Multisystem Inflammatory Syndrome in Adults (MIS-A) Reported in Patient with Hematological Malignancy: A Case Report
Abstract
:1. Background
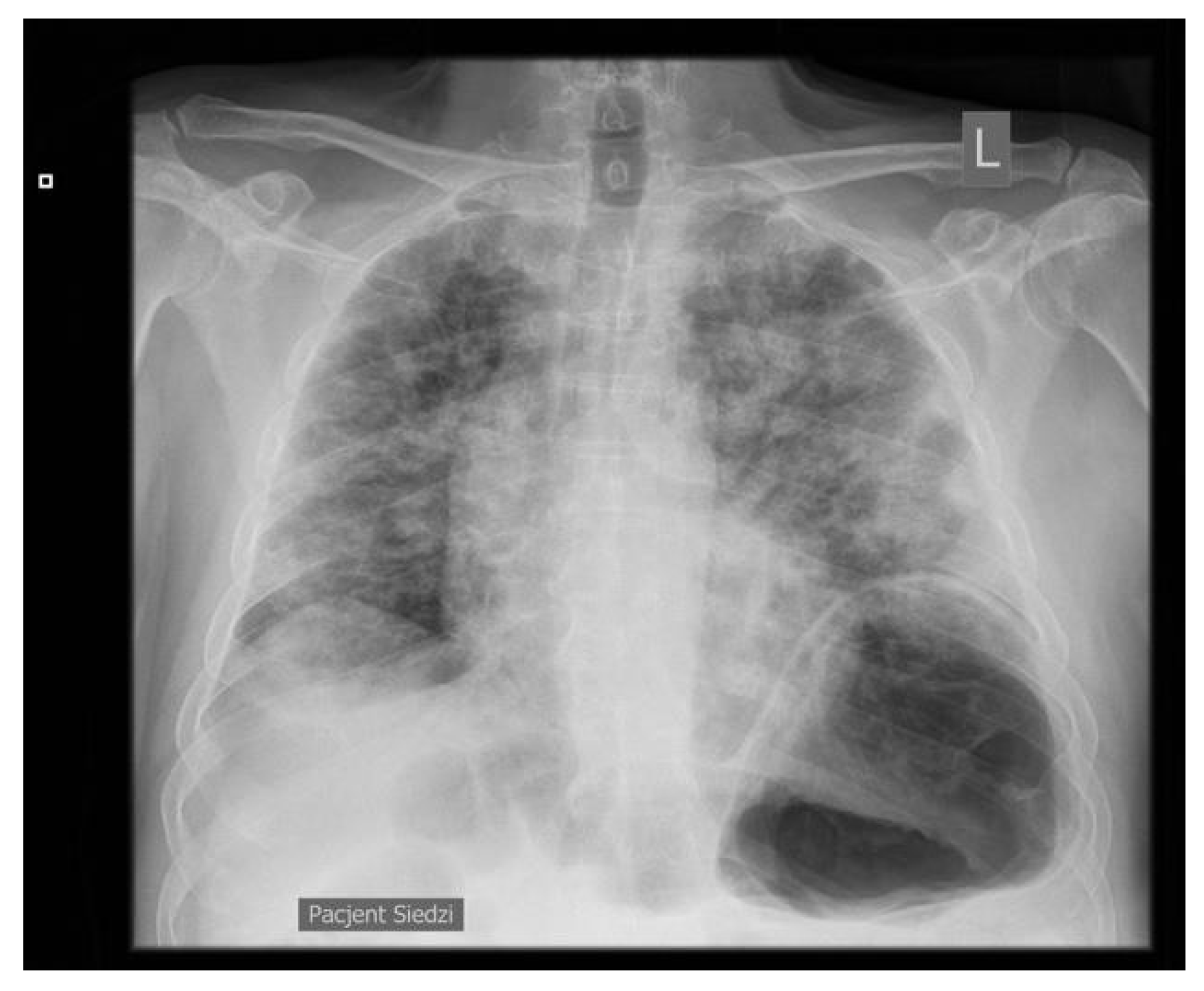
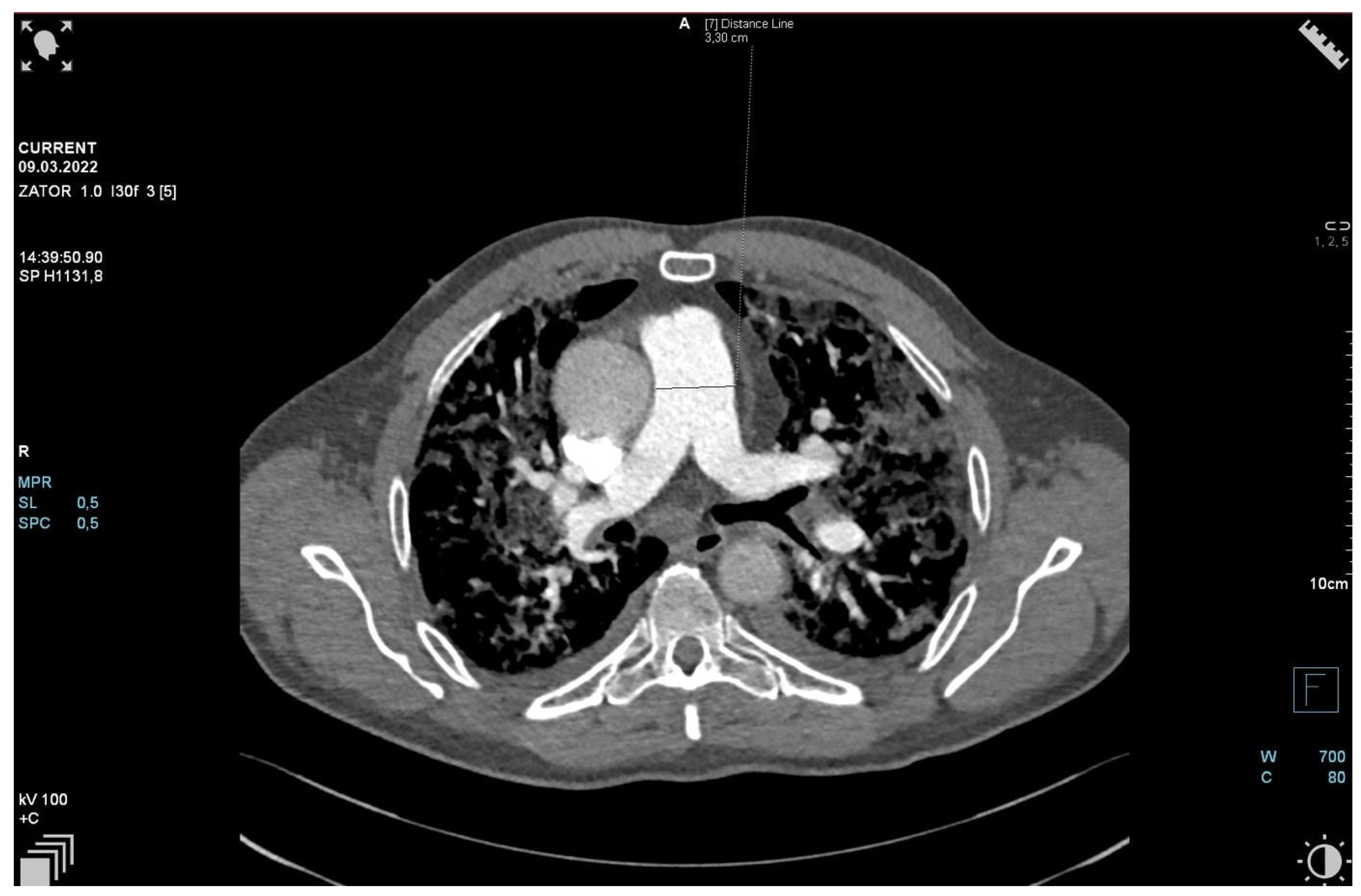
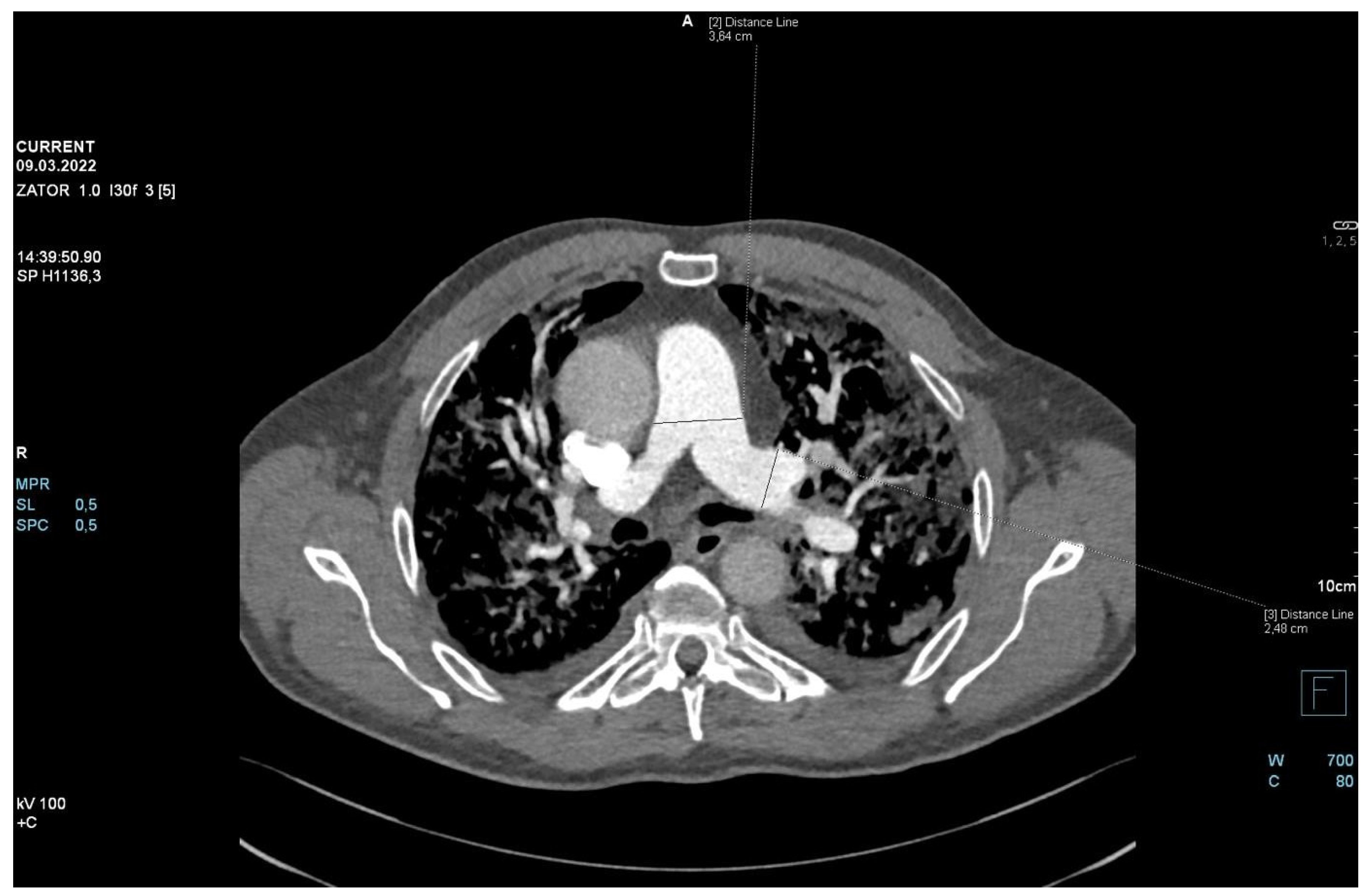
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Patel, P.; De Cuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw. Open. 2021, 4, e2126456. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Adults (MIS-A) Case Definition Infomation for Healthcare Providers. Available online: https://www.cdc.gov/mis/mis-a/hcp.html (accessed on 20 December 2022).
- Kalicińska, E.; Biernat, M.; Rybka, J.; Zińczuk, A.; Janocha-Litwin, J.; Rosiek-Biegus, M.; Morawska, M.; Waszczuk-Gajda, A.; Drozd-Sokołowska, J.; Szukalski, Ł.; et al. Endothelial Activation and Stress Index (EASIX) as an Early Predictor for Mortality and Overall Survival in Hematological and Non- Hematological Patients with COVID-19: Multicenter Cohort Study. J. Clin. Med. 2021, 10, 4373. [Google Scholar] [CrossRef] [PubMed]
- Weatherhead, J.E.; Clark, E.; Vogel, T.P.; Atmar, R.L.; Kulkarni, P.A. Inflammatory syndromes associated with SARS-CoV-2 infection: Dysregulation of the immune response across the age spectrum. J Clin Investig. 2020, 130, 6194–6197. [Google Scholar] [CrossRef] [PubMed]
- Tahaghoghi-Hajghorbani, S.; Zafari, P.; Masoumi, E.; Rajabinejad, M.; Jafari-Shakib, R.; Hasani, B.; Rafiei, A. The role of dysregulated immune responses in COVID-19 pathogenesis. Virus Res. 2020, 290, 198197. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, R.; Aguinaga, L.; Feliu, J.; Anton-Remirez, J.; Jorge-Del-Val, L.; Casajús- Navasal, A.; Nebot-Villacampa, M.J.; Daroca-Fernandez, I.; Domínguez-Garrido, E.; Rabasa, P.; et al. Obinutuzumab induces depletion of NK cells in patients with chronic lymphocytic leukemia. Immunotherapy 2018, 10, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ronit, A.; Jørgensen, S.E.; Roed, C.; Eriksson, R.; Iepsen, U.W.; Plovsing, R.R.; Storgaard, M.; Gustafsson, F.; Hansen, A.E.; Mogensen, T.H. Host Genetics and Antiviral Immune Responses in Adult Patients With Multisystem Inflammatory Syndrome. Front. Immunol. 2021, 12, 718744. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Kalicińska, E.; Szymczak, D.; Andrasiak, I.; Bogucka-Fedorczuk, A.; Zińczuk, A.; Szymański, W.; Biernat, M.; Rymko, M.; Semeńczuk, G.; Jabłonowska, P.; et al. Lymphocyte subsets in haematological patients with COVID-19: Multicentre prospective study. Transl. Oncol. 2021, 14, 100943. [Google Scholar] [CrossRef] [PubMed]
- Taeschler, P.; Adamo, S.; Deng, Y.; Cervia, C.; Zurbuchen, Y.; Chevrier, S.; Raeber, M.E.; Hasler, S.; Bächli, E.; Rudiger, A.; et al. T-cell recovery and evidence of persistent immune activation 12 months after severe COVID-19. Allergy 2022, 77, 2468–2481. [Google Scholar] [CrossRef] [PubMed]

| Variable | Normal Range | 2 Months before MIS-A | Day 1 | Day 3 | Day 5 | Day 7 | Day 10 | after 3 Months |
|---|---|---|---|---|---|---|---|---|
| C-reactive protein [mg/L] | 0.2–5.0 | 1.6 | 20.6 | 117 | 172 | 104 | 47 | 7.1 |
| Procalcitonin [ng/mL] | 0–0.05 | -- | 0.09 | 0.3 | 0.66 | 0.59 | 0.3 | 0.05 |
| Interleukin-6 [pg/mL] | 0–5.9 | -- | 36 | 197 | 282 | 11 | 2 | 1.2 |
| Ferritin [ug/L] | 20–290 | 111 | 390 | 1670 | 1810 | 1520 | 858 | 101 |
| Fibrinogen [g/L] | 2–4.39 | 2.77 | 5.16 | 5.89 | 6.22 | 4.39 | 3.44 | 3.77 |
| D-dimers [ug/mL] | 0–0.5 | 0.242 | 0.667 | 1.63 | 5.02 | 15.29 | 6.7 | 0.97 |
| LDH [U/L] | 125–220 | 169 | 295 | 560 | 588 | 642 | 440 | 206 |
| White blood cells [103/uL] | 4–10 | 3.83 | 2.36 | 6.81 | 8.16 | 12.58 | 9.17 | 5.99 |
| Lymphocytes [103/uL] | 1.5–3.5 | 1.44 | 0.59 | 0.65 | 0.59 | 0.59 | 0.71 | 1.76 |
| Neutrophiles [103/uL] | 2.5–6 | 1.7 | 1.31 | 5.58 | 7.19 | 11.67 | 8.14 | 3.36 |
| Hemoglobin [g/dL] | 14–18 | 14 | 13.7 | 13.7 | 11.6 | 11.4 | 10.3 | 13.2 |
| Platelets [103/uL] | 140–440 | 63 | 3 | 4 | 2 | 15 | 43 | 75 |
| BNP [pg/mL] | 0–125 | -- | 102 | 166 | 426 | 194 | 111 | 25.4 |
| NT-proBNP [pg/mL] | 0–125 | -- | 231 | 659 | 2354 | 899 | 305 | 68.6 |
| T CD3+ Lymphocytes | |||
|---|---|---|---|
| CD3+: 100% | CD8+: 65% | CD5+: 91% | CD56 + CD16+: negative |
| CD7+: 85% | CD4+: 35% | CD4+/CD8+ ratio: 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalicińska, E.; Jabłonowska, P.; Wróbel, T. Multisystem Inflammatory Syndrome in Adults (MIS-A) Reported in Patient with Hematological Malignancy: A Case Report. J. Pers. Med. 2023, 13, 178. https://doi.org/10.3390/jpm13020178
Kalicińska E, Jabłonowska P, Wróbel T. Multisystem Inflammatory Syndrome in Adults (MIS-A) Reported in Patient with Hematological Malignancy: A Case Report. Journal of Personalized Medicine. 2023; 13(2):178. https://doi.org/10.3390/jpm13020178
Chicago/Turabian StyleKalicińska, Elżbieta, Paula Jabłonowska, and Tomasz Wróbel. 2023. "Multisystem Inflammatory Syndrome in Adults (MIS-A) Reported in Patient with Hematological Malignancy: A Case Report" Journal of Personalized Medicine 13, no. 2: 178. https://doi.org/10.3390/jpm13020178
APA StyleKalicińska, E., Jabłonowska, P., & Wróbel, T. (2023). Multisystem Inflammatory Syndrome in Adults (MIS-A) Reported in Patient with Hematological Malignancy: A Case Report. Journal of Personalized Medicine, 13(2), 178. https://doi.org/10.3390/jpm13020178





