Efficacy of Low-Level Laser Therapy for Oral Mucositis in Hematologic Patients Undergoing Transplantation: A Single-Arm Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Criteria for Treatment Discontinuation
2.3. Prophylactic LLLT for Oral Mucositis
2.4. Oral Assessment and Measurements
2.5. Endpoints
2.6. Equipment Used and Adverse Events
2.7. Statistical Analysis
2.8. Sample Size
2.9. Data Management
3. Results
3.1. Patient Characteristics
3.2. Efficacy of Low-Level Laser Therapy for Oral Mucositis
3.3. Low-Level Laser Therapy Safety Profile
3.4. Comparison of Patient Characteristics Based on Oral Mucositis Severity
3.5. Temporal Changes in Oral Bacterial Count following Pre-Treatment Initiation
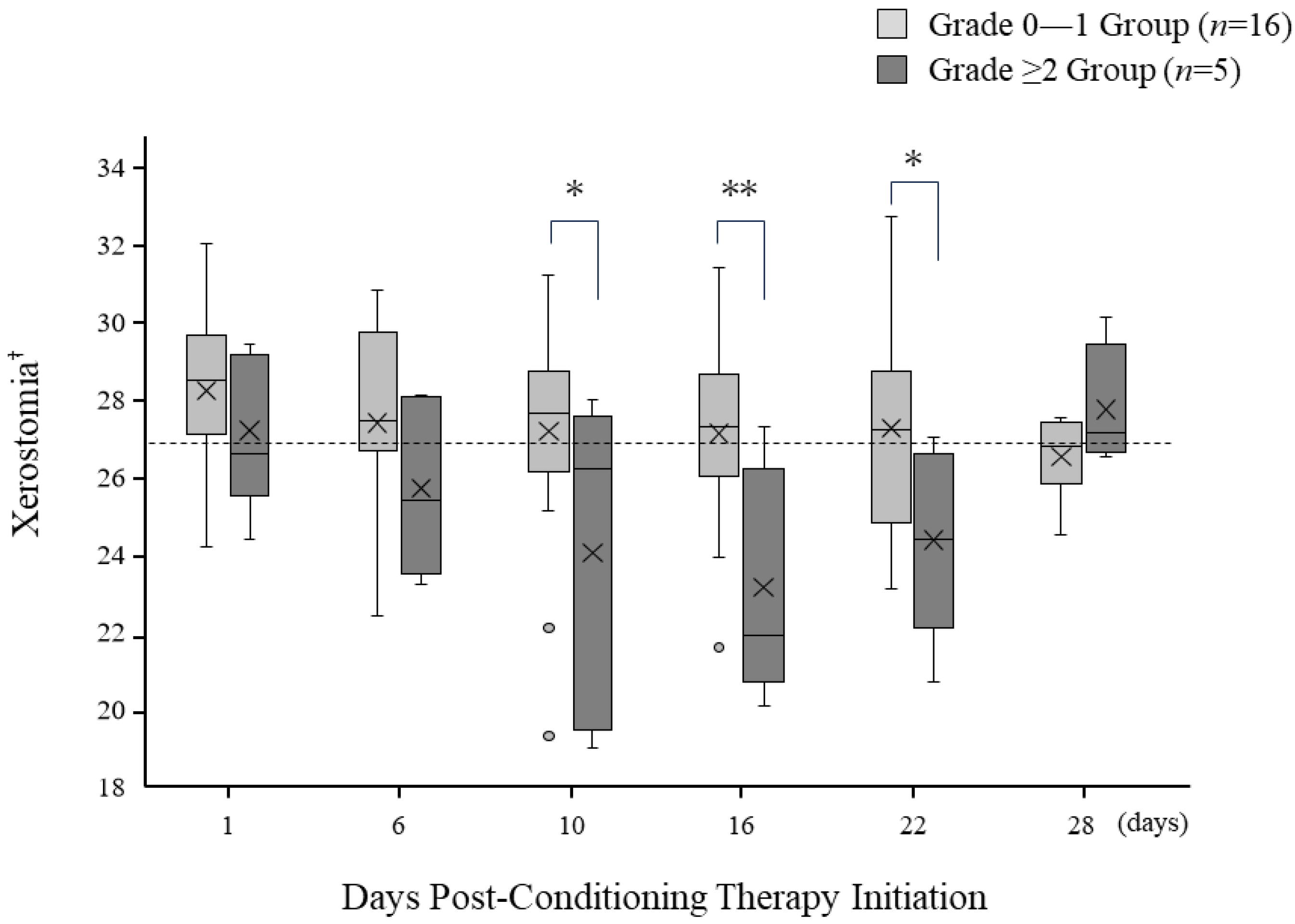
3.6. Temporal Changes in Oral Dryness following Pre-Treatment Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amiri Khosroshahi, R.; Talebi, S.; Zeraattalab-Motlagh, S.; Imani, H.; Rashidi, A.; Travica, N.; Mohammadi, H. Nutritional Interventions for the Prevention and Treatment of Cancer Therapy-Induced Oral Mucositis: An Umbrella Review of Systematic Reviews and Meta-Analysis. Nutr. Rev. 2023, 81, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The Effects of Swallowing Disorders, Dysgeusia, Oral Mucositis and Xerostomia on Nutritional Status, Oral Intake and Weight Loss in Head and Neck Cancer Patients: A Systematic Review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Vera-Llonch, M.; Oster, G.; Ford, C.M.; Lu, J.; Sonis, S. Oral Mucositis and Outcomes of Allogeneic Hematopoietic Stem-Cell Transplantation in Patients with Hematologic Malignancies. Support. Care Cancer 2007, 15, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, Y.; Imataki, O.; Uemura, M.; Takeuchi, A.; Aoki, S.; Tanaka, M.; Nakai, Y.; Nakai, F.; Miyake, M. Oral Microorganisms and Bloodstream Infection in Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Oral Investig. 2021, 25, 4359–4367. [Google Scholar] [CrossRef]
- Scully, C.; Epstein, J.; Sonis, S. Oral Mucositis: A Challenging Complication of Radiotherapy, Chemotherapy, and Radiochemotherapy. Part 2: Diagnosis and Management of Mucositis. Head Neck 2004, 26, 77–84. [Google Scholar] [CrossRef]
- Alsulami, F.J.; ul Shaheed, S. Oral Cryotherapy for Management of Chemotherapy-induced Oral Mucositis in Haematopoietic Cell Transplantation: A Systematic Review. BMC Cancer 2022, 22, 442. [Google Scholar] [CrossRef]
- Franco, R.; Lupi, E.; Iacomino, E.; Galeotti, A.; Capogreco, M.; Santos, J.M.M.; D’Amario, M. Low-Level Laser Therapy for the Treatment of Oral Mucositis Induced by Hematopoietic Stem Cell Transplantation: A Systematic Review with Meta-Analysis. Medicina 2023, 59, 1413. [Google Scholar] [CrossRef]
- Tam, S.Y.; Tam, V.C.W.; Ramkumar, S.; Khaw, M.L.; Law, H.K.W.; Lee, S.W.Y. Review on the Cellular Mechanisms of Low-Level Laser Therapy Use in Oncology. Front. Oncol. 2020, 10, 1255. [Google Scholar] [CrossRef]
- Ferreira, B.; da Motta Silveira, F.M.; de Orange, F.A. Low-Level Laser Therapy Prevents Severe Oral Mucositis in Patients Submitted to Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. Support. Care Cancer 2016, 24, 1035–1042. [Google Scholar] [CrossRef]
- Migliorati, C.; Hewson, I.; Lalla, R.V.; Antunes, H.S.; Estilo, C.L.; Hodgson, B.; Lopes, N.N.F.; Schubert, M.M.; Bowen, J.; Elad, S. Systematic Review of Laser and Other Light Therapy for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 333–341. [Google Scholar] [CrossRef]
- Logan, R.M.; Al-Azri, A.R.; Bossi, P.; Stringer, A.M.; Joy, J.K.; Soga, Y.; Ranna, V.; Vaddi, A.; Raber-Durlacher, J.E.; Lalla, R.V.; et al. Systematic Review of Growth Factors and Cytokines for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines. Support. Care Cancer 2020, 28, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kerio, A.A.; Khattak, T.A.; Hussain, M.; Khan, M.A.; Abbas, Y. Oral Mucositis in Patients Undergoing Hematopoietic Stem Cell Transplantation. J. Coll. Physicians Surg. Pak. 2023, 33, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hamada, R.; Nakano, M.; Suehiro, J.; Konishi, K.; Kikutani, T. Development of Rapid Oral Bacteria Detection Apparatus Based on Dielectrophoretic Impedance Measurement Method. IET Nanobiotechnol. 2011, 5, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Nishi, Y.; Kamashita, Y.; Nagaoka, E. Relationship between Medical Treatment and Oral Dryness Diagnosed by Oral Moisture-Checking Device in Patients with Maxillofacial Prostheses. J. Prosthodont. Res. 2009, 53, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal Inflamed Surface Area: Quantifying Inflammatory Burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Obayashi, T.; Ueda, T.; Ohta, K.; Shigeishi, H.; Munenaga, S.; Kono, T.; Yoshioka, Y.; Konishi, M.; Taga, R.; et al. Head and Neck Cancer Patients Show Poor Oral Health as Compared to Those with Other Types of Cancer. BMC Oral Health 2023, 23, 647. [Google Scholar] [CrossRef]
- Barolet, D.; Duplay, P.; Jacomy, H.; Auclair, M. Importance of Pulsing Illumination Parameters in Low-Level-Light Therapy. J. Biomed. Opt. 2010, 15, 048005. [Google Scholar] [CrossRef]
- Ichiro Watanabe, Y.M. LLLT Is Clinically Useful in Wound Healing, Musculoskeletal Disorders and Pain Management. Jpn. J. Rehabil. Med. 2001, 38, 587–595. [Google Scholar] [CrossRef]
- Heger, M.; Bezemer, R.; Huertas-Pérez, J.F.; Dekker, H.; Beek, J.F. Endovascular Laser-Tissue Interactions Redefined: Shining Light on Novel Windows of Therapeutic Opportunity beyond Selective Photothermolysis. Photomed. Laser Surg. 2010, 28, 569–572. [Google Scholar] [CrossRef]
- Baron, F.; Labopin, M.; Peniket, A.; Jindra, P.; Afanasyev, B.; Sanz, M.A.; Deconinck, E.; Nagler, A.; Mohty, M. Reduced-Intensity Conditioning with Fludarabine and Busulfan versus Fludarabine and Melphalan for Patients with Acute Myeloid Leukemia: A Report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2015, 121, 1048–1055. [Google Scholar] [CrossRef]
- Edahiro, T.; Kawase, T.; Nagoshi, H.; Fujino, K.; Toishigawa, K.; Miyama, T.; Mino, T.; Yoshida, T.; Morioka, T.; Hirata, Y.; et al. Allogeneic Hematopoietic Cell Transplantation Using Fludarabine plus Myeloablative Busulfan and Melphalan Confers Promising Survival in High-Risk Hematopoietic Neoplasms: A Single-Center Retrospective Analysis. Hematology 2021, 26, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.S.; De Azevedo, A.M.; Bouzas, L.F.D.S.; Adão, C.A.E.; Pinheiro, C.T.; Mayhe, R.; Pinheiro, L.H.; Azevedo, R.; De Matos, V.D.A.; Rodrigues, P.C.; et al. Low-Power Laser in the Prevention of Induced Oral Mucositis in Bone Marrow Transplantation Patients: A Randomized Trial. Blood 2007, 109, 2250–2255. [Google Scholar] [CrossRef][Green Version]
- Vibha, S.; Akhilesh Kumar, S. Oral Mucositis. Natl. J. Maxillofac. Surg. 2020, 112, 159–168. [Google Scholar] [CrossRef]
- Jones, B. Oral Hydration and Its Implications in Oral Mucositis: A Review. Oral Dis. 2017, 23, 189–190. [Google Scholar]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Radochová, V.; Šembera, M.; Slezák, R.; Heneberk, O.; Radocha, J. Oral Mucositis Association with Periodontal Status: A Retrospective Analysis of 496 Patients Undergoing Hematopoietic Stem Cell Transplantation. J. Clin. Med. 2021, 10, 5790. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis Among Patients With Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Emerging Evidence on the Pathobiology of Mucositis. Support. Care Cancer 2013, 21, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Miyano, K.; Ueno, T.; Yatsuoka, W.U.Y. Treatment for Cancer Patients with Oral Mucositis: Assessment Based on the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer in International Society of Oral Oncology (MASCC/ISOO) in 2013 and Proposal of Possible Novel Treatment with a Japanese Herbal Medicine. Curr. Pharm. Des. 2016, 22, 2270–2278. [Google Scholar] [CrossRef] [PubMed]


| Clinical Parameter | n = 21 |
|---|---|
| Age (mean ± SD) | 52.6 ± 12.4 |
| Gender (% male) | 13 (61.9) |
| BMI (mean ± SD) | 23.5 ± 3.1 |
| Disease, n (%) | |
| -Myeloid Leukemias | 8 (38.1) |
| -Lymphoid Leukemias/Lymphomas | 6 (28.6) |
| -Multiple Myeloma | 3 (14.3) |
| -Virus-Related Disorders | 2 (9.5) |
| -Other Disorders | 2 (9.5) |
| Type of Hematopoietic Stem Cell Transplantation (HSCT), n (%) | |
| -Autologous-HSCT | 10 (47.6) |
| -Allogeneic-HSCT | 11 (52.4) |
| Donor source, n (%) | |
| -Autologous Peripheral Blood Stem Cell | 10 (47.6) |
| -Related Bone Marrow | 1 (4.8) |
| -Unrelated Bone Marrow | 5 (23.8) |
| -Unrelated Cord Blood | 5 (23.8) |
| Conditioning regimen, n (%) | |
| -Reduced-Intensity Conditioning (RIC) | 9 (42.9) |
| -Myeloablative Conditioning (MAC) | 12 (57.1) |
| Total Body Irradiation (TBI), n (%) | |
| -Non-TBI | 14 (66.7) |
| -2Gy-TBI | 7 (33.3) |
| Parameter | Grade 0–1 Oral Mucositis (n = 16) | Grade ≥ 2 Oral Mucositis (n = 5) | p Value |
|---|---|---|---|
| Age, years | 55.2 ± 3.1 | 46.6 ± 5.6 | 0.19 |
| Gender (% male) | 9 (56.3) | 4 (80.0) | 0.32 |
| BMI (mean ± SD) | 22.8 ± 3.1 | 25.4 ± 2.2 | 0.10 |
| PESA (mm2), median (IQR) | 1240.8 (876.8–1353.4) | 1001.3 (823.2–1145.9) | 0.61 |
| PISA (mm2), median (IQR) | 45.8 (23.7–209.6) | 24.6 (3.0–112.2) | 0.31 |
| Oral bacteria count (×104 CFU/mL) | 478.2 ± 336.0 | 827.8 ± 365.9 | 0.09 |
| Xerostomia (mean ± SD) | 26.5 ± 3.3 | 27.8 ± 2.7 | 0.50 |
| Type of Hematopoietic Stem Cell Transplantation (HSCT), n (%) | 0.14 | ||
| -Autologous-HSCT | 9 (56.3) | 1 (20.0) | |
| -Allogeneic-HSCT | 7 (43.8) | 4 (80.0) | |
| Donor source, n (%) | <0.05 | ||
| -Autologous Peripheral Stem Cell | 9 (56.3) | 1 (20.0) | |
| -Related Bone Marrow | 0 (0.0) | 1 (20.0) | |
| -Unrelated Bone Marrow | 2 (12.5) | 3 (60.0) | |
| -Unrelated Cord Blood | 5 (31.3) | 0 (0.0) | |
| Conditioning regimen, n (%) | 0.88 | ||
| -Reduced-Intensity Conditioning | 7 (43.8) | 2 (40.0) | |
| -Myeloablative Conditioning | 9 (56.3) | 3 (60.0) | |
| Total Body Irradiation (TBI), n (%) | 0.45 | ||
| -Non-TBI | 10 (62.5) | 4 (80.0) | |
| -2Gy-TBI | 6 (37.5) | 1 (20.0) | |
| Days with Fever after Pre-treatment | 6.1 ± 4.2 | 6.8 ± 4.3 | 0.74 |
| Missed Meals after Pre-treatment | 6.2 ± 3.8 | 11.8 ± 6.8 | 0.48 |
| Days from Pre-treatment to Discharge | 41.5 ± 24.3 | 68.8 ± 24.4 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, H.; Horikoshi, S.; Ohta, K.; Yoshida, T.; Fukushima, N.; Oshita, K.; Munenaga, S.; Edahiro, T.; Ureshino, H.; Shigeishi, H.; et al. Efficacy of Low-Level Laser Therapy for Oral Mucositis in Hematologic Patients Undergoing Transplantation: A Single-Arm Prospective Study. J. Pers. Med. 2023, 13, 1603. https://doi.org/10.3390/jpm13111603
Nishi H, Horikoshi S, Ohta K, Yoshida T, Fukushima N, Oshita K, Munenaga S, Edahiro T, Ureshino H, Shigeishi H, et al. Efficacy of Low-Level Laser Therapy for Oral Mucositis in Hematologic Patients Undergoing Transplantation: A Single-Arm Prospective Study. Journal of Personalized Medicine. 2023; 13(11):1603. https://doi.org/10.3390/jpm13111603
Chicago/Turabian StyleNishi, Hiromi, Susumu Horikoshi, Kouji Ohta, Tetsumi Yoshida, Noriyasu Fukushima, Kyoko Oshita, Syuichi Munenaga, Taro Edahiro, Hiroshi Ureshino, Hideo Shigeishi, and et al. 2023. "Efficacy of Low-Level Laser Therapy for Oral Mucositis in Hematologic Patients Undergoing Transplantation: A Single-Arm Prospective Study" Journal of Personalized Medicine 13, no. 11: 1603. https://doi.org/10.3390/jpm13111603
APA StyleNishi, H., Horikoshi, S., Ohta, K., Yoshida, T., Fukushima, N., Oshita, K., Munenaga, S., Edahiro, T., Ureshino, H., Shigeishi, H., Yoshioka, Y., Konishi, M., Ide, N., Ogawa, Y., Marukawa, R., Shintani, T., Ino, N., Kajiya, M., Kakimoto, N., ... Kawaguchi, H. (2023). Efficacy of Low-Level Laser Therapy for Oral Mucositis in Hematologic Patients Undergoing Transplantation: A Single-Arm Prospective Study. Journal of Personalized Medicine, 13(11), 1603. https://doi.org/10.3390/jpm13111603







