Principles and Standards for Designing and Managing Integrable and Interoperable Transformed Health Ecosystems
Abstract
:1. Introduction
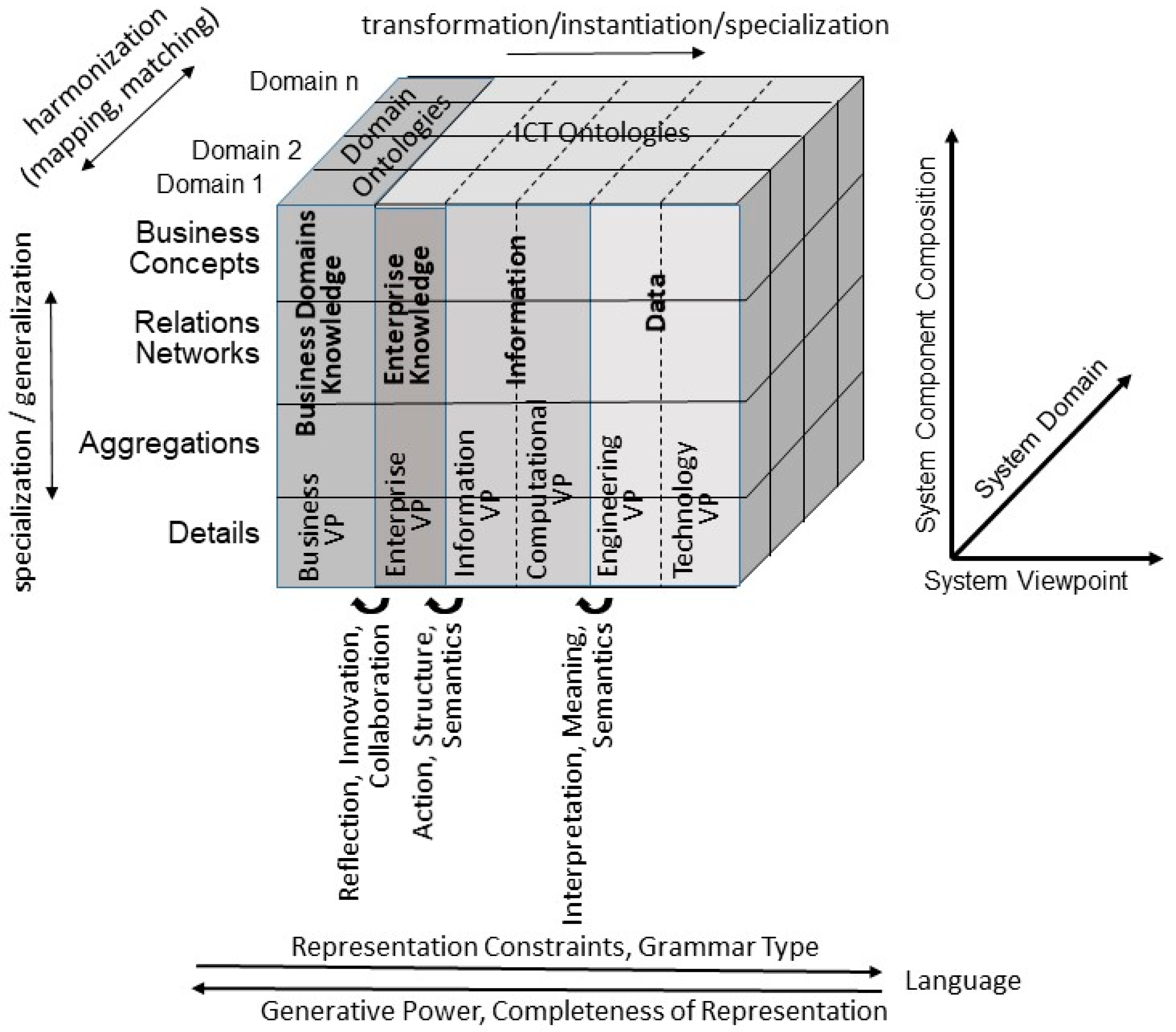
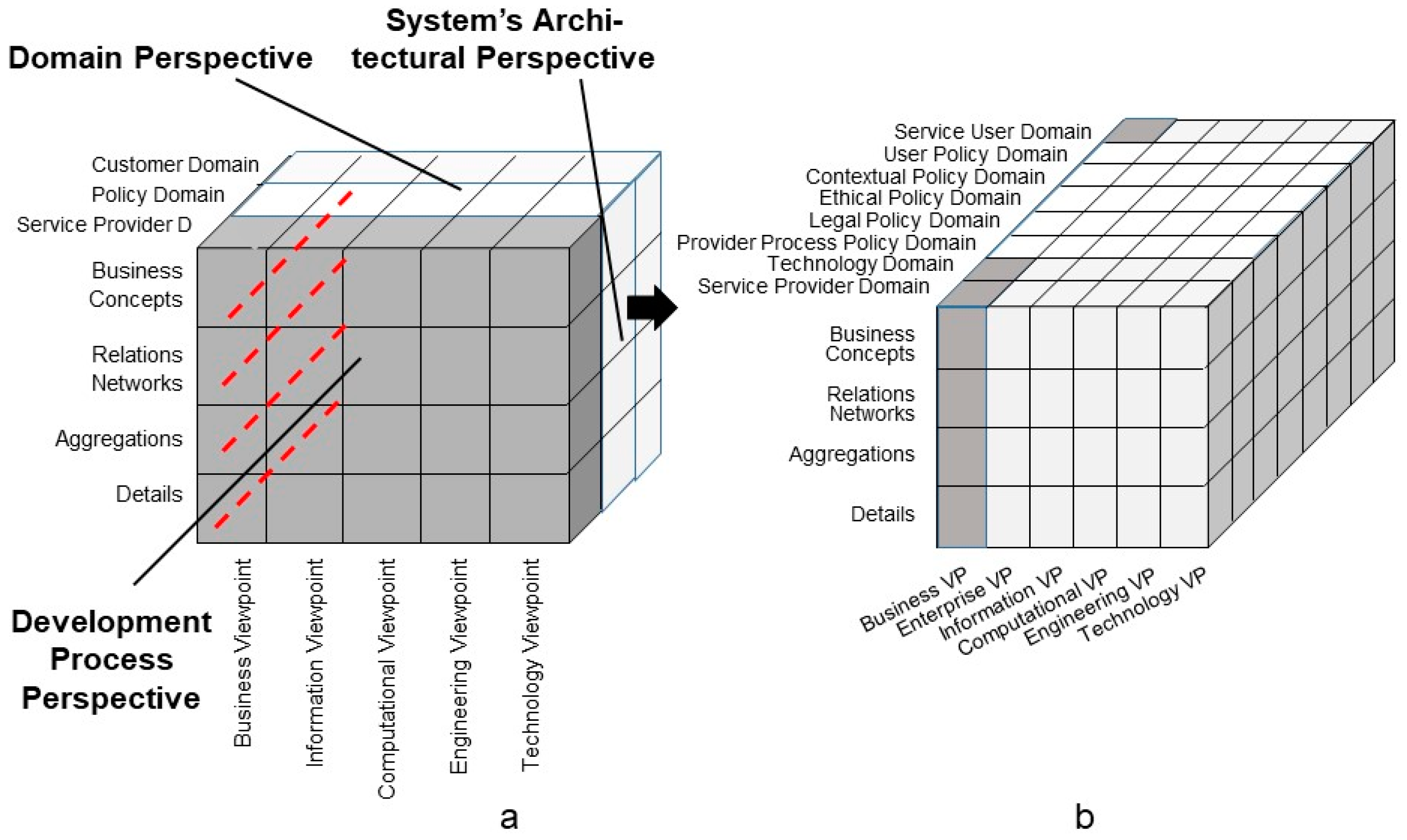
2. Representation of 5P Medicine Ecosystems
- The system’s architectural perspective, representing the system’s composition/decomposition or specialization/generalization;
- The system’s domain perspective, representing the involved domains and their actors;
- The system’s evolutionary or development perspective.
3. Standards for Modeling 5P Medicine Ecosystems
3.1. Architecture Standards
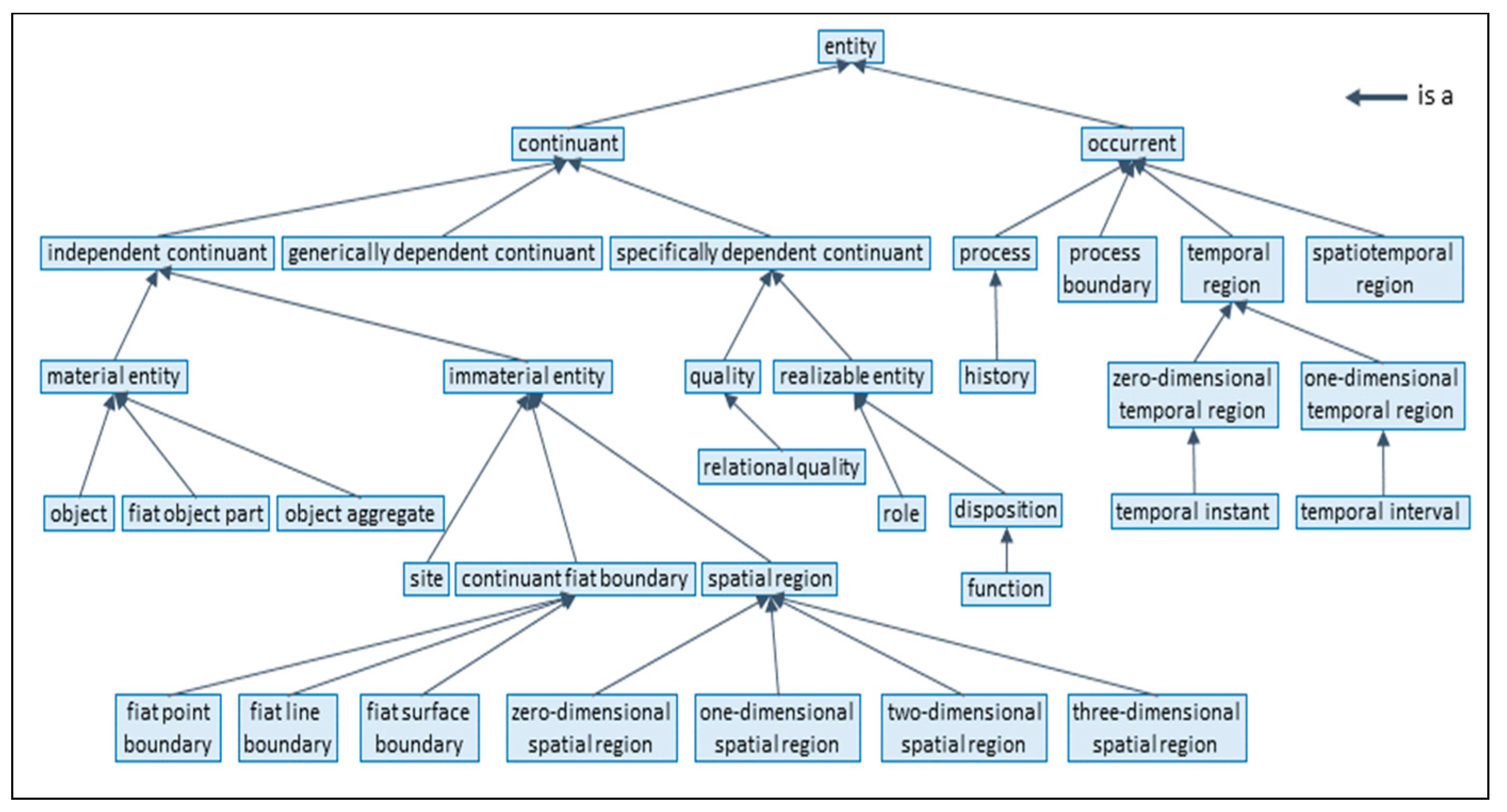
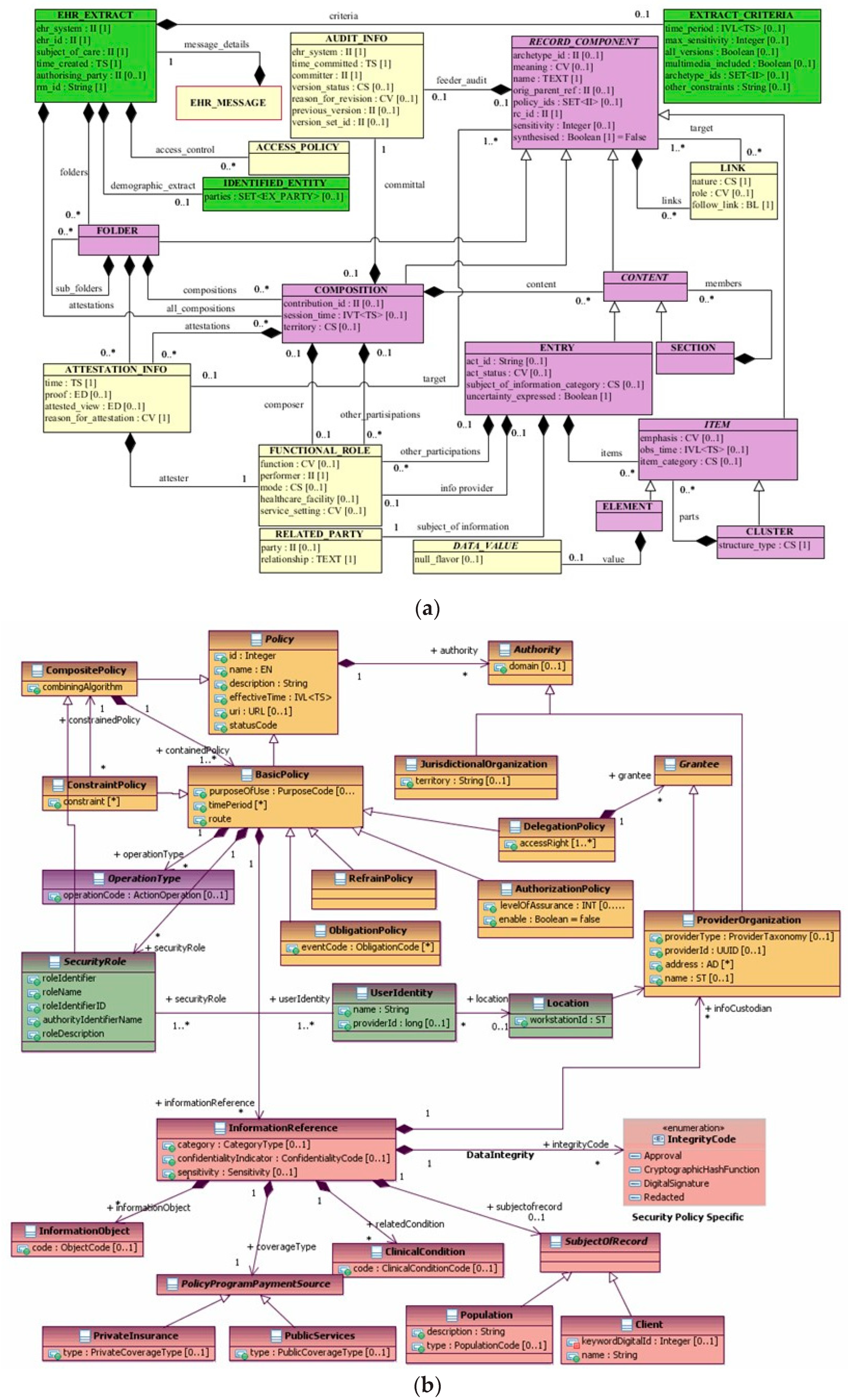
3.2. Knowledge Representation and Knowledge Management Standards
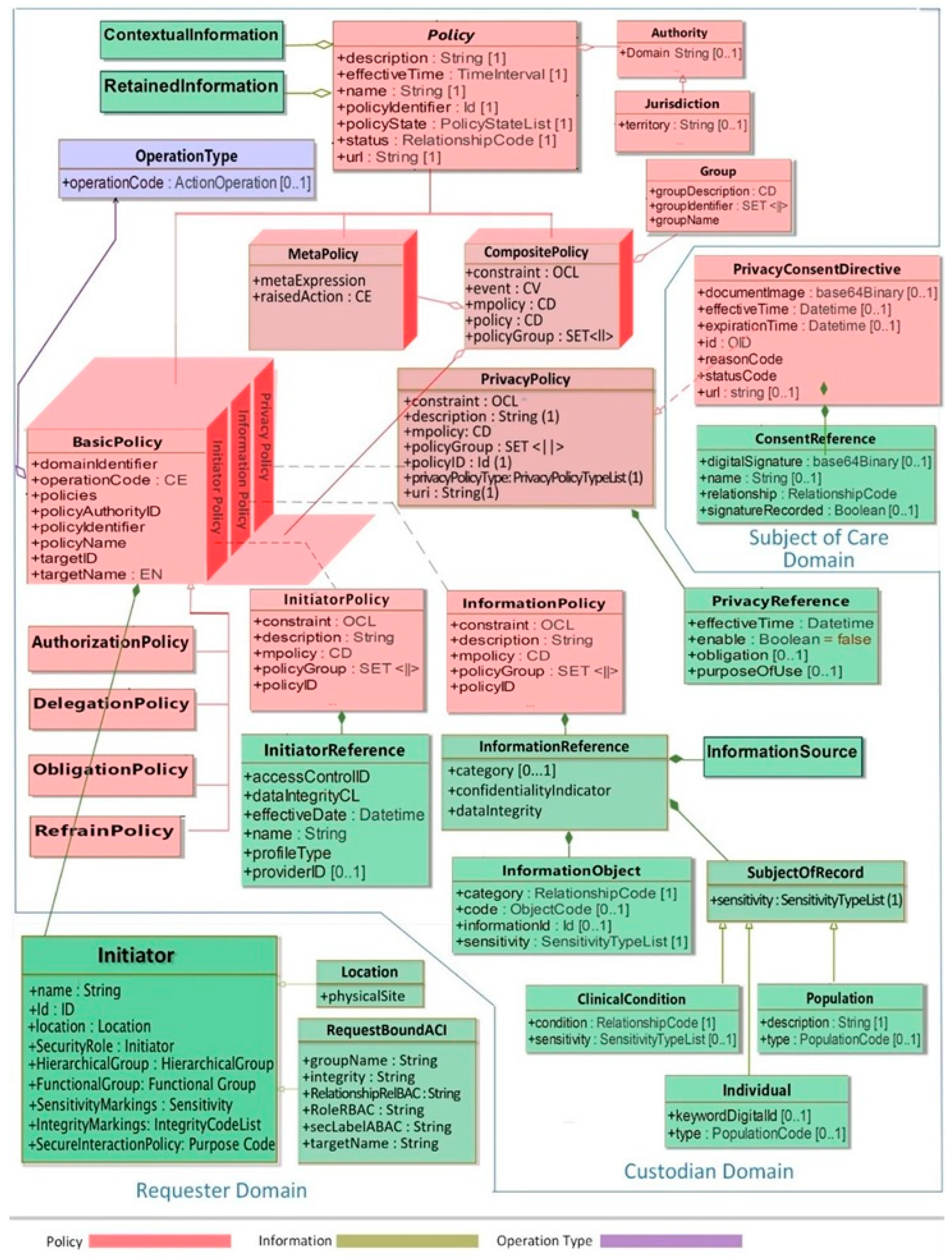
3.3. The Policy Domain
4. A Short Overview on Standard Classes and Related Specifications
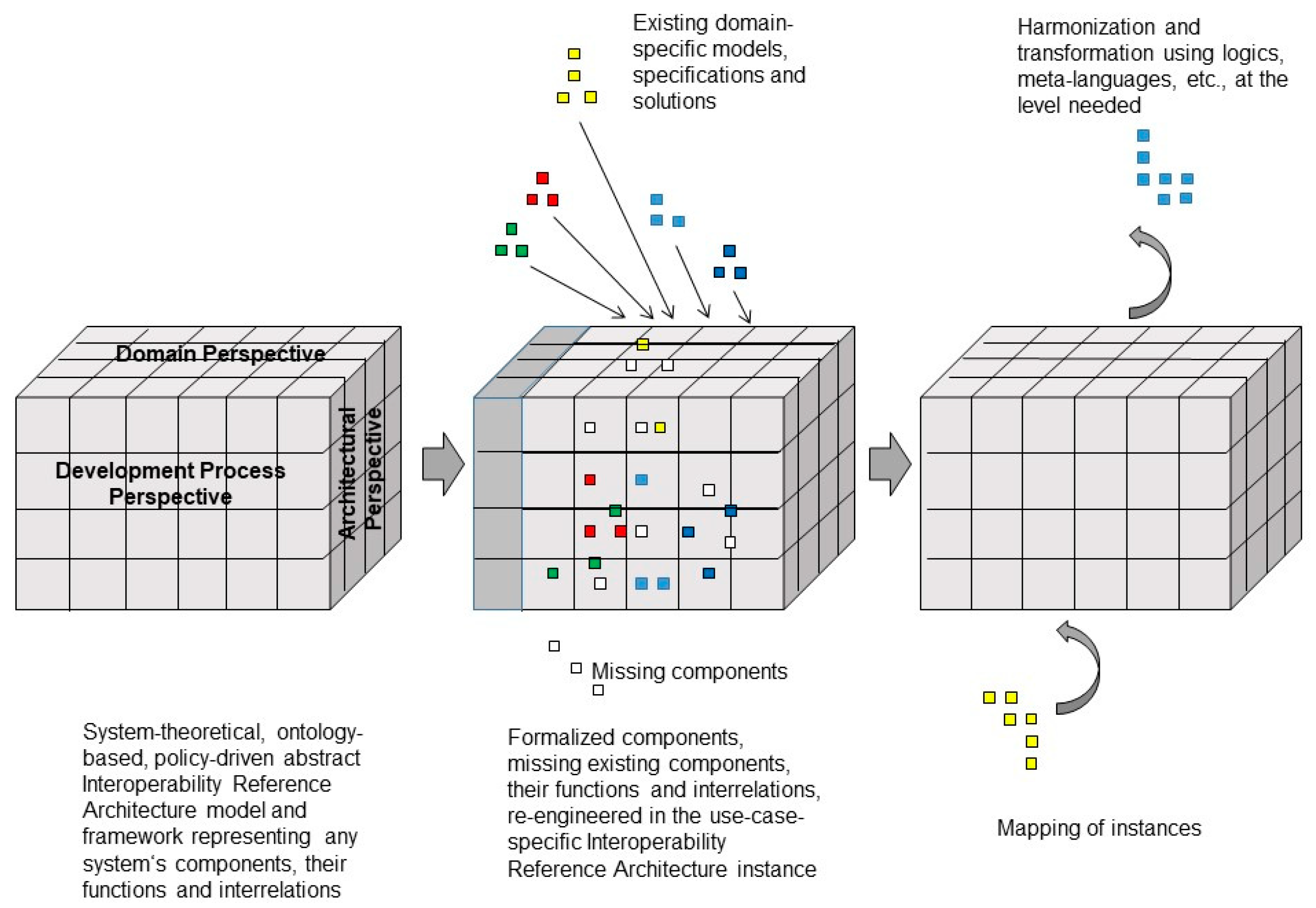
5. Managing the Modeling and Development Process of 5P Medicine Ecosystems
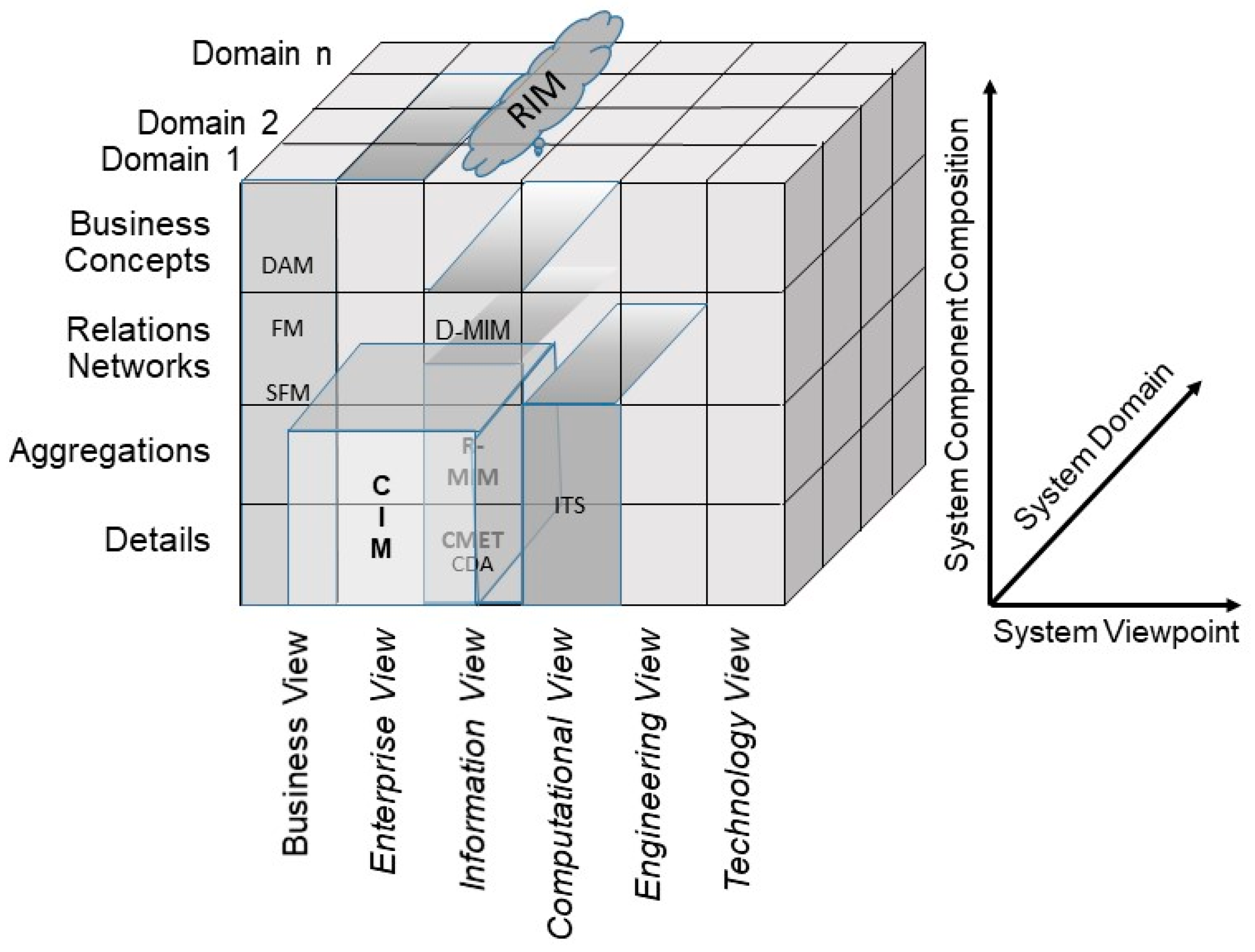
5.1. Representation of 5P Medicine Ecosystems through Standards
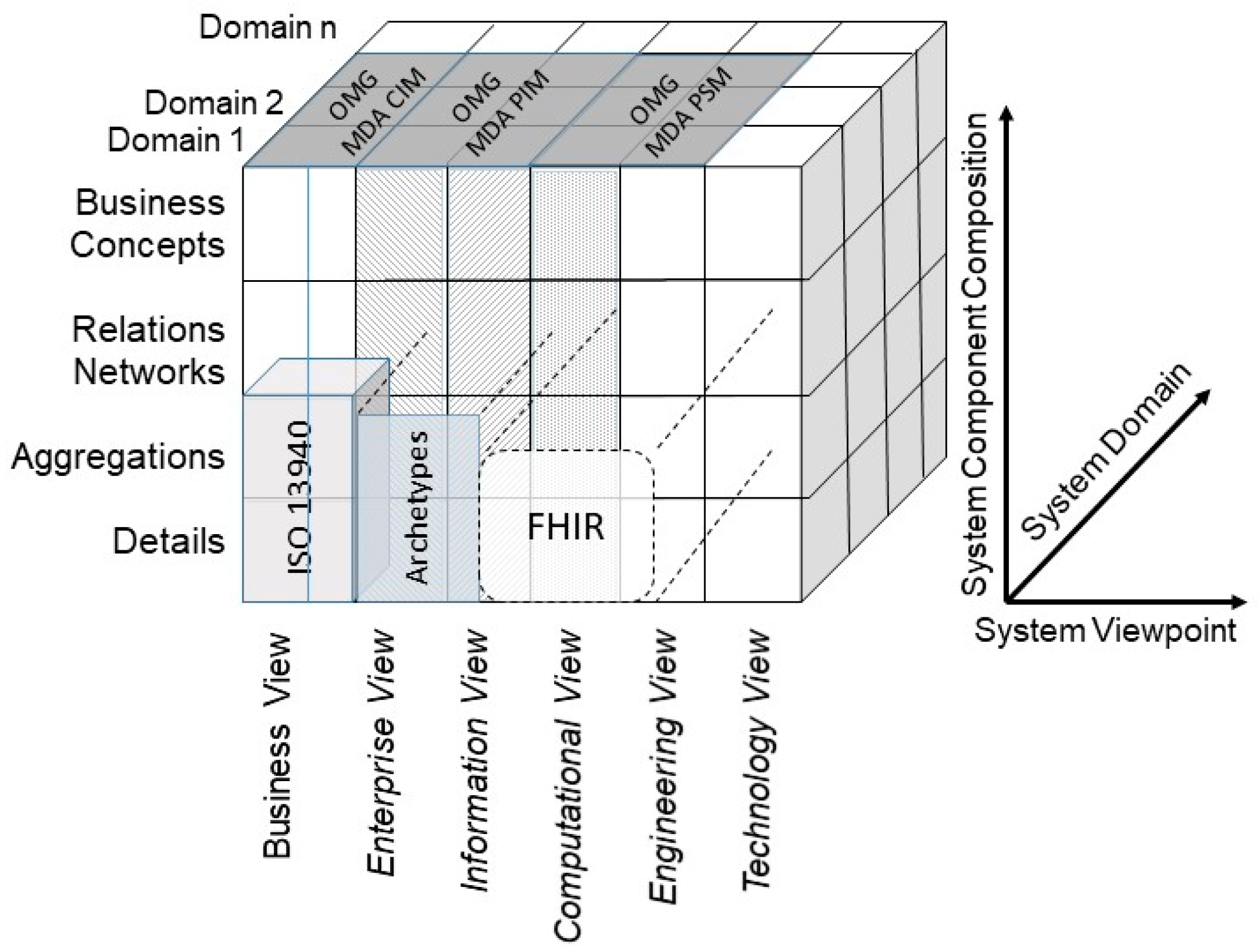
5.2. Integrating Existing Standards in 5P Medicine Ecosystems
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blobel, B.; Ruotsalainen, P.; Giacomini, M. Standards and Principles to Enable Interoperability and Integration of 5P Medicine Ecosystems. Stud. Health Technol. Inform. 2022, 299, 3–19. [Google Scholar] [PubMed]
- Byju’s. Structure, Functions, Units and Types of Ecosystem. Available online: https://byjus.com/biology (accessed on 28 June 2023).
- Blobel, B.; Ruotsalainen, P.; Brochhausen, M.; Prestes, E.; Houghtaling, M.A. Designing and Managing Advanced, Intelligent and Ethical Health and Social Care Ecosystems. J. Pers. Med. 2023, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Blobel, B.; Kalra, D. Managing Healthcare Transformation Towards 5P Medicine. Lausanne: Frontiers Media SA. Available online: https://www.frontiersin.org/research-topics/21049/managing-healthcare-transformation-towards-p5-medicine (accessed on 11 October 2023).
- Blobel, B. Challenges and Solutions for Designing and Managing pHealth Ecosystems. Front. Med. 2019, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Blobel, B.; Oemig, F.; Ruotsalainen, P.; Lopez, D.M. Transformation of Health and Social Care Systems—An Interdisciplinary Approach Toward a Foundational Architecture. Front. Med. 2022, 9, 802487. [Google Scholar] [CrossRef] [PubMed]
- Blobel, B.; Giacomini, M. Interoperability is more than just technology—Editorial. Eur. J. Biomed. Inform. 2016, 12, en1–en2. [Google Scholar] [CrossRef]
- Blobel, B.; Ruotsalainen, P.; Lopez, D.M.; Oemig, F. Requirements and Solutions for Personalized Health Systems. Stud. Health Technol. Inform. 2017, 237, 3–21. [Google Scholar] [PubMed]
- Blobel, B.; Ruotsalainen, P.; Oemig, F. Why Interoperability at Data Level Is Not Sufficient for Enabling pHealth? Stud. Health Technol. Inform. 2020, 273, 3–19. [Google Scholar] [PubMed]
- Blobel, B. Architectural approach to eHealth for enabling paradigm changes in health. Methods Inf. Med. 2010, 49, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kamareddine, F.; Laan, T.; Nederpelt, R. A Modern Perspective on Type Theory; Kluwer Academic Publishers: New York, NY, USA, 2004. [Google Scholar]
- Rebstock, M.; Fengel, J.; Paulheim, H. Ontologies-Based Business Integration; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- ISO/IEC 10746; Information Technology—Open Distributed Processing—Reference Model. International Organization for Standardization: Geneva, Switzerland, 2009.
- Hoberman, S.; Burbank, D.; Bradley, C. Data Modeling for the Business: A Handbook for Aligning the Business with IT Using High-Level Data Models; Technics Publications, LLC.: Bradley Beach, NJ, USA, 2009. [Google Scholar]
- ISO 23903:2021; Health Informatics—Interoperability and Integration Reference Architecture—Model and Framework. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- ComplianceForge. Introduction-Guide to Information-Security-Policy-Cybersecurity-Program-Documentation, Version 2022.3; ComplianceForge LLC: 2022. Available online: https://www.complianceforge.com (accessed on 28 June 2023).
- IEC 82304-2:2021; Health Software-Part 2: Health and wellness apps-Quality and reliability. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2021.
- ISO/IEC/IEEE 42010:2011; Systems and Software Engineering—Architecture Description. International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- ISO/IEC/IEEE 42020:2019; Software, Systems and Enterprise—Architecture Processes. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Péraire, C.; Edwards, M.; Fernandes, A.; Mancin, E.; Carroll, K. The IBM Rational Unified Process for System Z; IBM International Technical Support Organization: Armonk, NY, USA, 2007. [Google Scholar]
- ISO/IEC 7498-1:1994; Information technology—Open Systems Interconnection—Basic Reference Model: The Basic Model. International Organization for Standardization (ISO): Geneva, Switzerland, 2000.
- Object Management Group. Model Driven Architecture (MDA). MDA Guide rev. 2.0. Milford; Object Management Group: Needham, MA, USA, 2014. [Google Scholar]
- Roubi, S.; Erramdani, M.; Mbarki, S. A Model Driven Approach for generating Graphical User Interface for MVC Rich Internet Application. Comput. Inf. Sci. 2016, 9, 91–98. [Google Scholar]
- Blobel, B.; Oemig, F. The Importance of Architectures for Interoperability. Stud. Health Technol. Inform. 2015, 211, 18–56. [Google Scholar] [PubMed]
- Blobel, B.; Oemig, F. Solving the Modeling Dilemma as a Foundation for Interoperability. Eur. J. Biomed. Inform. 2018, 14, 3–12. [Google Scholar] [CrossRef]
- ISO 12967; Health Informatics—Service Architecture (HISA). International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Gruber, T.R. Toward principles for the design of ontologies used for knowledge sharing? Int. J. Hum. Comput. Stud. 1995, 43, 907–928. [Google Scholar] [CrossRef]
- ISO/IEC 21838:2021; Information Technology—Top-Level Ontologies (TLO). International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Bodenreider, O. The Unified Medical Language System (UMLS): Integrating Biomedical Terminology. Nucleic Acids Res. 2004, 32, D267–D270. [Google Scholar] [CrossRef] [PubMed]
- SNOMED International. Available online: https://www.snomed.org (accessed on 28 June 2023).
- ISO 25720:2009; Health informatics—Genomic Sequence Variation Markup Language (GSVML). International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- Robinson, P.N.; Mundlos, S. The human phenotype ontology. Clin. Genet. 2010, 77, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cowell, L.G.; Smith, B. Infectious disease ontology. In Infectious Disease Informatics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 373–395. [Google Scholar]
- Sahoo, S.S.; Lhatoo, S.D.; Gupta, D.K.; Cui, L.; Zhao, M.; Jayapandian, C.; Bozorgi, A.; Zhang, G.-Q. Epilepsy and seizure ontology: Towards an epilepsy informatics infrastructure for clinical research and patient care. J. Am. Med. Inform. Assoc. 2014, 21, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Younesi, E.; Gundel, M.; Muller, B.; Heneka, M.T.; Hofmann-Apitius, M. ADO: A disease ontology representing the domain knowledge specific to Alzheimer’s disease. Alzheimers Dement. 2014, 10, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. The Gene Ontology; Geneontology: 2022. Available online: http://geneontology.org (accessed on 28 June 2023).
- ISO 13940:2015; Health Informatics—System of Concepts to Support Continuity of Care. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Benis, A.; Dornauer, V.; Grosjean, J.; Crisan-Vida, M. Medical Informatics and Digital Health Multilingual Ontology (MIMO): A tool to improve international collaborations. Int. J. Med. Inform. 2022, 167, 104860. [Google Scholar] [CrossRef] [PubMed]
- Blobel, B.; Ruotsalainen, P.; Brochhausen, M. Autonomous systems and artificial intelligence—Hype or prerequisite for P5 medicine? Stud. Health Technol. Inform. 2021, 285, 3–14. [Google Scholar] [PubMed]
- Damianou, N.; Dulay, N.; Lupu, E.; Sloman, M. Ponder: A Language for Specifying Security and Management Policies for Distributed Systems; The Language Specification, Version 2.3. Imperial College Research Report DoC 2000/1. 2000. Available online: https://www.researchgate.net/publication/243241194_Ponder_A_Language_for_Specifying_Security_and_Management_Policies_for_Distributed_Systems(accessed on 7 September 2023).
- ISO 22600:2014; Health Informatics—Privilege Management and Access Control. International Organization for Standardization (ISO): Geneva, Switzerland, 2014.
- Health Level 7 International Inc. HL7 Privacy and Security Logical Data Model, Release 1, June 2021. Ann Arbor: HL7 International. 2021. Available online: https://www.hl7.org (accessed on 28 June 2023).
- Tsoumas, B.; Gritzalis, D. Towards an Ontology-based Security Management. In Proceedings of the 20th International Conference on Advanced Information Networking and Applications—Volume 1 (AINA’06), Vienna, Austria, 18–20 April 2006. [Google Scholar] [CrossRef]
- Palmirani, M.; Martoni, M.; Rossi, A.; Bartolini, C.; Robaldo, L. PrOnto: Privacy Ontology for Legal Reasoning. In Electronic Government and the Information Systems Perspective: 7th International Conference, EGOVIS 2018, Regensburg, Germany, 3–5 September 2018; Kő, A., Francesconi, E., Eds.; Proceedings 7; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 139–152. [Google Scholar]
- IEEE 7000:2021; IEEE Standard Model Process for Addressing Ethical Concerns during System Design. IEEE: Piscataway, NJ, USA, 2021.
- IEEE 7007:2021; IEEE Ontological Standard for Ethically Driven Robotics and Automation Systems. IEEE: Piscataway, NJ, USA, 2021.
- ISO 13972:2022; Health Informatics—Clinical Information Models—Characteristics, Structures and Requirements. International Organization for Standardization (ISO): Geneva, Switzerland, 2022.
- openEHR International. Available online: https://openehr.org (accessed on 28 June 2023).
- ISO 13606-1:2019; Health Informatics—Electronic Health Record Communication—Part 1: Reference Model. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- ISO 13606-3:2019; Health Informatics—Electronic Health Record Communication—Part 3: Reference Archetypes and Term Lists. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Health Level 7 International Inc. HL7 V3 Standard: Clinical Document Architecture, Release 2; HL7 International: Ann Arbor, MI, USA, 2021; Available online: https://www.hl7.org (accessed on 28 June 2023).
- Health Level 7 International Inc. HL7 Fast Healthcare Interoperability Resources Release 4B; HL7 International: Ann Arbor, MI, USA, 2021; Available online: https://hl7.org/FHIR/ (accessed on 28 June 2023).
- Health Level 7 International Inc. HL7 Version 3 Domain Analysis Model: Composite Security and Privacy, Release 1; HL7 International: Ann Arbor, MI, USA, 2020; Available online: https://www.hl7.org (accessed on 28 June 2023).
- Oemig, F. Development of an Ontology-Based Architecture for Ensuring Semantic Interoperability between Communication Standards in Healthcare. Ph.D. Thesis, Medical Faculty, University of Regensburg, Regensburg, Germany, 2011. (In German). Available online: http://epub.uni-regensburg.de/20076/1/Dissertation_v39_final.pdf (accessed on 28 June 2023).
- Oemig, F.; Blobel, B. A Communication Standards Ontology Using Basic Formal Ontologies. Stud. Health Technol. Inform. 2010, 156, 105–113. [Google Scholar] [PubMed]
- European Parliament and Council. Directive (EU) 95/46/EC Data Protection Directive; EC: Brussels, Belgium, 1995. [Google Scholar]
- European Parliament and Council. Regulation (EU) 2016/679 General Data Protection Regulation; EC: Brussels, Belgium, 2016. [Google Scholar]
- Blobel, B.; Ruotsalainen, P. How Does GDPR Support Healthcare Transformation to 5P Medicine? Stud. Health Technol. Inform. 2019, 264, 1135–1339. [Google Scholar] [PubMed]
- European Parliament and Council. Proposal for a Regulation on the European Health Data Space; EC: Strasbourg, France, 2022. [Google Scholar]


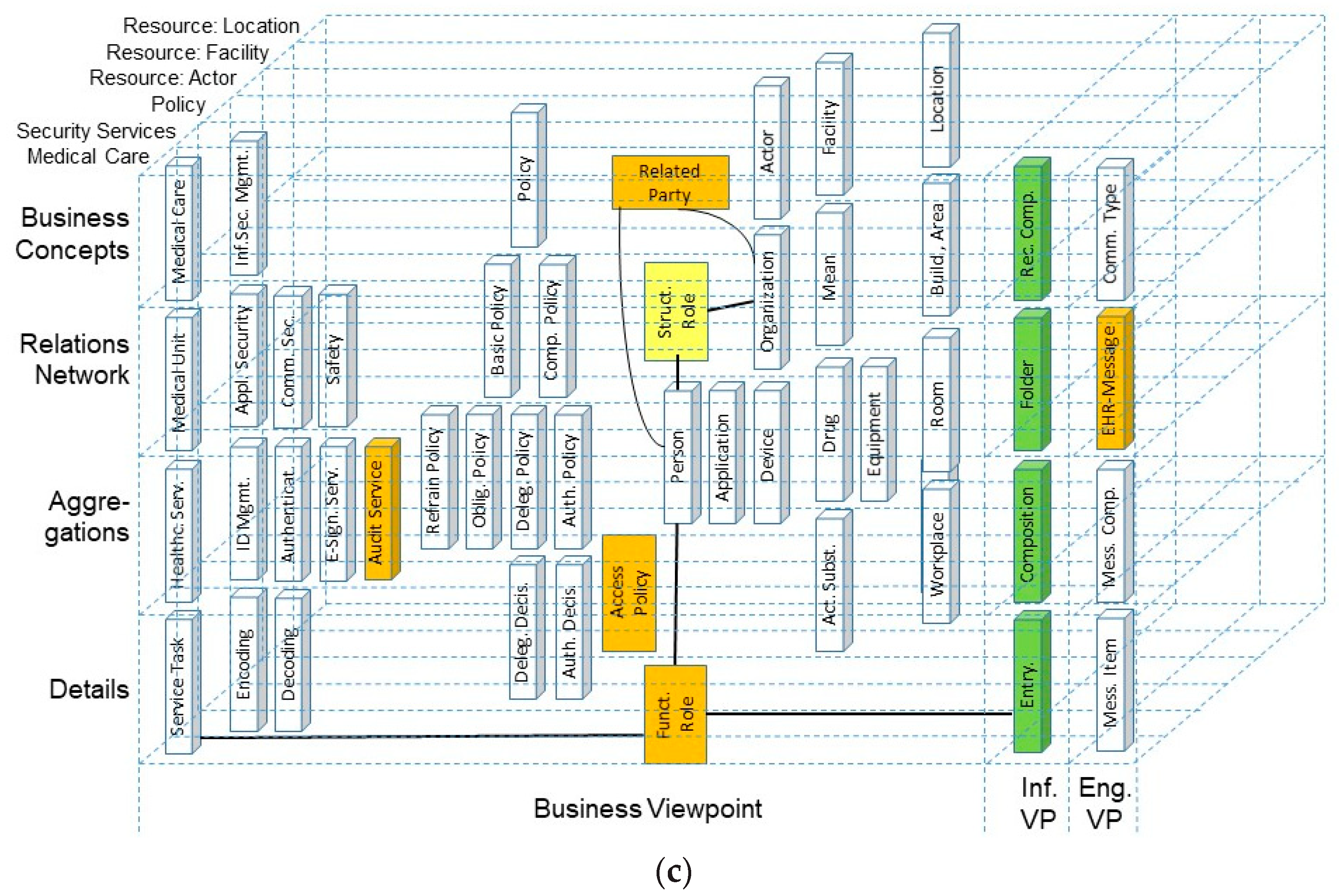
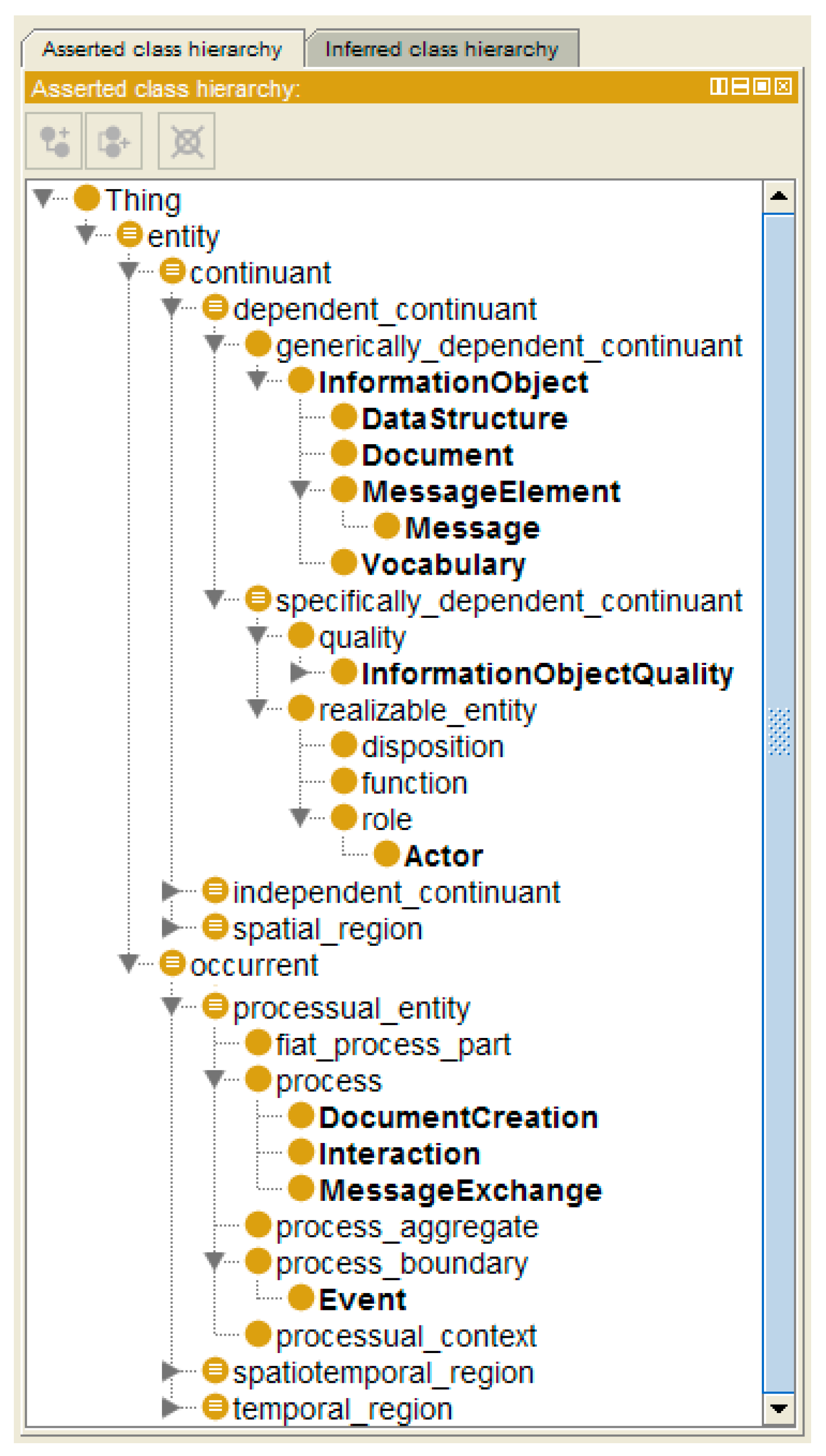
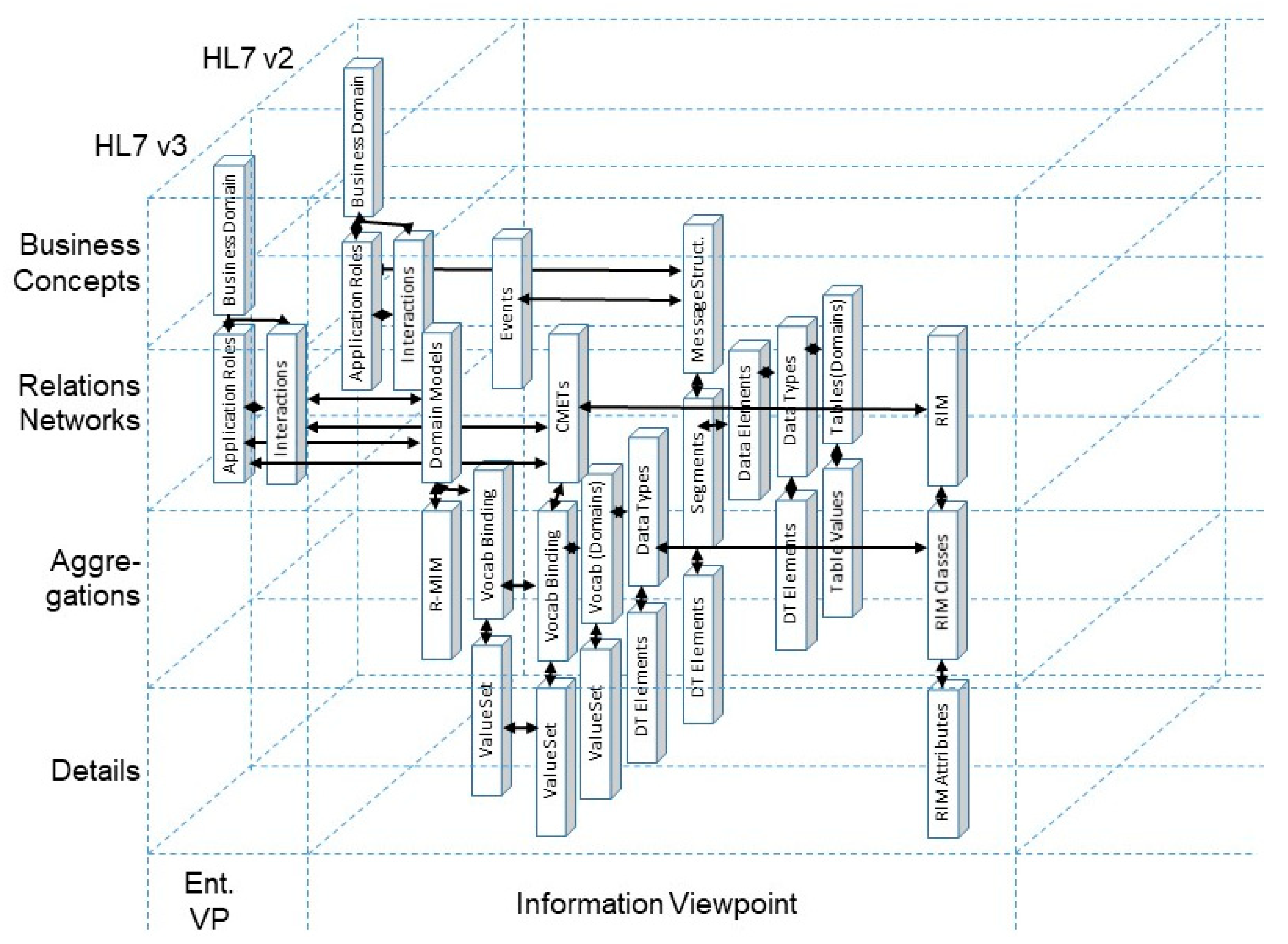
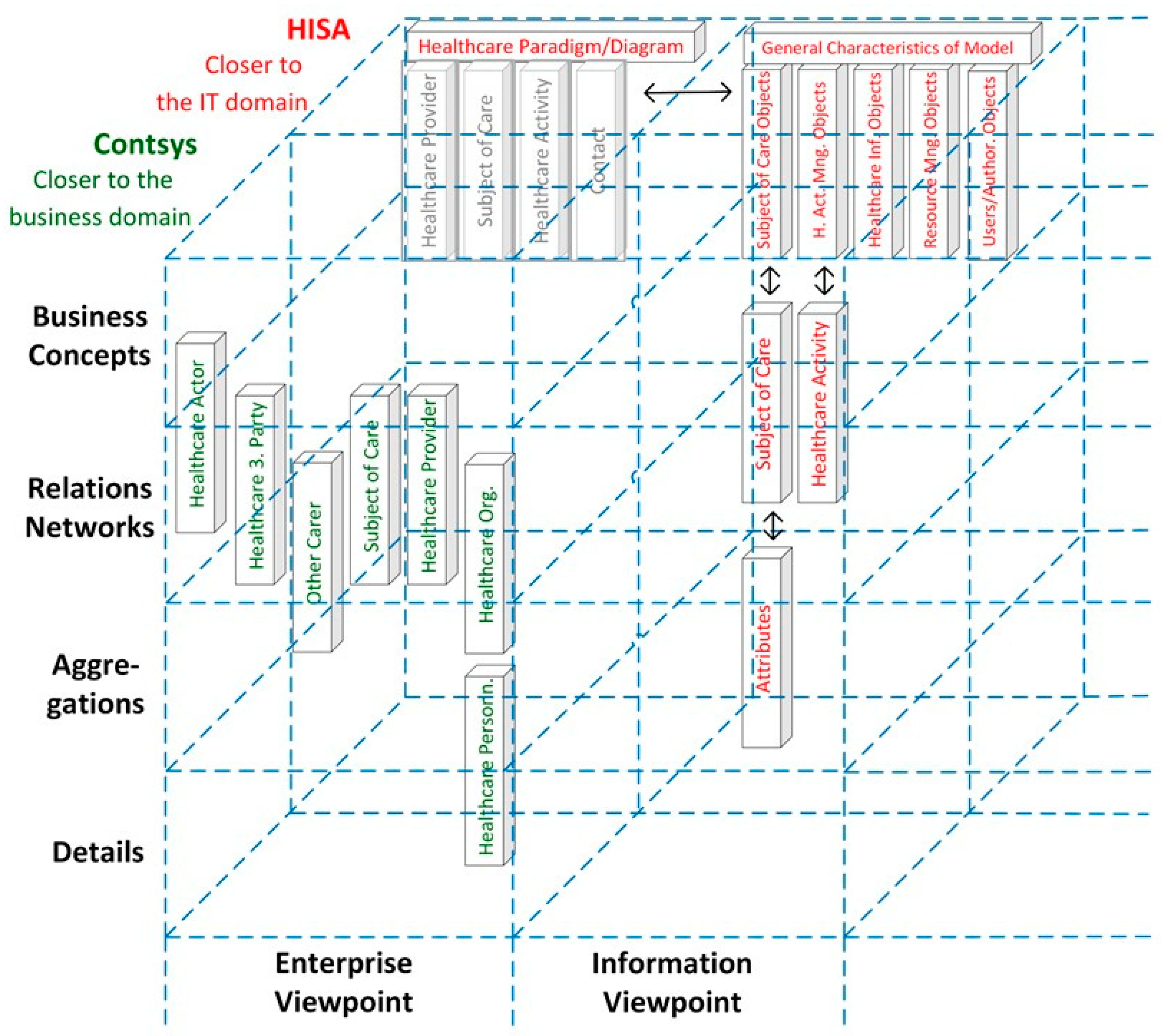
| Care Type | Organization, Service Provision | Actors | Services | Target | Way of Practicing | Justification | Representation Style | Electronic Comm./ Co-op. | Standard |
|---|---|---|---|---|---|---|---|---|---|
| Phenomenological medicine | Organization-centered, local services | Regulated professionals | Domain-specific general services | Humanity | Observation | Pattern recognition | Data | Local data repository; inside the unit | Data standards |
| Evidence-based medicine | Organization-centered, local services | Regulated professionals | Domain-specific, group-specific services | Disease-specific defined group | Observation with objective evaluation | Statistical justification, group-specific treatment outcome | Information | Central data repositories | Information standards |
| Person-centered medicine | Cross-organizational local services | Regulated professionals | Multiple domains’ services | Individual | Managed care | Process mgmt., best medical practice guidelines | Agreed terminology, DMP Best Practice Guidelines | Cross-organizational business process | Terminology standards; process standards |
| Personalized medicine | Distributed local and remote services | Regulated and non-regulated professionals, laymen, technical systems | Multiple domains’ services—telemedicine | Individual’s personal disposition | Considering the pathology of disease | Clinically justified individual status and context | Disciplinary concepts in situational context | Knowledge management | Domain ontology standards |
| 5P medicine | Distributed cross-domain services, smart healthcare | Regulated and non-regulated professionals, laymen, technical systems | Cross-domain services—consumerism, telemedicine | Individual in personal, environmental, social, occupational, and behavioral contexts | Understanding the pathology of disease | Scientifically justified individual status and context | Multidisciplinary concepts in comprehensive context | Knowledge space management | Multiple ontologies guided via top-level ontology standards |
| Ubiquitous personal health and social care | Ubiquitous services | Regulated and non-regulated professionals, laymen, technical systems | Integrated services—consumerism, ubiquitous medicine | Individual under comprehensive focus | Dynamically and scientifically justified individual status |
| Objective | Characteristics | Methodologies/Technologies |
|---|---|---|
| Provision of health services everywhere, anytime |
|
|
| Individualization of the system according to status, context, needs, expectations, wishes, environments, etc., of the subject of care |
|
|
| Integration of different actors from different disciplines/domains (incl. the participation/empowerment of the subject of care), using their own languages, methodologies, terminologies, ontologies, thereby meeting any behavioral aspects, rules, and regulations |
|
|
| Usability and acceptability of 5P Medicine solutions |
|
|
| Viewpoint | Language/Grammar | Representation Level |
|---|---|---|
| Business View | Domain-specific and/or natural languages | |
| Enterprise View | Business Process Modeling Language (BPML) | Very high |
| Information View | Unified Modeling Language (UML) | High level |
| Computational View | Object Constraint Language (OCL) | Logical level |
| Engineering View | Programming languages with different levels of grammar | Physical level |
| Technology View |
| Data Model Level | Modeling Actors | Model Scope | Dimension of Modeling | Interop. Reference Architecture | Examples | ||
|---|---|---|---|---|---|---|---|
| Very-high-level data model | Business domain stake-holders | Scope, requirements, and related basic concepts of business case | Knowledge space | Business View | OMG CIM | ISO 23903 Interoperability and Integration Reference Architecture | |
| High-level data model | Business domain stake-holders | Relevant information and representation and relationships of basic concepts | Knowledge | Enterprise View | OMG PIM, HL7 DCM, CSO | ISO 10746 ODP-RM | |
| Logical data model | Data modelers and analysts | Layout and types of data and object relationships | Information | Information View | OMG PSM, HL7 V3 (CMETs), HL7 CIMI, openEHR Archetypes, FHIM | ||
| Computational View | HL7 FHIR | ||||||
| Physical data model | Data modelers and developers | Implementation-related and platform-specific aspects | Data | Engineering View | |||
| Standards Classification | Examples |
|---|---|
| Architecture standards | HL7 Version 2.x/3, OMG CORBA, OMG MDA, ISO 12967 Health informatics–Service architecture (HISA), ISO 7498-2:1989, Information processing systems—Open Systems Interconnection—Basic Reference Model—Part 2: Security Architecture, ISO 13407:1999 Human-centred design processes for interactive systems |
| Modelling standards | OMG Unified Modeling Language (UML), ISO/IEC 19505-2:2012 Unified Modeling Language (UML), CEN 15300 CEN Report: Framework for formal modelling of healthcare security policies |
| Terminology and ontology standards | UMLS, Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT), Systematized Nomenclature of Medicine Clinical Term Ontology (SCTO), ISO 25720 Genomic sequence variation markup language, ISO/IEC 2382-8:1998 Information technology—Vocabulary—Part 8: Security, CEN-ENV 13608-1:2000 Health informatics—Security for healthcare communication—Part 1: Concepts and terminology, ISO 13940:2015 Health informatics—System of concepts to support continuity of care, Logical Observation Identifiers Names and Codes (LOINC), Unified Code for Units of Measure (UCUM) |
| Communication standards | ISO/IEC 7498-1:1994 Information technology—Open Systems Interconnection—Basic Reference Model: The Basic Model, HL7 V2.x/3, HL7 FHIR (Fast Healthcare Interoperability Resource), X12 EDI, UN EDIFACT, H.PRIM, xDT, Odette FTP, CEN 13606 Electronic healthcare record communication, ISO/IEEE 11073 Health informatics—Point-of-care medical device communication, ISO 17113 Health informatics–Exchange of information between healthcare information systems–Development of messages, CDISC and DICOM specifications, Classification Markup Language (ClaML), EN ISO 27269:2022 Health informatics-International patient summary (ISO 27269:2021) |
| Policy, security, and privacy standards | ISO/IEC 2700 Information security management, ISO 22600:2014 Health informatics–Privilege management and access control, ISO 17090 Public key infrastructure, ETSI TS 101733 Electronic Signature Formats, ASTM E1987-98 Standard guide for individual rights regarding health information, CEN 13608 Security for healthcare communication, CEN 13729 Secure user identification-Strong authentication using microprocessor cards, ISO 25237:2017 Health informatics—Pseudonymization, ISO/IEC PDTS Pseudonymisation Practices for the Protection of Personal Health Information and Health Related Services, ISO/IEC 27018:2019 Information technology—Security techniques—Code of practice for protection of personally identifiable information (PII) in public clouds acting as PII processors, ISO/IEC 29151:2017 Information technology—Security techniques—Code of practice for personally identifiable information protection, ISO 21298:2017 Health informatics–Functional and structural roles, ISO/IEC 9594-8:2008, Information technology—Open Systems Interconnection—The Directory: Public-key and attribute certificate frameworks, ISO/IEC 9798-3:1998, Information technology—Security techniques—Entity authentication—Part 3: Mechanisms using digital signature techniques, ISO/IEC 10181-1:1996, Information technology—Open Systems Interconnection—Security frameworks for open systems: Overview, ISO/TS 17090-1:2013 Health informatics—Public key infrastructure—Part 1: Overview of digital certificate services, ENV 13729:1999, Health informatics—Secure user identification for healthcare strong authentication using microprocessor cards, ISO 21091:2013 Health informatics—Directory services for healthcare providers, subjects of care and other entities, ISO/IEC 15408-1:2009 Information technology—Security techniques—Evaluation criteria for IT security—Part 1: Introduction and general model |
| Safety standards | CEN 13694 CEN Report: Safety and security related software quality standards for healthcare, ISO/DTS 25238 Classification of Safety Risks, IEC 82304-1 Health Software–Part 1: General requirements for product safety. IEC 82304-2 Health Software–Part 2: Health and wellness apps–Quality and reliability |
| Identifier and identification standards | LOINC, ASTM E1714-00 Standard guide for properties of a Universal Healthcare Identifier |
| Document standards | HL7 V3/CDA (Clinical Document Architecture), DICOM SR (Structured Reporting), HL7 FHIR Bundle+Composition |
| Data representation (visualization) standards | HTML, PDF, PDF/A, MS Word, ClaML |
| Encoding standards | XML, JSON, ASN.1, ER7, xDT |
| Character representation standards | ASCII, EBCDIC, Unicode |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blobel, B.; Ruotsalainen, P.; Oemig, F.; Giacomini, M.; Sottile, P.A.; Endsleff, F. Principles and Standards for Designing and Managing Integrable and Interoperable Transformed Health Ecosystems. J. Pers. Med. 2023, 13, 1579. https://doi.org/10.3390/jpm13111579
Blobel B, Ruotsalainen P, Oemig F, Giacomini M, Sottile PA, Endsleff F. Principles and Standards for Designing and Managing Integrable and Interoperable Transformed Health Ecosystems. Journal of Personalized Medicine. 2023; 13(11):1579. https://doi.org/10.3390/jpm13111579
Chicago/Turabian StyleBlobel, Bernd, Pekka Ruotsalainen, Frank Oemig, Mauro Giacomini, Pier Angelo Sottile, and Frederik Endsleff. 2023. "Principles and Standards for Designing and Managing Integrable and Interoperable Transformed Health Ecosystems" Journal of Personalized Medicine 13, no. 11: 1579. https://doi.org/10.3390/jpm13111579
APA StyleBlobel, B., Ruotsalainen, P., Oemig, F., Giacomini, M., Sottile, P. A., & Endsleff, F. (2023). Principles and Standards for Designing and Managing Integrable and Interoperable Transformed Health Ecosystems. Journal of Personalized Medicine, 13(11), 1579. https://doi.org/10.3390/jpm13111579







