Alloisio, M.; Testori, A. Lung Metastases: Current Surgical Indications and New Perspectives. Front. Surg. 2022, 9, 884915. [Google Scholar] [CrossRef] [PubMed]
- Digumarthy, S.R.; Mendoza, D.P.; Padole, A.; Chen, T.; Gabriel Peterson, P.; Piotrowska, Z.; Sequist, L.V. Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Cancers 2019, 11, 1360. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.; Ding, L.; Wang, Y.; Zhu, C.; Wang, K.; Li, J.; Sun, M.; Oupicky, D. Increased Survival by Pulmonary Treatment of Established Lung Metastases with Dual STAT3/CXCR4 Inhibition by siRNA Nanoemulsions. Mol. Ther. 2019, 27, 2100–2110. [Google Scholar] [CrossRef]
- Li, Z.; Shen, Y.; Wang, Y.; Zhu, L.; Zhu, C.; Qian, C.; Sun, M.; Oupicky, D. Perfluorocarbon Nanoemulsions for Combined Pulmonary siRNA Treatment of Lung Metastatic Osteosarcoma. Adv. Ther. 2019, 2, 1900039. [Google Scholar] [CrossRef]
- Sabatino, V.; Russo, U.; D’Amuri, F.; Bevilacqua, A.; Pagnini, F.; Milanese, G.; Gentili, F.; Nizzoli, R.; Tiseo, M.; Pedrazzi, G.; et al. Pneumothorax and Pulmonary Hemorrhage after CT-Guided Lung Biopsy: Incidence, Clinical Significance and Correlation. Radiol. Med. 2021, 126, 170–177. [Google Scholar] [CrossRef]
- Winokur, R.; Sullivan, B.; Madoff, D.; Pua, B. Percutaneous Lung Biopsy: Technique, Efficacy, and Complications. Semin. Interv. Radiol. 2013, 30, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ericson Lindquist, K.; Ciornei, C.; Westbom-Fremer, S.; Gudinaviciene, I.; Ehinger, A.; Mylona, N.; Urdar, R.; Lianou, M.; Svensson, F.; Seidal, T.; et al. Difficulties in Diagnostics of Lung Tumours in Biopsies: An Interpathologist Concordance Study Evaluating the International Diagnostic Guidelines. J. Clin. Pathol. 2022, 75, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.R.; Kim, M.-S.; Park, C.M.; Yoon, S.H.; Chae, K.J.; Lee, J. Patterns of Percutaneous Transthoracic Needle Biopsy (PTNB) of the Lung and Risk of PTNB-Related Severe Pneumothorax: A Nationwide Population-Based Study. PLoS ONE 2020, 15, e0235599. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Frank, N. An Overview of Percutaneous CT-Guided Lung Biopsies. J. Radiol. Nurs. 2018, 37, 2–8. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, Z.; Wang, X.; Ai, H.; Yang, C.; Luo, Y.; Jiang, X. Radiomics for Prediction of Response to EGFR-TKI Based on Metastasis/brain Parenchyma (M/BP)-Interface. Radiol. Med. 2022, 127, 1342–1354. [Google Scholar] [CrossRef]
- Agazzi, G.M.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D. CT Texture Analysis for Prediction of EGFR Mutational Status and ALK Rearrangement in Patients with Non-Small Cell Lung Cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef]
- Bracci, S.; Dolciami, M.; Trobiani, C.; Izzo, A.; Pernazza, A.; D’Amati, G.; Manganaro, L.; Ricci, P. Quantitative CT Texture Analysis in Predicting PD-L1 Expression in Locally Advanced or Metastatic NSCLC Patients. Radiol. Med. 2021, 126, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Stecco, A.; Micci, G.; Sconfienza, L.M.; Colagrande, S.; Reginelli, A.; Grassi, R.; Carriero, A.; Midiri, M.; Lagalla, R.; et al. Whole-Body Magnetic Resonance Imaging (WB-MRI) in Oncology: An Italian Survey. Radiol. Med. 2021, 126, 299–305. [Google Scholar] [CrossRef]
- Mungai, F.; Verrone, G.B.; Bonasera, L.; Bicci, E.; Pietragalla, M.; Nardi, C.; Berti, V.; Mazzoni, L.N.; Miele, V. Imaging Biomarkers in the Diagnosis of Salivary Gland Tumors: The Value of Lesion/parenchyma Ratio of Perfusion-MR Pharmacokinetic Parameters. Radiol. Med. 2021, 126, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Milanese, G.; Cademartiri, F.; Maffei, E.; Benedetti, G.; Goldoni, M.; Silva, M.; Sverzellati, N. Association of Hepatic Steatosis with Epicardial Fat Volume and Coronary Artery Disease in Symptomatic Patients. Radiol. Med. 2021, 126, 652–660. [Google Scholar] [CrossRef]
- Halefoglu, A.M.; Ozagari, A.A. Tumor Grade Estımatıon of Clear Cell and Papıllary Renal Cell Carcınomas Usıng Contrast-Enhanced MDCT and FSE T2 Weıghted MR ımagıng: Radıology-Pathology Correlatıon. Radiol. Med. 2021, 126, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs): A Radiomic Model to Predict Tumor Grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Mallio, C.A. Relationship between Visceral Adipose Tissue and Genetic Mutations (VHL and KDM5C) in Clear Cell Renal Cell Carcinoma. Radiol. Med. 2021, 126, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, F.; Gabelloni, M.; Gonnelli, A.; Faggioni, L.; Cantarella, M.; Montrone, S.; Gadducci, G.; Giannini, N.; Montemurro, N.; Mattioni, R.; et al. Impact of Temporalis Muscle Thickness in Elderly Patients with Newly Diagnosed Glioblastoma Treated with Radio or Radio-Chemotherapy. Radiol. Med. 2022, 127, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to Measure, When and Why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Orlandi, A.; Bufi, E.; Mercogliano, S.; Belli, P.; Manfredi, R. Automated Breast Volume Scanner (ABVS) Compared to Handheld Ultrasound (HHUS) and Contrast-Enhanced Magnetic Resonance Imaging (CE-MRI) in the Early Assessment of Breast Cancer during Neoadjuvant Chemotherapy: An Emerging Role to Monitoring Tumor Response? Radiol. Med. 2021, 126, 517–526. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-Based Radiomics for Prediction of Therapeutic Response to Everolimus in Metastatic Neuroendocrine Tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the Standardized Index of Shape Tool to Analyze DCE-MRI Data in the Assessment of Neo-Adjuvant Therapy in Locally Advanced Rectal Cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Shroff, G.S.; Benveniste, M.F.; de Groot, P.M.; Wu, C.C.; Viswanathan, C.; Papadimitrakopoulou, V.A.; Truong, M.T. Targeted Therapy and Imaging Findings. J. Thorac. Imaging 2017, 32, 313–322. [Google Scholar] [CrossRef]
- Souza, F.F.; Smith, A.; Araujo, C.; Jagannathan, J.; Johnston, C.; O’Regan, K.; Shinagare, A.; Ramaiya, N. New Targeted Molecular Therapies for Cancer: Radiological Response in Intrathoracic Malignancies and Cardiopulmonary Toxicity: What the Radiologist Needs to Know. Cancer Imaging 2014, 14, 26. [Google Scholar] [CrossRef]
- Carter, B.W.; Altan, M.; Shroff, G.S.; Truong, M.T.; Vlahos, I. Post-Chemotherapy and Targeted Therapy Imaging of the Chest in Lung Cancer. Clin. Radiol. 2022, 77, e1–e10. [Google Scholar] [CrossRef]
- Paulmurugan, R.; Oronsky, B.; Brouse, C.F.; Reid, T.; Knox, S.; Scicinski, J. Real Time Dynamic Imaging and Current Targeted Therapies in the War on Cancer: A New Paradigm. Theranostics 2013, 3, 437–447. [Google Scholar] [CrossRef]
- Del Re, M.; Cucchiara, F.; Rofi, E.; Fontanelli, L.; Petrini, I.; Gri, N.; Pasquini, G.; Rizzo, M.; Gabelloni, M.; Belluomini, L.; et al. A Multiparametric Approach to Improve the Prediction of Response to Immunotherapy in Patients with Metastatic NSCLC. Cancer Immunol. Immunother. 2020, 70, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef]
- Martins, I.; Ribeiro, I.P.; Jorge, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Melo, J.B.; Carreira, I.M. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid Biopsy of Cancer: A Multimodal Diagnostic Tool in Clinical Oncology. Ther. Adv. Med. Oncol. 2018, 10, 175883591879463. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Sousa, C.; Machado, F.; Serino, M.; Santos, V.; Cruz-Martins, N.; Teixeira, A.; Cunha, A.; Pereira, T.; Oliveira, H.P.; et al. The Role of Liquid Biopsy in Early Diagnosis of Lung Cancer. Front. Oncol. 2021, 11, 634316. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A Deep Look into Radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A Narrative Review on Current Imaging Applications of Artificial Intelligence and Radiomics in Oncology: Focus on the Three Most Common Cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta Radiomics: A Systematic Review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Gabelloni, M.; De Vietro, F.; Mendola, V.; Cattabriga, A.; Cocozza, M.A.; Vara, G.; Piccinino, A.; Lo Monaco, S.; et al. Human, All Too Human? An All-Around Appraisal of the “Artificial Intelligence Revolution” in Medical Imaging. Front. Psychol. 2021, 12, 710982. [Google Scholar] [CrossRef]
- Gabelloni, M.; Faggioni, L.; Neri, E. Imaging Biomarkers in Upper Gastrointestinal Cancers. BJR Open 2019, 1, 20190001. [Google Scholar] [CrossRef]
- Coppola, F.; Giannini, V.; Gabelloni, M.; Panic, J.; Defeudis, A.; Lo Monaco, S.; Cattabriga, A.; Cocozza, M.A.; Pastore, L.V.; Polici, M.; et al. Radiomics and Magnetic Resonance Imaging of Rectal Cancer: From Engineering to Clinical Practice. Diagnostics 2021, 11, 756. [Google Scholar] [CrossRef]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology Ex-Machina: Impact of Artificial Intelligence on Practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial Intelligence: Radiologists’ Expectations and Opinions Gleaned from a Nationwide Online Survey. Radiol. Med. 2021, 126, 63–71. [Google Scholar] [CrossRef]
- Satake, H.; Ishigaki, S.; Ito, R.; Naganawa, S. Radiomics in Breast MRI: Current Progress toward Clinical Application in the Era of Artificial Intelligence. Radiol. Med. 2022, 127, 39–56. [Google Scholar] [CrossRef]
- Wang, F.-H.; Zheng, H.-L.; Li, J.-T.; Li, P.; Zheng, C.-H.; Chen, Q.-Y.; Huang, C.-M.; Xie, J.-W. Prediction of Recurrence-Free Survival and Adjuvant Therapy Benefit in Patients with Gastrointestinal Stromal Tumors Based on Radiomics Features. Radiol. Med. 2022, 127, 1085–1097. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics Textural Features by MR Imaging to Assess Clinical Outcomes Following Liver Resection in Colorectal Liver Metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Yao, F.; Bian, S.; Zhu, D.; Yuan, Y.; Pan, K.; Pan, Z.; Feng, X.; Tang, K.; Yang, Y. Machine Learning-Based Radiomics for Multiple Primary Prostate Cancer Biological Characteristics Prediction with 18F-PSMA-1007 PET: Comparison among Different Volume Segmentation Thresholds. Radiol. Med. 2022, 127, 1170–1178. [Google Scholar] [CrossRef]
- Xue, K.; Liu, L.; Liu, Y.; Guo, Y.; Zhu, Y.; Zhang, M. Radiomics Model Based on Multi-Sequence MR Images for Predicting Preoperative Immunoscore in Rectal Cancer. Radiol. Med. 2022, 127, 702–713. [Google Scholar] [CrossRef]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT Radiomics in Predicting the Overall Survival of Patients with Stage III Clear Cell Renal Carcinoma after Radical Nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef]
- Gregucci, F.; Fiorentino, A.; Mazzola, R.; Ricchetti, F.; Bonaparte, I.; Surgo, A.; Figlia, V.; Carbonara, R.; Caliandro, M.; Ciliberti, M.P.; et al. Radiomic Analysis to Predict Local Response in Locally Advanced Pancreatic Cancer Treated with Stereotactic Body Radiation Therapy. Radiol. Med. 2022, 127, 100–107. [Google Scholar]
- Robertis, R.D.; De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver Metastases in Pancreatic Ductal Adenocarcinoma: A Predictive Model Based on CT Texture Analysis. Radiol. Med. 2022, 127, 1079–1084. [Google Scholar]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Liver Mucinous Colorectal Metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar]
- Autorino, R.; Gui, B.; Panza, G.; Boldrini, L.; Cusumano, D.; Russo, L.; Nardangeli, A.; Persiani, S.; Campitelli, M.; Ferrandina, G.; et al. Radiomics-Based Prediction of Two-Year Clinical Outcome in Locally Advanced Cervical Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Radiol. Med. 2022, 127, 498–506. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-Derived Radiomic Features to Discriminate Histologic Characteristics of Pancreatic Neuroendocrine Tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic Features for Prostate Cancer Grade Detection through Formal Verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Del Grande, F.; Sconfienza, L.M. An Update in Musculoskeletal Tumors: From Quantitative Imaging to Radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A Field Strength Independent MR Radiomics Model to Predict Pathological Complete Response in Locally Advanced Rectal Cancer. Radiol. Med. 2021, 126, 421–429. [Google Scholar] [CrossRef]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.-R.; He, Y.; Chen, J.-Q.; Yang, H. Magnetic Resonance Imaging (MRI) Radiomics of Papillary Thyroid Cancer (PTC): A Comparison of Predictive Performance of Multiple Classifiers Modeling to Identify Cervical Lymph Node Metastases before Surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar]
- Palatresi, D.; Fedeli, F.; Danti, G.; Pasqualini, E.; Castiglione, F.; Messerini, L.; Massi, D.; Bettarini, S.; Tortoli, P.; Busoni, S.; et al. Correlation of CT Radiomic Features for GISTs with Pathological Classification and Molecular Subtypes: Preliminary and Monocentric Experience. Radiol. Med. 2022, 127, 117–128. [Google Scholar]
- Cozzi, D.; Bicci, E.; Cavigli, E.; Danti, G.; Bettarini, S.; Tortoli, P.; Mazzoni, L.N.; Busoni, S.; Pradella, S.; Miele, V. Radiomics in Pulmonary Neuroendocrine Tumours (NETs). Radiol. Med. 2022, 127, 609–615. [Google Scholar]
- Gao, W.; Wang, W.; Song, D.; Yang, C.; Zhu, K.; Zeng, M.; Rao, S.-X.; Wang, M. A Predictive Model Integrating Deep and Radiomics Features Based on Gadobenate Dimeglumine-Enhanced MRI for Postoperative Early Recurrence of Hepatocellular Carcinoma. Radiol. Med. 2022, 127, 259–271. [Google Scholar]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed Tomography-Based Radiomics Approach in Pancreatic Tumors Characterization. Radiol. Med. 2021, 126, 1388–1395. [Google Scholar]
- Chiloiro, G.; Cusumano, D.; de Franco, P.; Lenkowicz, J.; Boldrini, L.; Carano, D.; Barbaro, B.; Corvari, B.; Dinapoli, N.; Giraffa, M.; et al. Does Restaging MRI Radiomics Analysis Improve Pathological Complete Response Prediction in Rectal Cancer Patients? A Prognostic Model Development. Radiol. Med. 2022, 127, 11–20. [Google Scholar] [CrossRef]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT Texture Analysis for Predicting Peritoneal Metastases in Patients with Gastric Cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar]
- Angus, L.; Starmans, M.P.A.; Rajicic, A.; Odink, A.E.; Jalving, M.; Niessen, W.J.; Visser, J.J.; Sleijfer, S.; Klein, S.; van der Veldt, A.A.M. The BRAF P.V600E Mutation Status of Melanoma Lung Metastases Cannot Be Discriminated on Computed Tomography by LIDC Criteria nor Radiomics Using Machine Learning. J. Pers. Med. 2021, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, S.; Huang, L.; Wang, J.; Shi, D.; Li, Y.; Tong, T.; Peng, W. A Clinical-Radiomics Nomogram for the Preoperative Prediction of Lung Metastasis in Colorectal Cancer Patients with Indeterminate Pulmonary Nodules. Eur. Radiol. 2019, 29, 439–449. [Google Scholar] [CrossRef]
- Kirienko, M.; Cozzi, L.; Rossi, A.; Voulaz, E.; Antunovic, L.; Fogliata, A.; Chiti, A.; Sollini, M. Ability of FDG PET and CT Radiomics Features to Differentiate between Primary and Metastatic Lung Lesions. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1649–1660. [Google Scholar]
- Liu, C.; Meng, Q.; Zeng, Q.; Chen, H.; Shen, Y.; Li, B.; Cen, R.; Huang, J.; Li, G.; Liao, Y.; et al. An Exploratory Study on the Stable Radiomics Features of Metastatic Small Pulmonary Nodules in Colorectal Cancer Patients. Front. Oncol. 2021, 11, 661763. [Google Scholar] [CrossRef]
- Miao, L.; Ma, S.-T.; Jiang, X.; Zhang, H.-H.; Wang, Y.-M.; Li, M. Prediction of the Therapeutic Efficacy of Epirubicin Combined with Ifosfamide in Patients with Lung Metastases from Soft Tissue Sarcoma Based on Contrast-Enhanced CT Radiomics Features. BMC Med. Imaging 2022, 22, 131. [Google Scholar] [CrossRef]
- Shang, H.; Li, J.; Jiao, T.; Fang, C.; Li, K.; Yin, D.; Zeng, Q. Differentiation of Lung Metastases Originated from Different Primary Tumors Using Radiomics Features Based on CT Imaging. Acad. Radiol. 2023, 30, 40–46. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, Z.; An, W.; Wang, B.; Zhang, H.; Liu, Y.; Liao, M. Radiomics Study for Discriminating Second Primary Lung Cancers From Pulmonary Metastases in Pulmonary Solid Lesions. Front. Oncol. 2021, 11, 801213. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, X.-L.; Zhang, T.; Wang, J.; Zhang, T.; Tian, R. Use of Radiomics Based on 18F-FDG PET/CT and Machine Learning Methods to Aid Clinical Decision-Making in the Classification of Solitary Pulmonary Lesions: An Innovative Approach. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2904–2913. [Google Scholar] [CrossRef]
- Silva, M.; Picozzi, G.; Sverzellati, N.; Anglesio, S.; Bartolucci, M.; Cavigli, E.; Deliperi, A.; Falchini, M.; Falaschi, F.; Ghio, D.; et al. Low-Dose CT for Lung Cancer Screening: Position Paper from the Italian College of Thoracic Radiology. Radiol. Med. 2022, 127, 543–559. [Google Scholar]
- Voulaz, E.; Novellis, P.; Rossetti, F.; Solinas, M.; Rossi, S.; Alloisio, M.; Pelosi, G.; Veronesi, G. Distinguishing Multiple Lung Primaries from Intra-Pulmonary Metastases and Treatment Implications. Expert Rev. Anticancer Ther. 2020, 20, 985–995. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeong, W.G.; Kim, Y.-H. Differentiation of Primary Lung Cancer from Solitary Lung Metastasis in Patients with Colorectal Cancer: A Retrospective Cohort Study. World J. Surg. Oncol. 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Perdigón Martinelli, C.; Morell, C.; González, C.; Nova-Lozano, C. Metastatic Pulmonary Dissemination as Differential Diagnosis of COVID-19 Disease. BMJ Case Rep. 2020, 13, e237453. [Google Scholar] [CrossRef]
- Vanfleteren, M.J.E.G.W.; Dingemans, A.-M.C.; Surmont, V.F.; Vermaelen, K.Y.; Postma, A.A.; Oude Lashof, A.M.L.; Pitz, C.C.M.; Hendriks, L.E.L. Invasive Aspergillosis Mimicking Metastatic Lung Cancer. Front. Oncol. 2018, 8, 188. [Google Scholar] [CrossRef]
- Deniffel, D.; Sauter, A.; Fingerle, A.; Rummeny, E.J.; Makowski, M.R.; Pfeiffer, D. Improved Differentiation between Primary Lung Cancer and Pulmonary Metastasis by Combining Dual-Energy CT–derived Biomarkers with Conventional CT Attenuation. Eur. Radiol. 2021, 31, 1002–1010. [Google Scholar] [CrossRef]
- Borghesi, A.; Tironi, A.; Michelini, S.; Scrimieri, A.; Benetti, D.; Maroldi, R. Two Synchronous Lung Metastases from Malignant Melanoma: The Same Patient but Different Morphological Patterns. Eur. J. Radiol. Open 2019, 6, 287–290. [Google Scholar] [CrossRef]
- Deniffel, D.; Sauter, A.; Dangelmaier, J.; Fingerle, A.; Rummeny, E.J.; Pfeiffer, D. Differentiating Intrapulmonary Metastases from Different Primary Tumors via Quantitative Dual-Energy CT Based Iodine Concentration and Conventional CT Attenuation. Eur. J. Radiol. 2019, 111, 6–13. [Google Scholar] [CrossRef]
- Seo, J.B.; Im, J.G.; Goo, J.M.; Chung, M.J.; Kim, M.Y. Atypical Pulmonary Metastases: Spectrum of Radiologic Findings. Radiographics 2001, 21, 403–417. [Google Scholar] [CrossRef]
- Grasso, R.F.; Bernetti, C.; Pacella, G.; Altomare, C.; Castiello, G.; Andresciani, F.; Sarli, M.; Zobel, B.B.; Faiella, E. A Comparative Analysis of Thermal Ablation Techniques in the Treatment of Primary and Secondary Lung Tumors: A Single-Center Experience. Radiol. Med. 2022, 127, 714–724. [Google Scholar] [CrossRef]
- Mega, S.; Fiore, M.; Carpenito, M.; Novembre, M.L.; Miele, M.; Trodella, L.E.; Grigioni, F.; Ippolito, E.; Ramella, S. Early GLS Changes Detection after Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer (NSCLC). Radiol. Med. 2022, 127, 1355–1363. [Google Scholar] [CrossRef]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C.; et al. Home-Based Pulmonary Rehabilitation in Patients Undergoing (chemo)radiation Therapy for Unresectable Lung Cancer: A Prospective Explorative Study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef]
- Lancellotta, V.; Fanetti, G.; Monari, F.; Mangoni, M.; Mazzarotto, R.; Tagliaferri, L.; Gobitti, C.; Lodi Rizzini, E.; Talomo, S.; Turturici, I.; et al. Stereotactic Radiotherapy (SRT) for Differentiated Thyroid Cancer (DTC) Oligometastases: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) Systematic Review. Radiol. Med. 2022, 127, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative Radiotherapy Indications during the COVID-19 Pandemic and in Future Complex Logistic Settings: The NORMALITY Model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef] [PubMed]
- Bellometti, S.; Nube, G.; Alongi, F.; Baiocchi, C.; Corti, L.; Di Biase, S.; Fiorica, F.; Gava, A.; Iannone, T.; Rumeileh, I.A.; et al. Radiotherapy Activities and Technological Equipment in Veneto, Italy: A Report from the Rete Radioterapica Veneta. Radiol. Med. 2021, 126, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, L.; Mendichi, M.; Chierchini, S.; Tenti, M.V.; Bellavita, R.; Saldi, S.; Ingrosso, G.; Reggioli, V.; Bini, V.; Aristei, C. Pulmonary Function in Stereotactic Body Radiotherapy with Helical Tomotherapy for Primary and Metastatic Lung Lesions. Radiol. Med. 2021, 126, 163–169. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Sánchez, I.; Díaz, O.; Valles, A.; Balderrama, R.; Fuentes, J.; Lara, B.; Olimón, C.; Ruiz, V.; Rodríguez, J.; et al. Current Evidence for Stereotactic Body Radiotherapy in Lung Metastases. Curr. Oncol. 2021, 28, 2560–2578. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Fan, W. Value of Ablation Therapy in the Treatment of Lung Metastases. Thorac. Cancer 2018, 9, 199–207. [Google Scholar] [CrossRef]
- Stork, T.; Boemans, R.; Hardes, J.; Streitbürger, A.; Dirksen, U.; Pöttgen, C.; Schildhaus, H.-U.; Bauer, S.; Collaud, S.; Aigner, C. Number of Metastases and Their Response to Chemotherapy Impact Survival of Patients with Isolated Lung Metastases from Bone-Derived Sarcoma. BMC Cancer 2021, 21, 375. [Google Scholar] [CrossRef]
- Okazaki, Y.; Shibutani, M.; Wang, E.; Nagahara, H.; Fukuoka, T.; Iseki, Y.; Maeda, K.; Hirakawa, K.; Ohira, M. Efficacy of Adjuvant Chemotherapy after Complete Resection of Pulmonary Metastasis from Colorectal Cancer. Mol. Clin. Oncol. 2021, 15, 205. [Google Scholar] [CrossRef]
- Schlachtenberger, G.; Doerr, F.; Menghesha, H.; Lauinger, P.; Wolber, P.; Sabashnikov, A.; Popov, A.-F.; Macherey-Meyer, S.; Bennink, G.; Klussmann, J.P.; et al. Patients with Pulmonary Metastases from Head and Neck Cancer Benefit from Pulmonary Metastasectomy, A Systematic Review. Medicina 2022, 58, 1000. [Google Scholar] [CrossRef]
- Hassan, M.; Ehle, B.; Passlick, B.; Grapatsas, K. Lung Resections for Elderly Patients with Lung Metastases: A Comparative Study of the Postoperative Complications and Overall Survival. Curr. Oncol. 2022, 29, 4511–4521. [Google Scholar] [CrossRef] [PubMed]
- Grapatsas, K.; Papaporfyriou, A.; Leivaditis, V.; Ehle, B.; Galanis, M. Lung Metastatectomy: Can Laser-Assisted Surgery Make a Difference? Curr. Oncol. 2022, 29, 6968–6981. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of Cutaneous Melanoma: Radiologists Challenging and Risk Assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, D.; Kim, H.S.; Park, S.Y.; Kim, J.Y.; Cho, S.J.; Shin, J.H.; Kim, J.H. Quality of science and reporting of radiomics in oncologic studies: Room for improvement according to radiomics quality score and TRIPOD statement. Eur. Radiol. 2020, 30, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Volovici, V.; Syn, N.L.; Ercole, A.; Zhao, J.J.; Liu, N. Steps to avoid overuse and misuse of machine learning in clinical research. Nat. Med. 2022, 28, 1996–1999. [Google Scholar] [CrossRef]
- Faiella, E.; Santucci, D.; Calabrese, A.; Russo, F.; Vadalà, G.; Zobel, B.B.; Soda, P.; Iannello, G.; de Felice, C.; Denaro, V. Artificial Intelligence in Bone Metastases: An MRI and CT Imaging Review. Int. J. Environ. Res. Public Health 2022, 19, 1880. [Google Scholar] [CrossRef]
- Stanzione, A.; Gambardella, M.; Cuocolo, R.; Ponsiglione, A.; Romeo, V.; Imbriaco, M. Prostate MRI Radiomics: A Systematic Review and Radiomic Quality Score Assessment. Eur. J. Radiol. 2020, 129, 109095. [Google Scholar] [CrossRef] [PubMed]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid Review: Radiomics and Breast Cancer. Breast Cancer Res. Treat. 2018, 169, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, A.; Santucci, D.; Landi, R.; Zobel, B.B.; Faiella, E.; de Felice, C. Radiomics MRI for Lymph Node Status Prediction in Breast Cancer Patients: The State of Art. J. Cancer Res. Clin. Oncol. 2021, 147, 1587–1597. [Google Scholar] [CrossRef]
- Montella, M.; Ciani, G.; Granata, V.; Fusco, R.; Grassi, F.; Ronchi, A.; Cozzolino, I.; Franco, R.; Zito Marino, F.; Urraro, F.; et al. Preliminary Experience of Liquid Biopsy in Lung Cancer Compared to Conventional Assessment: Light and Shadows. J. Pers. Med. 2022, 12, 1896. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.V.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Meglio, N.D.; Roscio, D.D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative Imaging Decision Support (QIDS) Tool Consistency Evaluation and Radiomic Analysis by Means of 594 Metrics in Lung Carcinoma on Chest CT Scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Mattace Raso, M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.C.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Dell’ Aversana, F.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Pucciarelli, F.; Zerunian, M.; Ganeshan, B.; De Santis, D.; Polici, M.; Rucci, C.; Polidori, T.; Guido, G.; Bracci, B.; et al. Chest CT Texture-Based Radiomics Analysis in Differentiating COVID-19 from Other Interstitial Pneumonia. Radiol. Med. 2021, 126, 1415–1424. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Belli, A.; Borzillo, V.; Palumbo, P.; Bruno, F.; Grassi, R.; Ottaiano, A.; Nasti, G.; Pilone, V.; et al. Conventional, Functional and Radiomics Assessment for Intrahepatic Cholangiocarcinoma. Infect. Agents Cancer 2022, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.; Muzic, S.I.; Marchetti, F.; Farina, L.M.; Bastianello, S.; Pichiecchio, A. Differential Imaging of Atypical Demyelinating Lesions of the Central Nervous System. Radiol. Med. 2021, 126, 827–842. [Google Scholar] [CrossRef]
- Cilla, S.; Macchia, G.; Lenkowicz, J.; Tran, E.H.; Pierro, A.; Petrella, L.; Fanelli, M.; Sardu, C.; Re, A.; Boldrini, L.; et al. CT Angiography-Based Radiomics as a Tool for Carotid Plaque Characterization: A Pilot Study. Radiol. Med. 2022, 127, 743–753. [Google Scholar] [CrossRef]
- Scaglione, M.; Galluzzo, M.; Santucci, D.; Trinci, M.; Messina, L.; Laccetti, E.; Faiella, E.; Beomonte Zobel, B. Small bowel obstruction and intestinal ischemia: Emphasizing the role of MDCT in the management decision process. Abdom. Radiol. 2022, 47, 1541–1555. [Google Scholar] [CrossRef]
- Cellina, M.; Pirovano, M.; Ciocca, M.; Gibelli, D.; Floridi, C.; Oliva, G. Radiomic Analysis of the Optic Nerve at the First Episode of Acute Optic Neuritis: An Indicator of Optic Nerve Pathology and a Predictor of Visual Recovery? Radiol. Med. 2021, 126, 698–706. [Google Scholar] [CrossRef]
- Sarkar, B.; Ganesh, T.; Munshi, A.; Manikandan, A.; Roy, S.; Krishnankutty, S.; Chitral, L.; Sathiya, J.; Pradhan, A.; Mohanti, B.K. Rotational Positional Error-Corrected Linear Set-up Margin Calculation Technique for Lung Stereotactic Body Radiotherapy in a Dual Imaging Environment of 4-D Cone Beam CT and ExacTrac Stereoscopic Imaging. Radiol. Med. 2021, 126, 979–988. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- El Ayachy, R.; Giraud, N.; Giraud, P.; Durdux, C.; Giraud, P.; Burgun, A.; Bibault, J.E. The Role of Radiomics in Lung Cancer: From Screening to Treatment and Follow-Up. Front. Oncol. 2021, 11, 603595. [Google Scholar] [CrossRef]
- Fornacon-Wood, I.; Mistry, H.; Ackermann, C.J.; Blackhall, F.; McPartlin, A.; Faivre-Finn, C.; Price, G.J.; O’Connor, J.P.B. Reliability and Prognostic Value of Radiomic Features are Highly Dependent on Choice of Feature Extraction Platform. Eur. Radiol. 2020, 30, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Thawani, R.; Mustafa, S.A. The future of radiomics in lung cancer. Lancet Digit. Health 2020, 2, e103. [Google Scholar] [CrossRef] [PubMed]
- Gabelloni, M.; Di Nasso, M.; Morganti, R.; Faggioni, L.; Masi, G.; Falcone, A.; Neri, E. Application of the ESR iGuide clinical decision support system to the imaging pathway of patients with hepatocellular carcinoma and cholangiocarcinoma: Preliminary findings. Radiol. Med. 2020, 125, 531–537. [Google Scholar] [CrossRef]
- Neri, E.; Gabelloni, M.; Bäuerle, T.; Beets-Tan, R.; Caruso, D.; D’Anastasi, M.; Dinkel, J.; Fournier, L.S.; Gourtsoyianni, S.; Hoffmann, R.T.; et al. Involvement of radiologists in oncologic multidisciplinary team meetings: An international survey by the European Society of Oncologic Imaging. Eur. Radiol. 2021, 31, 983–991. [Google Scholar] [CrossRef]
- Morin, O.; Vallières, M.; Jochems, A.; Woodruff, H.C.; Valdes, G.; Braunstein, S.E.; Wildberger, J.E.; Villanueva-Meyer, J.E.; Kearney, V.; Yom, S.S.; et al. A Deep Look Into the Future of Quantitative Imaging in Oncology: A Statement of Working Principles and Proposal for Change. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).