48-Month Clinical Outcomes and Prognostic Factors in an All-Comers Population with Acute Coronary Syndrome and Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention with a Sirolimus-Eluting Stent
Abstract
1. Introduction
2. Materials and Methods
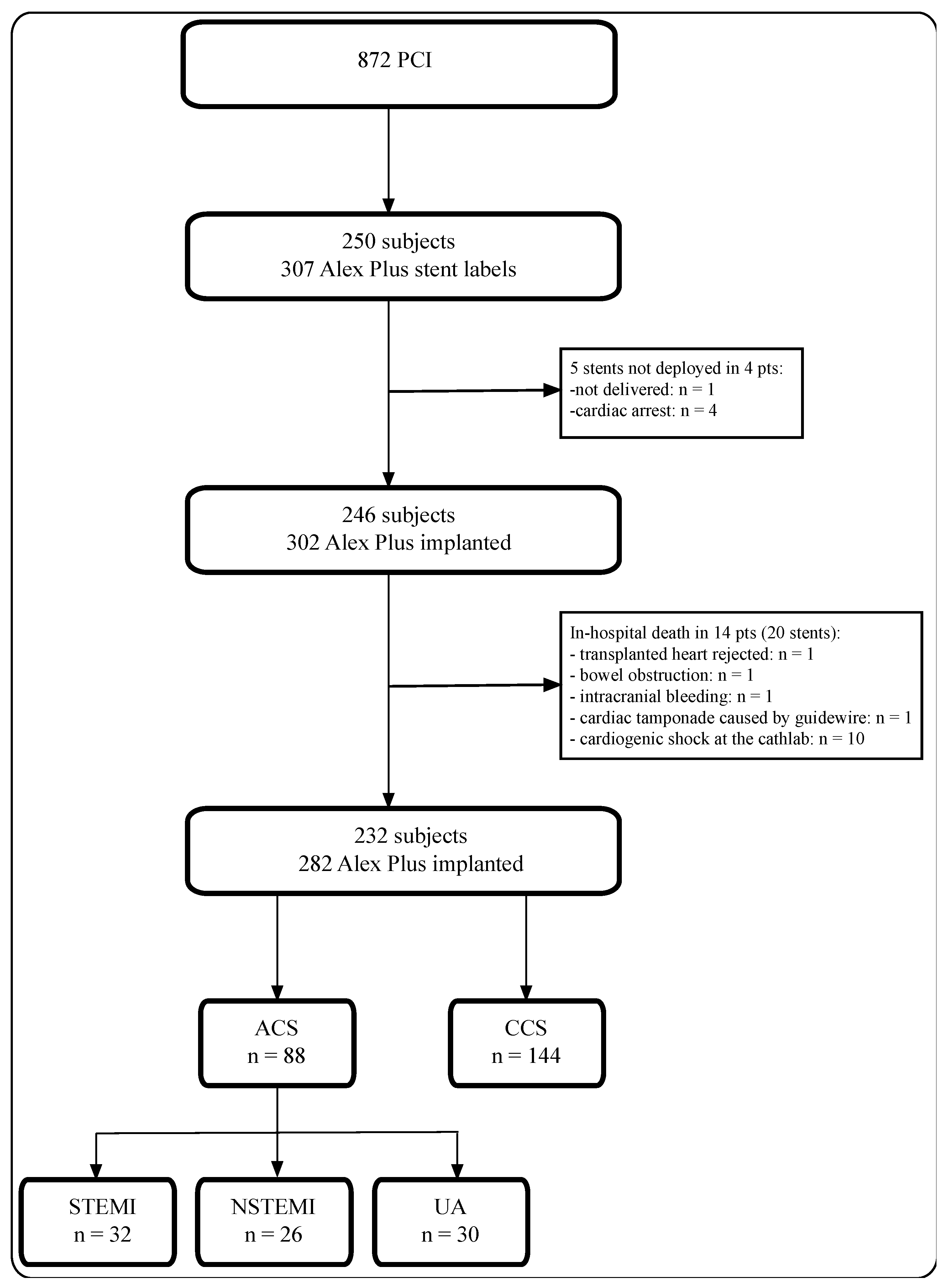
2.1. Study Design and Study Population
2.2. Alex plus Stent Characteristics
2.3. Data Collection
2.4. Study Endpoints
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Procedure Characteristics
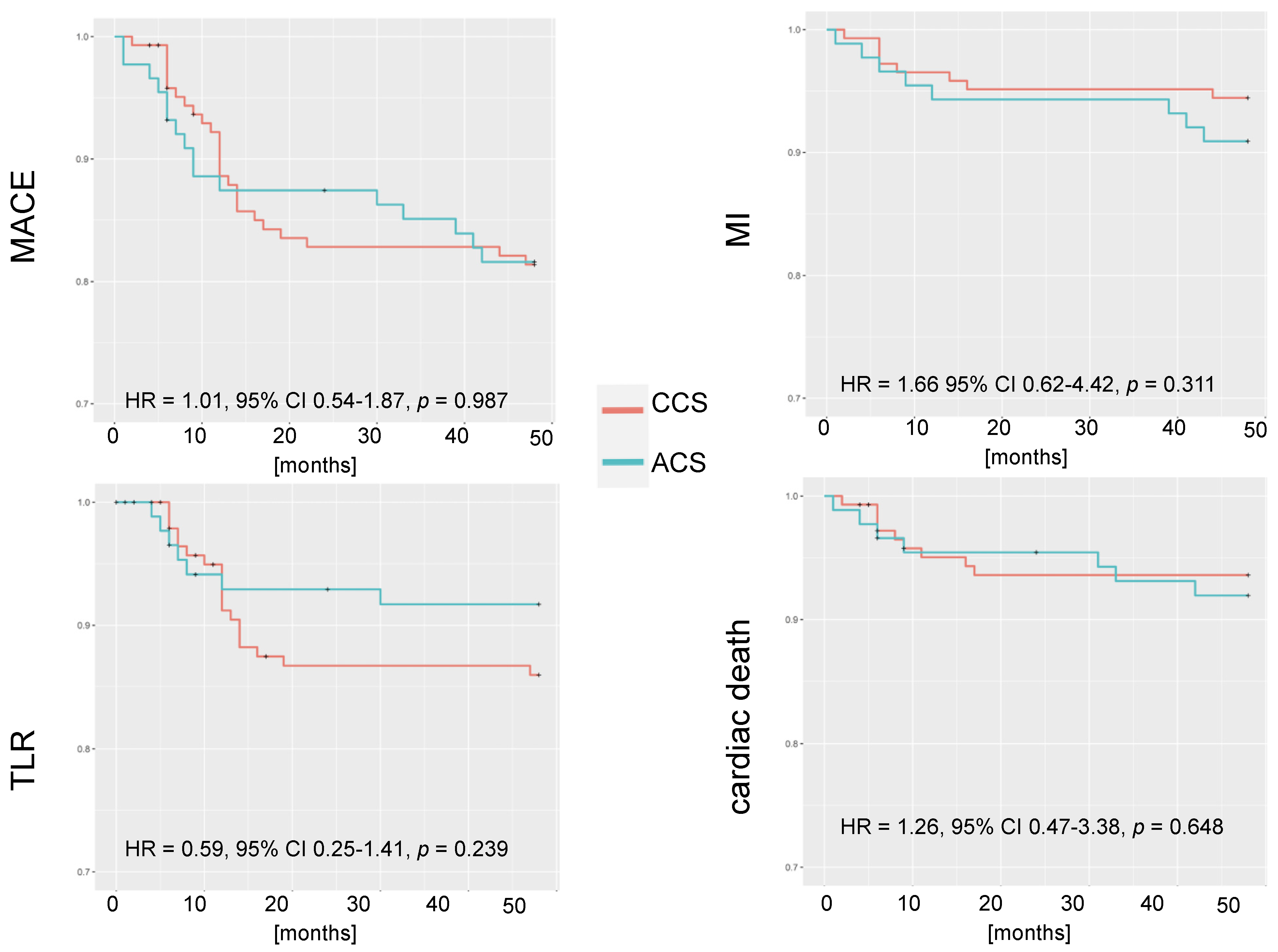
3.3. 4-Year Outcomes
3.4. Cox Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Marc, M.; Ruzsa, Z. Surgical Turned-Downed CHIP Cases-Can PCI Save the Day? Front. Cardiovasc. Med. 2022, 9, 872398. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.A.; Tyler, J.M.; Henry, T.D.; Garberich, R.; Sharkey, S.W.; Schmidt, C.W.; Henry, J.T.; Eckman, P.; Megaly, M.; Brilakis, E.S.; et al. Clinical Characteristics and Outcomes of STEMI Patients With Cardiogenic Shock and Cardiac Arrest. JACC Cardiovasc. Interv. 2020, 13, 1211–1219. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Bil, J.; Gil, R.J.; Kern, A.; Pawlowski, T.; Seweryniak, P.; Sliwinski, Z. Novel sirolimus-eluting stent Prolim(R) with a biodegradable polymer in the all-comers population: One year clinical results with quantitative coronary angiography and optical coherence tomography analysis. BMC Cardiovasc. Disord. 2015, 15, 150. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef]
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef]
- Liu, H.L.; Jin, Z.G.; Yang, S.L.; Han, W.; Jing, Q.M.; Zhang, L.; Luo, J.P.; Ma, D.X.; Liu, Y.; Yang, L.X.; et al. Five-year outcomes of ST-elevation myocardial infarction versus non-ST-elevation acute coronary syndrome treated with biodegradable polymer-coated sirolimus-eluting stents: Insights from the CREATE trial. J. Cardiol. 2017, 69, 149–155. [Google Scholar] [CrossRef][Green Version]
- Gherasie, F.A.; Valentin, C.; Busnatu, S.S. Is There an Advantage of Ultrathin-Strut Drug-Eluting Stents over Second- and Third-Generation Drug-Eluting Stents? J. Pers. Med. 2023, 13, 753. [Google Scholar] [CrossRef]
- Leone, A.; Simonetti, F.; Avvedimento, M.; Angellotti, D.; Immobile Molaro, M.; Franzone, A.; Esposito, G.; Piccolo, R. Ultrathin Struts Drug-Eluting Stents: A State-of-the-Art Review. J. Pers. Med. 2022, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Abbott, J.D. Coronary Stents: History, Design, and Construction. J. Clin. Med. 2018, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Jezewski, M.P.; Kubisa, M.J.; Eyileten, C.; De Rosa, S.; Christ, G.; Lesiak, M.; Indolfi, C.; Toma, A.; Siller-Matula, J.M.; Postula, M. Bioresorbable Vascular Scaffolds-Dead End or Still a Rough Diamond? J. Clin. Med. 2019, 8, 2167. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Alampi, C.; Krivoshei, L.; Leibundgut, G. In vitro effect of intravascular lithotripsy on the polymer of a drug-eluting stent. EuroIntervention 2022, 18, e333–e334. [Google Scholar] [CrossRef]
- Tyczyński, M.; Kern, A.; Buller, P.; Wańha, W.; Gil, R.J.; Bil, J. Clinical Outcomes and Prognostic Factors in Complex, High-Risk Indicated Procedure (CHIP) and High-Bleeding-Risk (HBR) Patients Undergoing Percutaneous Coronary Intervention with Sirolimus-Eluting Stent Implantation: 4-Year Results. J. Clin. Med. 2023, 12, 5313. [Google Scholar] [CrossRef]
- Buszman, P.P.; Michalak, M.J.; Pruski, M.; Fernandez, C.; Jelonek, M.; Janas, A.; Savard, C.; Gwiazdowska-Nowotka, B.; Zurakowski, A.; Wojakowski, W.; et al. Comparable vascular response of a new generation sirolimus eluting stents when compared to fluoropolymer everolimus eluting stents in the porcine coronary restenosis model. Cardiol. J. 2016, 23, 657–666. [Google Scholar] [CrossRef]
- Dobrolinska, M.; Gasior, P.; Roleder, T.; Roleder-Dylewska, M.; Smolka, G.; Ochala, A.; Kedhi, E.; Wojakowski, W. Short-term healing response after implantation of the thin-strut, fast-releasing sirolimus-eluting biodegradable polymer-coated Alex Plus stent: Optical coherence tomography study. Postepy Kardiol. Interwencyjnej 2020, 16, 187–191. [Google Scholar] [CrossRef]
- Ryan, T.J.; Faxon, D.P.; Gunnar, R.M.; Kennedy, J.W.; King, S.B., 3rd; Loop, F.D.; Peterson, K.L.; Reeves, T.J.; Williams, D.O.; Winters, W.L., Jr.; et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988, 78, 486–502. [Google Scholar] [CrossRef]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R., Jr.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Lancellotti, P.; Zamorano, J.; Habib, G.; Badano, L. The EACVI Textbook of Echocardiography; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Egami, Y.; Kawanami, S.; Sugae, H.; Ukita, K.; Nakamura, H.; Matsuhiro, Y.; Yasumoto, K.; Tsuda, M.; Okamoto, N.; et al. Preferable vascular healing of ultrathin strut biodegradable-polymer sirolimus-eluting stents in patients with acute coronary syndrome. Cardiovasc. Interv. Ther. 2022, 37, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Matsuhiro, Y.; Egami, Y.; Okamoto, N.; Kusuda, M.; Sakio, T.; Nohara, H.; Sugae, H.; Kawanami, S.; Kawamura, A.; Ukita, K.; et al. Early vascular healing of ultra-thin strut polymer-free sirolimus-eluting stents in acute coronary syndrome: USUI-ACS study. Cardiovasc. Interv. Ther. 2023, 38, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, P.P.; Varho, V.; Nammas, W.; Mikkelsson, J.; Pietila, M.; Ylitalo, A.; Airaksinen, J.K.; Sia, J.; Nyman, K.; Biancari, F.; et al. Early neointimal coverage and vasodilator response following biodegradable polymer sirolimus-eluting vs. durable polymer zotarolimus-eluting stents in patients with acute coronary syndrome -HATTRICK-OCT trial. Circ. J. 2015, 79, 360–367. [Google Scholar] [CrossRef]
- Iannaccone, M.; Barbero, U.; De Benedictis, M.; Imori, Y.; Quadri, G.; Trabattoni, D.; Ryan, N.; Venuti, G.; Montabone, A.; Wojakowski, W.; et al. Comparison of bioresorbable vs durable polymer drug-eluting stents in unprotected left main (from the RAIN-CARDIOGROUP VII Study). BMC Cardiovasc. Disord. 2020, 20, 225. [Google Scholar] [CrossRef]
- Saito, Y.; Wijns, W.; Baumbach, A.; Xu, B.; Kelbaek, H.; Zheng, M.; Morel, M.A.; Anderson, R.; Schachinger, V.; Lansky, A.; et al. Differential impact of abluminal groove-filled biodegradable-polymer sirolimus-eluting stent versus durable-polymer everolimus-eluting stent on and off dual antiplatelet therapy. Catheter. Cardiovasc. Interv. 2022, 99, 357–365. [Google Scholar] [CrossRef]
- Valgimigli, M.; Wlodarczak, A.; Tolg, R.; Merkely, B.; Kelbaek, H.; Legutko, J.; Galli, S.; Godin, M.; Toth, G.G.; Lhermusier, T.; et al. Biodegradable-Polymer or Durable-Polymer Stents in Patients at High Bleeding Risk: A Randomized, Open-Label Clinical Trial. Circulation 2023, 148, 989–999. [Google Scholar] [CrossRef]
- Jolly, S.S.; Yusuf, S.; Cairns, J.; Niemela, K.; Xavier, D.; Widimsky, P.; Budaj, A.; Niemela, M.; Valentin, V.; Lewis, B.S.; et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011, 377, 1409–1420. [Google Scholar] [CrossRef]
- Araujo, G.N.; Machado, G.P.; Moura, M.; Silveira, A.D.; Bergoli, L.C.; Fuchs, F.C.; Goncalves, S.C.; Wainstein, R.V.; Lemos, P.A.; Quadros, A.S.; et al. Real-World Assessment of an Ultrathin Strut, Sirolimus-Eluting Stent in Patients with ST-Elevation Myocardial Infarction Submitted to Primary Percutaneous Coronary Intervention (INSTEMI Registry). Arq. Bras. Cardiol. 2023, 120, e20220594. [Google Scholar] [CrossRef]
- Jimenez, V.A.; Iniguez, A.; Baz, J.A.; Valdes, M.; Ortiz, A.; Vuilliomenet, A.; Mainar, V.; Dudek, D.; Banai, S.; Tuller, D.; et al. A randomized comparison of novel bioresorbable polymer sirolimus-eluting stent and durable polymer everolimus-eluting stent in patients with acute coronary syndromes: The CENTURY II high risk ACS substudy. Cardiovasc. Revasc. Med. 2016, 17, 355–361. [Google Scholar] [CrossRef]
- Iglesias, J.F.; Heg, D.; Roffi, M.; Degrauwe, S.; Tuller, D.; Muller, O.; Brinkert, M.; Cook, S.; Weilenmann, D.; Kaiser, C.; et al. Five-Year Outcomes With Biodegradable-Polymer Sirolimus-Eluting Stents Versus Durable-Polymer Everolimus-Eluting Stents in Patients With Acute Coronary Syndrome: A Subgroup Analysis of the BIOSCIENCE Trial. Cardiovasc. Revasc. Med. 2022, 34, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tousek, P.; Lazarak, T.; Varvarovsky, I.; Novackova, M.; Neuberg, M.; Kocka, V. Comparison of a Bioresorbable, Magnesium-Based Sirolimus-Eluting Stent with a Permanent, Everolimus-Eluting Metallic Stent for Treating Patients with Acute Coronary Syndrome: The PRAGUE-22 Study. Cardiovasc. Drugs Ther. 2022, 36, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A.; Kandzari, D.E.; Marcusohn, E.; Koolen, J.J.; Doros, G.; Massaro, J.M.; Garcia-Garcia, H.M.; Bennett, J.; Gharib, E.G.; Cutlip, D.E.; et al. Subgroup Analysis Comparing Ultrathin, Bioresorbable Polymer Sirolimus-Eluting Stents Versus Thin, Durable Polymer Everolimus-Eluting Stents in Acute Coronary Syndrome Patients. Circ. Cardiovasc. Interv. 2018, 11, e007331. [Google Scholar] [CrossRef] [PubMed]
- Hemetsberger, R.; Abdelghani, M.; Toelg, R.; Garcia-Garcia, H.M.; Farhan, S.; Mankerious, N.; Elbasha, K.; Allali, A.; Windecker, S.; Lefevre, T.; et al. Complex vs. non-complex percutaneous coronary intervention with newer-generation drug-eluting stents: An analysis from the randomized BIOFLOW trials. Clin. Res. Cardiol. 2022, 111, 795–805. [Google Scholar] [CrossRef]
| Variable | Total Population N = 232 (%) | ACS N = 88 (%) | CCS N = 144 (%) | p |
|---|---|---|---|---|
| Sex: female | 64 (27.6) | 28 (32) | 36 (25) | 0.260 |
| Age (years) | 68 ± 11 | 67 ± 13 | 68 ± 9 | 0.788 |
| Acute coronary syndrome type upon presentation | ||||
| Unstable angina | 30 (12.9) | 30 (34.2) | 0 | <0.001 |
| Non-ST-elevation MI | 26 (11.2) | 26 (29.5) | 0 | |
| ST-elevation MI | 32 (13.8) | 32 (36.3) | 0 | |
| Cardiogenic shock | 6 (2.6) | 6 (6.8) | 0 | 0.003 |
| Arterial hypertension | 213 (91.8) | 75 (85.2) | 138 (95.8) | 0.004 |
| Type 2 diabetes | 97 (41.8) | 32 (36.4) | 65 (45.1) | 0.189 |
| Dyslipidemia | 177 (76.3) | 59 (67.0) | 118 (81.9) | 0.010 |
| Prior myocardial infarction | 113 (48.7) | 30 (34.1) | 83 (57.6) | <0.001 |
| Prior PCI | 130 (56.0) | 31 (35.2) | 99 (68.8) | <0.001 |
| Prior CABG | 22 (9.5) | 7 (8.0) | 15 (10.4) | 0.535 |
| Chronic kidney disease | 42 (18.1) | 20 (22.7) | 22 (15.3) | 0.153 |
| Prior stroke | 17 (7.3) | 8 (9.1) | 9 (6.2) | 0.420 |
| Peripheral artery disease | 25 (10.8) | 8 (9.1) | 17 (11.8) | 0.518 |
| Chronic obstructive pulmonary disease | 13 (5.6) | 6 (6.8) | 7 (4.9) | 0.565 |
| Echocardiographic parameters | ||||
| Left-ventricular end-diastolic diameter (mm) | 50.4 ± 9.0 | 50.6 ± 7.3 | 50.2 ± 10.2 | 0.665 |
| Intraventricular septal diameter (mm) | 11.4 ± 2.1 | 11.2 ± 2.5 | 11.6 ± 1.8 | 0.197 |
| Posterior wall diastolic diameter (mm) | 10.5 ± 1.6 | 10.2 ± 1.8 | 10.7 ± 1.4 | 0.124 |
| left atrium (mm) | 40.4 ± 5.9 | 39.4 ± 5.9 | 41.3 ± 5.8 | 0.033 |
| TAPSE (mm) | 22.0 ± 4.3 | 21.0 ± 3.2 | 22.0 ± 3.8 | 0.609 |
| LVEF [%] | 49.5 ± 10.5 | 48.0 ± 11.1 | 50.6 ± 9.9 | 0.075 |
| Severe mitral insufficiency | 6 (3.1) | 3 (3.5) | 3 (2.8) | 0.999 |
| Severe aortic insufficiency | 1 (0.5) | 1 (1.2) | 0 (0.0) | 0.438 |
| Severe aortic stenosis | 4 (2.1) | 1 (1.2) | 3 (2.8) | 0.633 |
| Variable | Total Population N = 232 | ACS N = 88 (%) | CCS N = 144 (%) | p |
|---|---|---|---|---|
| White blood cells (109/L) | 8.5 ± 2.7 | 9.7 ± 3.0 | 7.8 ± 2.1 | <0.002 |
| Hemoglobin (g/dL) | 13.4 ± 1.7 | 13.4 ± 1.7 | 13.3 ± 1.6 | 0.861 |
| Red blood cells (1012/L) | 4.4 ± 0.5 | 4.5 ± 0.5 | 4.4 ± 0.5 | 0.477 |
| Platelets (109/L) | 222.9 ± 65 | 226.7 ± 67.2 | 220.3 ± 63.5 | 0.580 |
| Glucose (mg/dL) | 136.4 ± 64.9 | 147.6 ± 72.1 | 125.4 ± 55.1 | 0.005 |
| HbA1c (%) | 6.3 (6.0–7.3) | 6.3 (5.8–7.3) | 6.3 (6.1–7.3) | 0.592 |
| Total cholesterol (mg/dL) | 163.9 ± 50.9 | 170.5 ± 52.2 | 157.5 ± 49.0 | 0.067 |
| HDL (mg/dL) | 45.7 ± 14.6 | 46.2 ± 16.7 | 45.2 ± 12.3 | 0.764 |
| LDL (mg/dL) | 89.8 ± 40.5 | 94.7 ± 43.0 | 85.0 ± 37.5 | 0.135 |
| Triglycerides (mg/dL) | 142 ± 33.9 | 147.2 ± 126.3 | 137.0 ± 141.4 | 0.564 |
| Creatine (mg/dL) | 1.1 ± 0.7 | 1.2 ± 1.0 | 1.1 ± 0.4 | 0.244 |
| eGFR (mL/min/1.73 m2) | 70.5 ± 23.2 | 68.5 ± 25.9 | 71.9 ± 21.1 | 0.269 |
| Troponin I upon admission (ng/mL) | 108 (15.8–235) | 281.4 (50.5–213) | 44 (9.5–119.5) | <0.001 |
| Troponin I max (ng/mL) | 1110 (49.8–11,573) | 5294 (688–32,561) | 53.8 (14–538) | <0.001 |
| CK at admission (IU/L) | 134.5 (84–326) | 232 (124–626) | 85 (67–121) | <0.001 |
| CK max (IU/L) | 173 (90–473) | 295 (164–963) | 89 (67–152) | <0.001 |
| CK-MB at admission (IU/L) | 18 (13.5–30) | 23 (17–47) | 15 (12–18.2) | <0001 |
| CK-MB max (U/L) | 22.5 (15–48.5) | 41 (22–116.5) | 16 (13–22.8) | <0.001 |
| Variable | Total Population N = 232 (%) | ACS N = 88 (%) | CCS N = 144 (%) | p |
|---|---|---|---|---|
| Coronary artery with the target lesion | ||||
| LM | 9 (3.9) | 5 (5.7) | 4 (2.8) | 0.305 |
| LAD | 72 (31) | 29 (33.0) | 43 (29.9) | |
| LCx | 61 (26.3) | 23 (26.1) | 38 (26.4) | |
| RCA | 90 (38.8) | 32 (36.4) | 58 (40.3) | |
| VG | 6 (2.6) | 5 (5.7) | 1 (0.7) | |
| Type of the target lesion | ||||
| A | 38 (16.4) | 13 (14.8) | 25 (17.4) | 0.495 |
| B1 | 66 (28.4) | 30 (34.1) | 36 (25.0) | |
| B2 | 37 (15.9) | 12 (13.6) | 25 (17.4) | |
| C | 91 (39.2) | 33 (37.5) | 58 (40.3) | |
| Heavy calcification | 18 (7.8) | 4 (4.5) | 14 (9.7) | 0.153 |
| Coronary bifurcation | 23 (9.9) | 8 (9.1) | 15 (10.4) | 0.743 |
| SYNTAX | 13.9 ± 8.7 | 16.0 ± 8.4 | 12.9 ± 8.6 | 0.008 |
| SYNTAX II PCI | 32.9 ± 11.0 | 35.6 ± 10.1 | 31.6 ± 11.2 | 0.003 |
| SYNTAX II CABG | 29.1 ± 10.8 | 29.9 ± 10.5 | 28.6 ± 11.1 | 0.491 |
| EuroScore II | 1.6 (0.9–3.3) | 2.5 (1.3–4.3) | 1.3 (0.8–2.5) | <0.001 |
| Lesion pre-dilatation | 143 (61.6) | 47 (53.4) | 96 (66.7) | 0.043 |
| Stent diameter (mm) | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.1 ± 0.5 | 0.069 |
| Stent length (mm) | 21.2 ± 10.9 | 21.9 ± 12 | 20.8 ± 10.2 | 0.821 |
| Stent pressure (atm) | 15.3 ± 2.7 | 15.5 (2.6) | 15.2 (2.7) | 0.578 |
| 2nd stent implantation | 90 (39) | 33 (37.5) | 57 (39.6) | 0.803 |
| Stent post-dilatation | 88 (37.9) | 34 (38.6) | 54 (37.5) | 0.852 |
| Access site * | ||||
| Transradial | 193 (83.2) | 64 (72.7) | 129 (89.3) | 0.013 |
| Transfemoral | 43 (18.5) | 24 (27.3) | 19 (13.2) | |
| Guiding catheter * | ||||
| 6F | 222 (95.7) | 87 (98.9) | 135 (93.8) | 0.094 |
| 7F | 11 (4.7) | 1 (1.1) | 10 (6.9) | |
| Coronary dissection | 16 (6.9) | 5 (5.7) | 11 (7.6) | 0.568 |
| MI type 4a | 5 (2.2) | 0 | 5 (3.5) | 0.159 |
| Variable | Total Population N = 232 (%) | ACS N = 88 (%) | CCS N = 144 (%) | p |
|---|---|---|---|---|
| Acetylsalicylic acid | 232 (100) | 88 (100.0) | 144 (100.0) | - |
| P2Y12 | ||||
| Clopidogrel | 214 (92.2) | 78 (88.6) | 136 (94.4) | 0.065 |
| Prasugrel | 1 (0.4) | 0 | 1 (0.7) | |
| Ticagrelor | 17 (7.3) | 10 (11.4) | 7 (4.9) | |
| Beta-blocker | 223 (96.1) | 84 (95.5) | 139 (96.5) | 0.733 |
| Ca-blocker | 53 (22.8) | 15 (17.0) | 38 (26.4) | 0.100 |
| Angiotensin-converting enzyme inhibitor | 190 (81.9) | 74 (84.1) | 116 (80.6) | 0.497 |
| Angiotensin receptor blocker | 36 (15.5) | 10 (11.4) | 26 (18.1) | 0.172 |
| Diuretic | 125 (53.9) | 48 (54.5) | 77 (53.5) | 0.874 |
| Mineralocorticoid receptor antagonist | 48 (20.7) | 23 (26.1) | 25 (17.4) | 0.109 |
| Nitrates | 13 (5.6) | 3 (3.4) | 10 (6.9) | 0.380 |
| Vitamin K antagonist | 17 (7.3) | 8 (9.1) | 9 (6.2) | 0.420 |
| Non-vitamin K oral anticoagulant | 11 (4.7) | 1 (1.1) | 10 (7.0) | 0.265 |
| Statin | 230 (99.1) | 88 (100.0) | 142 (98.6) | 0.527 |
| Hypoglycemic medications | 62 (26.7) | 16 (18.2) | 46 (31.9) | 0.022 |
| Insulin | 33 (14.2) | 16 (18.2) | 17 (11.8) | 0.177 |
| Year | Death | Cardiac Death | TLR | MI | MACE |
|---|---|---|---|---|---|
| 1 | 5 (5.7) | 4 (4.5) | 6 (6.8) | 4 (4.5) | 11 (12.5) |
| 2 | 6 (6.8) | 4 (4.5) | 7 (7.9) | 4 (4.5) | 12 (13.6) |
| 3 | 8 (9.1) | 6 (6.8) | 8 (9.1) | 4 (4.5) | 15 (17.1) |
| 4 | 10 (11.4) | 7 (7.9) | 9 (10.2) | 8 (9.1) | 21 (23.9) |
| Variable | Multivariable Analysis for MACE | ||
|---|---|---|---|
| HR | 95% CI | p | |
| High bleeding risk | 1.63 | 0.44–6.02 | 0.461 |
| Calcification | 9.00 | 1.75–46.3 | 0.009 |
| Post-dilatation | 3.78 | 1.28–11.2 | 0.016 |
| Prior coronary artery bypass grafting | 6.64 | 1.62–27.1 | 0.008 |
| VKA—vitamin K antagonist use | 5.99 | 1.29–27.8 | 0.022 |
| Rivaroxaban use | 51.7 | 4.48–596 | 0.002 |
| Variable | Multivariable Analysis for TLR | ||
|---|---|---|---|
| HR | 95% CI | p | |
| Calcifications | 10.2 | 1.72–60.9 | 0.011 |
| 2nd stent | 11.8 | 1.22–114 | 0.033 |
| SYNTAX 23–32 | 6.79 | 1.12–41.1 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyczynski, M.; Kern, A.; Buller, P.; Gil, R.J.; Bil, J. 48-Month Clinical Outcomes and Prognostic Factors in an All-Comers Population with Acute Coronary Syndrome and Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention with a Sirolimus-Eluting Stent. J. Pers. Med. 2023, 13, 1573. https://doi.org/10.3390/jpm13111573
Tyczynski M, Kern A, Buller P, Gil RJ, Bil J. 48-Month Clinical Outcomes and Prognostic Factors in an All-Comers Population with Acute Coronary Syndrome and Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention with a Sirolimus-Eluting Stent. Journal of Personalized Medicine. 2023; 13(11):1573. https://doi.org/10.3390/jpm13111573
Chicago/Turabian StyleTyczynski, Maciej, Adam Kern, Patryk Buller, Robert J. Gil, and Jacek Bil. 2023. "48-Month Clinical Outcomes and Prognostic Factors in an All-Comers Population with Acute Coronary Syndrome and Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention with a Sirolimus-Eluting Stent" Journal of Personalized Medicine 13, no. 11: 1573. https://doi.org/10.3390/jpm13111573
APA StyleTyczynski, M., Kern, A., Buller, P., Gil, R. J., & Bil, J. (2023). 48-Month Clinical Outcomes and Prognostic Factors in an All-Comers Population with Acute Coronary Syndrome and Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention with a Sirolimus-Eluting Stent. Journal of Personalized Medicine, 13(11), 1573. https://doi.org/10.3390/jpm13111573







