Cerebral Venous Thrombosis during Thyrotoxicosis: Case Report and Literature Update
Abstract
1. Introduction
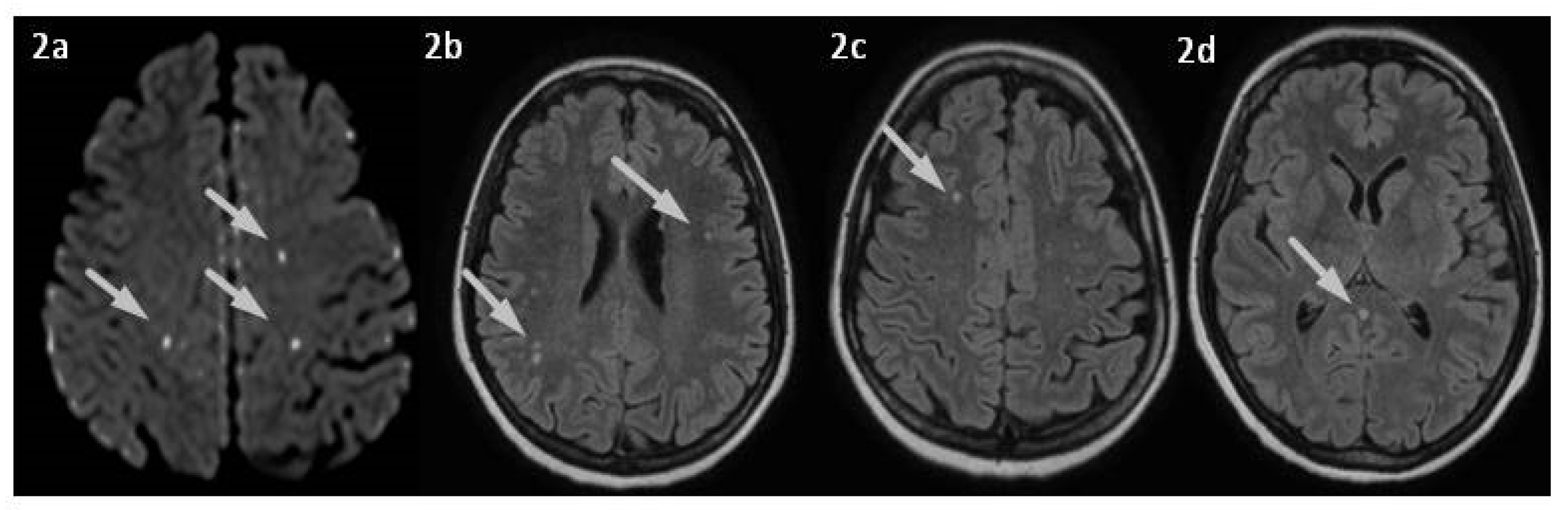
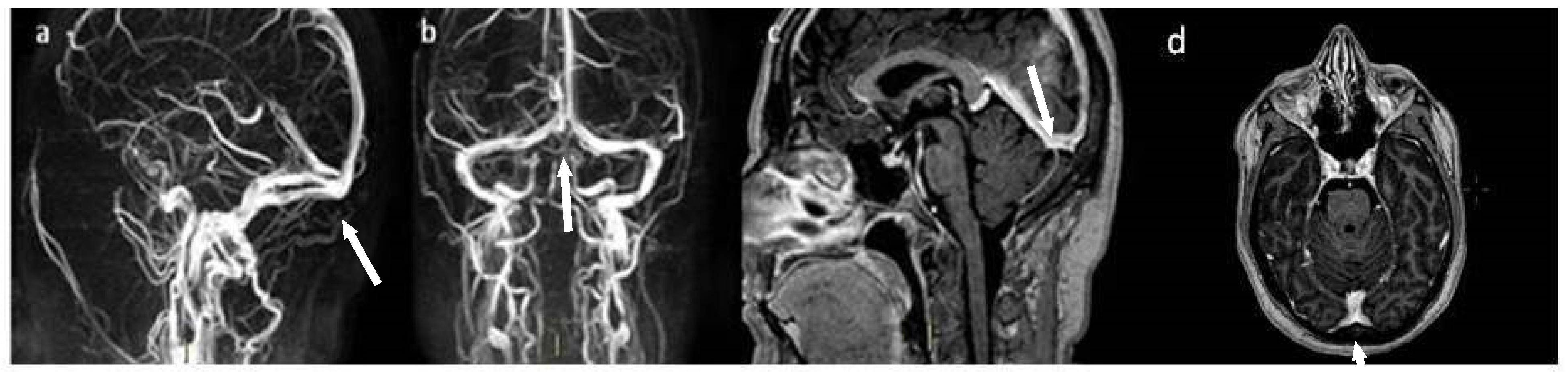
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silvis, S.M.; de Sousa, D.A.; Ferro, J.M.; Coutinho, J.M. Cerebral venous thrombosis. Nat. Rev. Neurol. 2017, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Bousser, M.G.; Ferro, J.M. Cerebral venous thrombosis: An update. Lancet Neurol. 2007, 6, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lauw, M.N.; Barco, S.; Coutinho, J.M.; Middeldorp, S. Cerebral venous thrombosis and thrombophilia: A systematic review and meta-analysis. Semin. Thromb. Hemost. 2013, 39, 913–927. [Google Scholar] [CrossRef]
- Dangal, G.; Thapa, L.B. Cerebral venous sinus thrombosis presenting in pregnancy and puerperium. BMJ Case Rep. 2009, 2009, bcr0620092045. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, S.F.; Stam, J.; Koopman, M.M.; Vandenbroucke, J.P. Case-control study of risk of cerebral sinus thrombosis in oral contraceptive users and in [correction of who are] carriers of hereditary prothrombotic conditions. The Cerebral Venous Sinus Thrombosis Study Group. BMJ 1998, 316, 589–592. [Google Scholar] [CrossRef]
- De Freitas, G.R.; Bogousslavsky, J. Risk factors of cerebral vein and sinus thrombosis. Front. Neurol. Neurosci. 2008, 23, 23–54. [Google Scholar] [CrossRef] [PubMed]
- Lemke, D.M.; Hacein-Bey, L. Cerebral venous sinus thrombosis. J. Neurosci. Nurs. 2005, 37, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, M.A.; Mottamedi, M.; Azimi, A.R.; Moghimi, B. Androgen-induced cerebral venous sinus thrombosis in a young body builder: Case report. BMC Neurol. 2004, 4, 22. [Google Scholar] [CrossRef]
- Waheed, W.; Aljerdi, S.; Decker, B.; Cushman, M.; Hamill, R.W. Cerebral venous thrombosis associated with thyrotoxicosis, the use of desmopressin and elevated factor VIII/von Willebrand factor. BMJ Case Rep. 2016, 2016, bcr2016216584. [Google Scholar] [CrossRef]
- Mouton, S.; Nighoghossian, N.; Berruyer, M.; Derex, L.; Philippeau, F.; Cakmak, S.; Honnorat, J.; Hermier, M.; Trouillas, P. Hyperthyroidism and cerebral venous thrombosis. Eur. Neurol. 2005, 54, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Bischof, J.; Dirk, A.L.; Friedrich, N.; Hammer, E.; Thiele, T.; Führer, D.; Homuth, G.; Brabant, G.; Völker, U. Effect of Experimental Thyrotoxicosis onto Blood Coagulation: A Proteomics Study. Eur. Thyroid. J. 2015, 4 (Suppl. 1), 119–124. [Google Scholar] [CrossRef] [PubMed]
- Horacek, J.; Maly, J.; Svilias, I.; Smolej, L.; Cepkova, J.; Vizda, J.; Sadilek, P.; Fatorova, I.; Zak, P. Prothrombotic changes due to an increase in thyroid hormone levels. Eur. J. Endocrinol. 2015, 172, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Stuijver, D.J.; van Zaane, B.; Romualdi, E.; Brandjes, D.P.; Gerdes, V.E.; Squizzato, A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: A systematic review and meta-analysis. Thromb. Haemost. 2012, 108, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.E. Thyroid disease in women. Med. Clin. N. Am. 1998, 82, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Pegoraro, S.; Ageno, W. Cerebral venous thrombosis. Minerva Med. 2021, 112, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, H.; Wei, H.; Liu, L.; Zhou, C.; Ji, X. Venous stroke-a stroke subtype that should not be ignored. Front. Neurol. 2022, 13, 1019671. [Google Scholar] [CrossRef] [PubMed]
- Idiculla, P.S.; Gurala, D.; Palanisamy, M.; Vijayakumar, R.; Dhandapani, S.; Nagarajan, E. Cerebral Venous Thrombosis: A Comprehensive Review. Eur. Neurol. 2020, 83, 369–379. [Google Scholar] [CrossRef]
- Bajko, Z.; Motataianu, A.; Stoian, A.; Barcutean, L.; Andone, S.; Maier, S.; Drăghici, I.-A.; Cioban, A.; Balasa, R. Gender Differences in Risk Factor Profile and Clinical Characteristics in 89 Consecutive Cases of Cerebral Venous Thrombosis. J. Clin. Med. 2021, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Hieber, M.; von Kageneck, C.; Weiller, C.; Lambeck, J. Thyroid Diseases Are an Underestimated Risk Factor for Cerebral Venous Sinus Thrombosis. Front. Neurol. 2020, 11, 561656. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Yamashita, R.; Furuya, M.; Yamazaki, M.; Koyama, K.; Tanaka, F. A Case of Cerebral Venous Thrombosis and Deep Venous Thrombosis Due to Hyperthyroidism with Increased Factor VIII Activity. J. Stroke Cerebrovasc Dis. 2019, 28, 104364. [Google Scholar] [CrossRef] [PubMed]
- Kraut, E.; Sarkar, R.; Houlden, R.L. Cerebral Venous Thrombosis Associated With Graves Hyperthyroidism. AACE Clin. Case Rep. 2017, 3, e70–e73. [Google Scholar] [CrossRef]
- Hieber, M.; Lambeck, J. Cerebral Venous Sinus Thrombosis in a Patient With Graves’ Disease. J. Endocrinol. Metab. 2016, 6, 162–164. [Google Scholar] [CrossRef]
- Srikant, B.; Balasubramaniam, S. Grave’s disease with transverse and sigmoid sinus thrombosis needing surgical intervention. Asian J. Neurosurg. 2013, 8, 162. [Google Scholar] [CrossRef]
- Anuszkiewicz, K.; Szerszenowicz, A.; Dzwilewski, K.; Geryk, N.; Zawadzka, M.; Radoń-Proskura, J.; Mazurkiewicz-Bełdzińska, M. Unstable Graves’ disease as a precipitating factor for cerebral sinus venous thrombosis. Endokrynol. Pol. 2021, 72, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.; Mariën, P.; De Deyn, P.P. Sinus sigmoideus thrombosis secondary to graves’ disease: A case description. Case Rep. Neurol. 2011, 3, 203–209. [Google Scholar] [CrossRef]
- Elhassan, A.E.E.; Ali, M.O.K.; Bougaila, A.; Abdelhady, M.; Abuzaid, H. Hyperthyroidism as a Precipitant Factor for Cerebral Venous Thrombosis: A Case Report. J. Investig. Med. High Impact. Case Rep. 2020, 8, 2324709620949309. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Hiraoka, E.; Hoshino, M.; Deshpande, G.A.; Sawada, K.; Norisue, Y.; Tsukuda, J.; Suzuki, T. Progressive Ischemic Stroke due to Thyroid Storm-Associated Cerebral Venous Thrombosis. Am. J. Case Rep. 2017, 18, 194–197. [Google Scholar] [CrossRef]
- Rehman, A.; Husnain, M.G.; Mushtaq, K.; Eledrisi, M.S. Cerebral venous sinus thrombosis precipitated by Graves’ disease. BMJ Case Rep. 2018, 2018, bcr2017224143. [Google Scholar] [CrossRef]
- Chee, Y.C.; Abdul Halim, S. Cerebral venous sinus thrombosis in a patient with concomitant Graves’ disease and squamous cell carcinoma of the cervix. BMJ Case Rep. 2020, 13, e236730. [Google Scholar] [CrossRef] [PubMed]
- Knudsen-Baas, K.M.; Kråkenes, J.; Thordarson, H.B.; Sjo, M.; Waje-Andreassen, U. Cerebral Venous Thrombosis and Hyperthyroidism. Intern. Med. 2014, 4, 136. [Google Scholar] [CrossRef]
- Gomes, R. Graves Thyrotoxicosis and Cerebral Venous Sinus Thrombosis Causality or Chance Alone? Int. Arch. Endocrinol. Clin. Res. 2021, 7, 26. [Google Scholar] [CrossRef]
- Kim, D.D.; Chunilal, S.; Young, S.; Cutfield, R. A study of venous thrombosis incidence in patients with acute hyperthyroidism. Intern. Med. J. 2013, 43, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, M.; Yilmaz, S.; Ersel, M.; Sarisoy, H.T. A rare cause of headache: Cerebral venous sinus thrombosis due to hyperthyroidism. Am. J. Emerg. Med. 2008, 26, 383.e1–383.e2. [Google Scholar] [CrossRef]
- Bensalah, M.; Squizzato, A.; Ould Kablia, S.; Menia, H.; Kemali, Z. Cerebral vein and sinus thrombosis and hyperthyrodism: A case report and a systematic review of the literature. Thromb. Res. 2011, 128, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.U.; Kwon, K.Y.; Hur, J.W.; Lee, J.W.; Lee, H.K. The role of hyperthyroidism as the predisposing factor for superior sagittal sinus thrombosis. J. Cerebrovasc. Endovasc. Neurosurg. 2012, 14, 251–254. [Google Scholar] [CrossRef]
- Ra, C.S.; Lui, C.C.; Liang, C.L.; Chen, H.J.; Kuo, Y.L.; Chen, W.F. Superior sagittal sinus thrombosis induced by thyrotoxicosis. Case report. J. Neurosurg. 2001, 94, 130–132. [Google Scholar] [CrossRef]
- Kim, B.R.; Jung, J.H.; Hahm, J.R.; Jung, J.; Park, H.J.; Kim, S.K. A Case of Cerebral Venous Thrombosis in a Patient with Graves’ Disease. Kosin Med. J. 2016, 31, 179–183. [Google Scholar] [CrossRef][Green Version]
- Liu, J.C.; Huang, H.Y.; Hsu, Y.T. Hyperthyroidism and thrombophilia in cerebral arterial and venous thrombosis: A case report and critical review. Neurologist 2015, 19, 53–55. [Google Scholar] [CrossRef]
- Fandler-Höfler, S.; Pilz, S.; Ertler, M.; Haidegger, M.; Kneihsl, M.; Wünsch, G.; Gary, T.; Enzinger, C.; Gattringer, T. Thyroid dysfunction in cerebral venous thrombosis: A retrospective cohort study. J. Neurol. 2022, 269, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Hong, W.; Wu, J.; Xu, J.; Zhao, J.; Zhang, X.; Liu, Y.; Yu, R.G. Cerebral venous sinus thrombosis caused by traumatic brain injury complicating thyroid storm: A case report and discussion. BMC Neurol. 2022, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xi, G.; Fan, W.; Wang, G.; Li, J.; Huang, J. Cerebrovascular intervention therapy worked positively in one patient with severe cerebral venous sinus thrombosis due to hyperthyroidism: A case report and review of the literature. J. Med. Case Rep. 2022, 16, 250. [Google Scholar] [CrossRef]
- Migeot, M.; Rutgers, M.P.; Gille, M. Puerperal cerebral sinus venous thrombosis and acute hyperthyroidism in Graves’ disease. Acta Neurol. Belg. 2013, 113, 331–333. [Google Scholar] [CrossRef]
- Verberne, H.J.; Fliers, E.; Prummel, M.F.; Stam, J.; Brandjes, D.P.; Wiersinga, W.M. Thyrotoxicosis as a predisposing factor for cerebral venous thrombosis. Thyroid 2000, 10, 607–610. [Google Scholar] [CrossRef]
- Son, H.M. Massive cerebral venous sinus thrombosis secondary to Graves’ disease. Yeungnam Univ. J. Med. 2019, 36, 273–280. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sharma, N. Cerebral venous sinus thrombosis with autoimmune thyroiditis. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. 1), S176–S177. [Google Scholar] [CrossRef]
- Janovsky, C.C.; Fukuda, T.G.; Silva, G.S.; Martins, J.R. An unusual association between acute ischaemic stroke and cerebral venous thrombosis with thyrotoxic state. BMJ Case Rep. 2013, 2013, bcr2013201130. [Google Scholar] [CrossRef]
- Dai, A.; Wasay, M.; Dubey, N.; Giglio, P.; Bakshi, R. Superior sagittal sinus thrombosis secondary to hyperthyroidism. J. Stroke Cerebrovasc. Dis. 2000, 9, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Elbers, L.P.; van Zaane, B.; Gerdes, V.E.; Coutinho, J.M.; Bisschop, P.H.; Fliers, E. Venous thromboembolism in overt hyperthyroidism—A direct association with clinical implications? Neth. J. Med. 2014, 72, 242–244. [Google Scholar]
- Maes, J.; Michotte, A.; Velkeniers, B.; Stadnik, T.; Jochmans, K. Hyperthyroidism with increased factor VIII procoagulant protein as a predisposing factor for cerebral venous thrombosis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 458. [Google Scholar] [CrossRef][Green Version]
- Madan, S.; Chaudhuri, Z. Cerebral venous thrombosis with auto-immune hyperthyroidism. Indian J. Ophthalmol. 2018, 66, 1649–1651. [Google Scholar] [CrossRef]
- Silburn, P.A.; Sandstrom, P.A.; Staples, C.; Mowat, P.; Boyle, R.S. Deep cerebral venous thrombosis presenting as an encephalitic illness. Postgrad. Med. J. 1996, 72, 355–357. [Google Scholar] [CrossRef][Green Version]
- Situmeang, R.F.V.; Stevano, R.; Sutanto, R. COVID-19 as a trigger of cerebral venous sinus thrombosis in a patient with autoimmune hyperthyroidism: A case report. Egypt J. Neurol. Psychiatr. Neurosurg. 2022, 58, 40. [Google Scholar] [CrossRef] [PubMed]
- Strada, L.; Gandolfo, C.; Del Sette, M. Cerebral sinus venous thrombosis in a subject with thyrotoxicosis and MTHFR gene polymorphism. Neurol. Sci. 2008, 29, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Kira, Y.; Maeda, N. Hyperthyroidism-induced Cerebral Venous Thrombosis Presenting as Chronic Isolated Intracranial Hypertension. Intern. Med. 2023, 62, 3021–3025. [Google Scholar] [CrossRef]
- Usami, K.; Kinoshita, T.; Tokumoto, K.; Ino, T.; Ozawa, K.; Kimura, T.; Nakamura, S. Successful treatment of plasma exchange for severe cerebral venous thrombosis with thyrotoxicosis. J. Stroke Cerebrovasc. Dis. 2009, 18, 239–243. [Google Scholar] [CrossRef]
- Van Eimeren, V.F.; Billinghurst, L.; Askalan, R.; Laughlin, S.; Brandão, L.R.; Williams, S.; Kahr, W.H. Cerebral sinus venous thrombosis in a child with hyperthyroidism. Pediatr. Blood Cancer 2012, 58, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, T.; Akakin, A. Anatomy of cerebral veins and sinuses. Front. Neurol. Neurosci. 2008, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B. Physiology of cerebral venous blood flow: From experimental data in animals to normal function in humans. Brain Res. Brain Res. Rev. 2004, 46, 243–260. [Google Scholar] [CrossRef]
- Arun, A.; Amans, M.R.; Higgins, N.; Brinjikji, W.; Sattur, M.; Satti, S.R.; Nakaji, P.; Luciano, M.; Huisman, T.A.; Moghekar, A.; et al. A proposed framework for cerebral venous congestion. Neuroradiol. J. 2022, 35, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.H.; Klein, J.P. Cerebral Venous Thrombosis. N. Engl. J. Med. 2021, 385, 59–64. [Google Scholar] [CrossRef]
- Van Zaane, B.; Squizzato, A.; Debeij, J.; Dekkers, O.M.; Meijers, J.C.; Van Zanten, A.P.; Büller, H.R.; Gerdes, V.E.; Cannegieter, S.C.; Brandjes, D.P. Alterations in coagulation and fibrinolysis after levothyroxine exposure in healthy volunteers: A controlled randomized crossover study. J. Thromb. Haemost. 2011, 9, 1816–1824. [Google Scholar] [CrossRef]
- Ordookhani, A.; Burman, K.D. Hemostasis in Hypothyroidism and Autoimmune Thyroid Disorders. Int. J. Endocrinol. Metab. 2017, 15, e42649. [Google Scholar] [CrossRef] [PubMed]
- Squizzato, A.; Gerdes, V.E.; Brandjes, D.P.; Büller, H.R.; Stam, J. Thyroid diseases and cerebrovascular disease. Stroke 2005, 36, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Comlekci, A.; Ozcan, M.A. The alteration of coagulation in patients with thyroid dysfunction. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Federici, A.B. Acquired von Willebrand syndrome associated with hypothyroidism: A mild bleeding disorder to be further investigated. Semin. Thromb. Hemost. 2011, 37, 35–40. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Kramer, C.K.; Marroni, C.P.; Leães, C.G.; Viana, L.; Roithman, S.; Schmaedecke, A.; Pereira-Lima, J.F. Acquired factor VIII and von Willebrand factor (aFVIII/VWF) deficiency and hypothyroidism in a case with hypopituitarism. Clin. Appl. Thromb. Hemost. 2010, 16, 107–109. [Google Scholar] [CrossRef] [PubMed]
- McQuade, C.; Skugor, M.; Brennan, D.M.; Hoar, B.; Stevenson, C.; Hoogwerf, B.J. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS database study. Thyroid 2011, 21, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Cantürk, Z.; Cetinarslan, B.; Tarkun, I.; Cantürk, N.Z.; Ozden, M.; Duman, C. Hemostatic system as a risk factor for cardiovascular disease in women with subclinical hypothyroidism. Thyroid 2003, 13, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Tsakiris, D.A.; Roth, C.B.; Guglielmetti, M.; Staub, J.J.; Marbet, G.A. Haemostatic profile in hypothyroidism as potential risk factor for vascular or thrombotic disease. Eur. J. Clin. Investig. 2001, 31, 131–137. [Google Scholar] [CrossRef]
- Erden, S.; Buyukozturk, S.; Vural, P.; Değirmencioğlu, S. Acute-phase reactans in Hashimoto thyroiditis. Int. Immunopharmacol. 2008, 8, 1863–1865. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hua, S.-C.; Chang, C.-H.; Kao, W.-Y.; Lee, H.-L.; Chuang, L.-M.; Huang, Y.-T.; Lai, M.-S. High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker. J. Clin. Med. 2019, 8, 817. [Google Scholar] [CrossRef]
- Peralta, A.R.; Canhão, P. Hypothyroidism and cerebral vein thrombosis—A possible association. J. Neurol. 2008, 255, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.A.; Manson, J.E.; Michels, K.B.; Alexander, E.K.; Willett, W.C.; Utiger, R.D. Smoking and other lifestyle factors and the risk of Graves’ hyperthyroidism. Arch. Intern. Med. 2005, 165, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Cappellani, D.; Bartalena, L.; Bogazzi, F. Short review: Novel concepts in the approach to patients with amiodarone-induced thyrotoxicosis. J. Endocrinol. Investig. 2023. ahead of print. [Google Scholar] [CrossRef]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef]
- Silvestri, R.; De Domenico, P.; Raffaele, M.; Lombardo, N.; Casella, C.; Gugliotta, M.A.; Meduri, M. Vascular compression from goiter as an unusual cause of cerebrovascular accident. Ital. J. Neurol. Sci. 1990, 11, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Elbers, L.P.B.; Fliers, E.; Cannegieter, S.C. The influence of thyroid function on the coagulation system and its clinical consequences. J. Thromb. Haemost. 2018, 16, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Coban, E.; Aydemir, M. Levels of plasma fibrinogen and D-dimer in subjects with subclinical hyperthyroidism. Med. Sci. Monit. 2008, 14, CR42–CR46. [Google Scholar] [PubMed]
- Marongiu, F.; Conti, M.; Mameli, G.; Murtas, M.L.; Balzano, S.; Sorano, G.; Mamusa, A.M.; Martino, E. Fibrinogen and fibrinolytic activity in hyperthyroidism before and after antithyroid treatment. J. Endocrinol. Investig. 1988, 11, 723–725. [Google Scholar] [CrossRef]
- Farid, N.R.; Griffiths, B.L.; Collins, J.R.; Marshall, W.H.; Ingram, D.W. Blood coagulation and fibrinolysis in thyroid disease. Thromb. Haemost. 1976, 35, 415–422. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G.; Targher, G. Hyperthyroidism and venous thrombosis: A casual or causal association? A systematic literature review. Clin. Appl. Thromb. Hemost. 2011, 17, 387–392. [Google Scholar] [CrossRef]
- Homoncik, M.; Gessl, A.; Ferlitsch, A.; Jilma, B.; Vierhapper, H. Altered platelet plug formation in hyperthyroidism and hypothyroidism. J. Clin. Endocrinol. Metab. 2007, 92, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Schechter, G.P. New Interfaces of Thyroid Hormone Actions With Blood Coagulation and Thrombosis. Clin. Appl. Thromb. Hemost. 2018, 24, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Debeij, J.; van Zaane, B.; Dekkers, O.M.; Doggen, C.J.; Smit, J.W.; van Zanten, A.P.; Brandjes, D.P.; Büller, H.R.; Gerdes, V.E.; Rosendaal, F.R.; et al. High levels of procoagulant factors mediate the association between free thyroxine and the risk of venous thrombosis: The MEGA study. J. Thromb. Haemost. 2014, 12, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, C.J.; Stuijver, D.J.; van Zaane, B.; Squizzato, A.; Brandjes, D.P.; Büller, H.R.; Meijers, J.C.; Gerdes, V.E. Thrombin-activatable fibrinolysis inhibitor in hypothyroidism and hyperthyroxinaemia. Thromb. Haemost. 2013, 109, 214–220. [Google Scholar] [CrossRef]
- Hooper, J.M.; Stuijver, D.J.; Orme, S.M.; van Zaane, B.; Hess, K.; Gerdes, V.E.; Phoenix, F.; Rice, P.; Smith, K.A.; Alzahrani, S.H.; et al. Thyroid dysfunction and fibrin network structure: A mechanism for increased thrombotic risk in hyperthyroid individuals. J. Clin. Endocrinol. Metab. 2012, 97, 1463–1473. [Google Scholar] [CrossRef]
- Pietzner, M.; Engelmann, B.; Kacprowski, T.; Golchert, J.; Dirk, A.L.; Hammer, E.; Iwen, K.A.; Nauck, M.; Wallaschofski, H.; Führer, D.; et al. Plasma proteome and metabolome characterization of an experimental human thyrotoxicosis model. BMC Med. 2017, 15, 6. [Google Scholar] [CrossRef]
- Erem, C. Thyroid Disorders and Hypercoagulability. Semin. Thromb. Hemost. 2011, 37, 017–026. [Google Scholar] [CrossRef]
- Ellervik, C.; Mora, S.; Kuś, A.; Åsvold, B.O.; Marouli, E.; Deloukas, P.; Sterenborg, R.B.; Teumer, A.; Burgess, S.; Sabater-Lleal, M.; et al. Effects of Thyroid Function on Hemostasis, Coagulation, and Fibrinolysis: A Mendelian Randomization Study. Thyroid® 2021, 31, 1305–1315. [Google Scholar] [CrossRef]
- Strozyk, E.A.; Desch, A.; Poeppelmann, B.; Magnolo, N.; Wegener, J.; Huck, V.; Schneider, S.W. Melanoma-derived IL-1 converts vascular endothelium to a proinflammatory and procoagulatory phenotype via NFκB activation. Exp. Dermatol. 2014, 23, 670–676. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Incerpi, S.; Davis, F.B.; Mousa, S.A.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K. Molecular mechanisms of actions of formulations of the thyroid hormone analogue, tetrac, on the inflammatory response. Endocr. Res. 2013, 38, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Wijayagunaratne, R.C.; D’Souza, D.G.; Darras, V.M.; Van Herck, S.L. Transport of thyroid hormones via the choroid plexus into the brain: The roles of transthyretin and thyroid hormone transmembrane transporters. Front. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The blood–brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 2019, 15, 444–455. [Google Scholar] [CrossRef]
- Davis, F.B.; Tang, H.Y.; Shih, A.; Keating, T.; Lansing, L.; Hercbergs, A.; Fenstermaker, R.A.; Mousa, A.; Mousa, S.A.; Davis, P.J.; et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006, 66, 7270–7275. [Google Scholar] [CrossRef] [PubMed]
- DiNubile, M.J. Septic thrombosis of the cavernous sinuses. Arch. Neurol. 1988, 45, 567–572. [Google Scholar] [CrossRef]
- Ivey, K.J.; Smith, H. Hypopituitarism associated with cavernous sinus thrombosis. Report of a case. J. Neurol. Neurosurg. Psychiatry 1968, 31, 187–189. [Google Scholar] [CrossRef]
- Joubert, M.; Verdon, R.; Reznik, Y. Transient pituitary enlargement with central hypogonadism secondary to bilateral cavernous sinus thrombosis: Pituitary oedema? Eur. J. Endocrinol. 2009, 160, 873–875. [Google Scholar] [CrossRef]
- Press, C.A.; Lindsay, A.; Stence, N.V.; Fenton, L.Z.; Bernard, T.J.; Mirsky, D.M. Cavernous Sinus Thrombosis in Children: Imaging Characteristics and Clinical Outcomes. Stroke 2015, 46, 2657–2660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kau, H.C.; Wu, S.B.; Tsai, C.C.; Liu, C.J.; Wei, Y.H. Cigarette Smoke Extract-Induced Oxidative Stress and Fibrosis-Related Genes Expression in Orbital Fibroblasts from Patients with Graves’ Ophthalmopathy. Oxid. Med. Cell. Longev. 2016, 2016, 4676289. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Smoking and thyroid. Clin. Endocrinol. 2013, 79, 145–151. [Google Scholar] [CrossRef]
- Vestergaard, P. Smoking and thyroid disorders--a meta-analysis. Eur. J. Endocrinol. 2002, 146, 153–161. [Google Scholar] [CrossRef]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, B.; Bednarczuk, T.; Kostrzewa, G.; Kosińska, J.; Miśkiewicz, P.; Płazińska, M.T.; Bar-Andziak, E.; Królicki, L.; Krajewski, P.; Płoski, R. Polymorphism of the oestrogen receptor beta gene (ESR2) is associated with susceptibility to Graves’ disease. Clin. Endocrinol. 2008, 68, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.S.G.; Dovom, M.R.; Tehrani, F.R. The Menstrual Disturbances in Endocrine Disorders: A Narrative Review. Int. J. Endocrinol. Metab. 2020, 18. [Google Scholar] [CrossRef]
- Nabriski, D.; Ellis, M.; Ness-Abramof, R.; Shapiro, M.; Shenkman, L. Autoimmune thyroid disease and antiphospholipid antibodies. Am. J. Hematol. 2000, 64, 73–75. [Google Scholar] [CrossRef]
- Tatlisumak, T.; Jood, K.; Putaala, J. Cerebral Venous Thrombosis: Epidemiology in Change. Stroke 2016, 47, 2169–2170. [Google Scholar] [CrossRef]
- Jafari, A.; Rajabi, A.; Gholian-Aval, M.; Peyman, N.; Mahdizadeh, M.; Tehrani, H. National, regional, and global prevalence of cigarette smoking among women/females in the general population: A systematic review and meta-analysis. Environ. Health Prev. Med. 2021, 26, 1–13. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, A.I. Cerebral venous sinus thrombosis after vaccination: The UK experience. Lancet 2021, 398, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, B.; Betterle, C.; Zanoni, G. Vaccinations and Autoimmune Diseases. Vaccines 2021, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Berlit, P.; Diener, H.C.; Gerloff, C.; Greinacher, A.; Klein, C.; Petzold, G.C.; Piccininni, M.; Poli, S.; Röhrig, R.; et al. COVID-19 Vaccine-Associated Cerebral Venous Thrombosis in Germany. Ann. Neurol. 2021, 90, 627–639. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2021, 43, 3–40. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chakravarty, A. Neurological Complications Following COVID-19 Vaccination. Curr. Neurol. Neurosci. Rep. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Sharma, A.; Babazadeh, A.; Alizadeh-Khatir, A.; Sio, T.T.; Moghadam, M.A.T.; Pirzaman, A.T.; Mojadad, A.; Hosseinzadeh, R.; Barary, M.; et al. A review of the potential neurological adverse events of COVID-19 vaccines. Acta Neurol. Belg. 2022, 123, 9–44. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. Cerebral Venous Thrombosis Developing after COVID-19 Vaccination: VITT, VATT, TTS, and More. Semin. Thromb. Hemost. 2022, 48, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Kowarz, E.; Krutzke, L.; Külp, M.; Streb, P.; Larghero, P.; Reis, J.; Bracharz, S.; Engler, T.; Kochanek, S.; Marschalek, R. Vaccine-induced COVID-19 mimicry syndrome. eLife 2022, 211, e74974. [Google Scholar] [CrossRef]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines 2021, 9, 559. [Google Scholar] [CrossRef]
- Saadoun, D.; Wechsler, B.; Resche-Rigon, M.; Trad, S.; Le Thi Huong, D.; Sbai, A.; Dormont, D.; Amoura, Z.; Cacoub, P.; Piette, J.C. Cerebral venous thrombosis in Behçet’s disease. Arthritis Rheum. 2009, 61, 518–526. [Google Scholar] [CrossRef]
- Lang, Y.; Zhang, W.; Wu, X.; Deng, F.; Cui, L. Sjögren’s Syndrome with Cerebral Venous Sinus Thrombosis: A Case Report and Literature Review. Ann. Indian Acad. Neurol. 2020, 23, 110–112. [Google Scholar] [CrossRef]
- Vidailhet, M.; Piette, J.C.; Wechsler, B.; Bousser, M.G.; Brunet, P. Cerebral venous thrombosis in systemic lupus erythematosus. Stroke 1990, 21, 1226–1231. [Google Scholar] [CrossRef]
- Zhang, B.; Lang, Y.; Zhang, W.; Cui, L.; Deng, F. Characteristics and Management of Autoimmune Disease-Associated Cerebral Venous Sinus Thrombosis. Front. Immunol. 2021, 12, 671101. [Google Scholar] [CrossRef]
- Normand, S.L.; Sykora, K.; Li, P.; Mamdani, M.; Rochon, P.A.; Anderson, G.M. Readers guide to critical appraisal of cohort studies: 3. Analytical strategies to reduce confounding. BMJ 2005, 330, 1021–1023. [Google Scholar] [CrossRef]
- Ferro, J.M.; Canhão, P.; Stam, J.; Bousser, M.G.; Barinagarrementeria, F.; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004, 35, 664–670. [Google Scholar] [CrossRef]


| First Author/Year | Age/Sex | Neurological Symptoms | Site of Thrombosis | FVIII Activity | Acute Treatment | Oral Contraceptives | Smoker | Coagulopathy | Malignancies | Infections | Graves’ Disease | Other Prothrombotic Conditions | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yokoyama, 2019 | 48 F | Fever, H | SSS | Increased | LMWH | - | - | - | - | Viral meningitis | - | - | CR | [22] |
| Kraut, 2017 | 62 F | H, GS | SSS, RTS | Increased | LMWH | - | - | - | - | - | + | - | CR | [23] |
| Hieber, 2016 | 52 F | H | LSS, LTS | - | LMWH | - | - | + | - | - | + | - | MD | [24] |
| Srikant, 2013 | 42 F | H, drowsiness, LHe | RSS, RTS | NA | LMWH + decompressive craniectomy | NA | Na | + | NA | NA | + | - | NA | [25] |
| Anuszkiewicz, 2021 | 15 M | H, RHe, Ap | SSS, LTS, LSS | Increased | UH | / | - | - | - | - | + | - | CR | [26] |
| Mouton, 2005 | 32 F | H, vertigo, right arm paresthesia | LTS | Increased | UH | + | - | - | - | - | - (post-partum thyroiditis) | Puerperium | CR | [10] |
| Mouton, 2005 | 49 F | H, left arm weakness, dysarthria | SSS, RTS | Increased | NA | + | - | - | - | - | + | - | CR | [10] |
| Mouton, 2005 | 50 F | H, blurred vision, RVFD | CV, LTS | Increased | NA | - | + | - | - | - | + | - | CR | [10] |
| Mouton, 2005 | 39 F | H, FS | SSS, RTS | Increased | NA | - | + | - | - | - | + | - | CR | [10] |
| Hermans, 2011 | 22 F | GS | LSS | - | UH | + | - | - | - | - | + | - | PI | [27] |
| Elhassa, 2020 | 41 M | GS | SSS, CV | - | LMWH | / | - | - | - | - | + | - | CR | [28] |
| Tanabe, 2017 | 49 F | H, LHe | LTS, LSS, LIJV | - | UH | - | - | - | - | - | + | - | PI | [29] |
| Waheed, 2016 | 48 F | Drowsiness, H, V | CV, SS | Increased | UH | - | - | + | - | - | + | - | NA | [9] |
| Rehman, 2018 | 31 M | Drowsiness, H, V | SSS, SS, RTS, LTS | - | LMWH | / | - | + | - | - | + | - | CR | [30] |
| Chee, 2020 | 40 F | H, Ap | LSS, LTS, LIJV | NA | LMWH+ decompressive craniectomy | - | - | - | + | - | + | - | NA | [31] |
| Knudsen-Baas, 2014 | 17 F | H, S, coma | SSS, RTS, RSS, RIJV | NA | UH | + | - | + | - | - | + | - | CR | [32] |
| Gomes, 2021 | 23 F | H, V, GS | RTS, RSS, SS | NA | LMWH | - | - | - | - | - | + | - | CR | [33] |
| Kim, 2013 | 23 F | NS | NS | NA | NS | + | - | - | - | - | + | - | NA | [34] |
| Pekdemir, 2008 | 28 M | H, V, papilledema | LSS, LTS | NA | UH | / | NA | NA | NA | NA | - (chronic thyroiditis) | NA | MD | [35] |
| Bensalah, 2011 | 23 M | H | SSS, RTS, RSS | NA | LMWH | / | NS | - | - | - | + | Steroid therapy | NA | [36] |
| Hwang, 2012 | 31 M | H, S, CI | SSS | - | Warfarin | / | - | - | - | - | - | - | CR | [37] |
| Ra, 2001 | 60 M | H, GS, LHe | SSS, LTS | - | Urokinase | / | NA | - | - | - | + | - | PI | [38] |
| Kim,2016 | 39 M | GS, LHe | SSS | - | UH | / | - | + | - | - | + | - | CR | [39] |
| Liu, 2015 | 44 F | H, cortical blindness | LTS, LSS | Increased | UH | + | - | + | - | - | + | - | SD | [40] |
| Fandler-Hofler, 2022 | 60 F | H | LTS, LSS | - | LMWH | - | - | - | - | - | + | - | CR | [41] |
| Fandler-Hofler,2022 | 33 F | H | LTS, SS | - | LMWH | + | - | - | - | - | + | - | CR | [41] |
| Gong, 2022 | 29 M | Coma | Multiple sites | LMWH | / | - | + | - | - | + | Head trauma | MD | [42] | |
| Jia, 2022 | 44 F | H, drowsiness, RHe | RTS, RSS, SS, SSS, LTS, LSS | - | LMWH, urokinase, alteplase, thrombus aspiration | - | - | - | - | - | + | - | PI | [43] |
| Migeot, 2013 | 26 F | H, LHe, LVFD | SSS, RTS | Increased | Warfarin | - | - | - | + (papillary thyroid carcinoma) | - | + | Puerperium | CR | [44] |
| Verberne, 2000 | 28 F | Drowsiness | LTS, SS, LIJV | Increased | LMWH | + | - | + | - | - | + | - | CR | [45] |
| Son, 2019 | 31 M | S | SSS, RTS, RSS | NA | LMWH | / | NA | - | - | - | + | - | CR | [46] |
| Aggarwal, 2013 | 44 F | H, V, RHe | SSS, SS | - | LMWH | - | - | - | - | - | - (Hashimoto thyroiditis) | - | CR | [47] |
| Janovsky, 2013 | 21 F | Ap, RHe | SSS, LTS, LSS | Increased | LMWH | - | - | + | - | - | + | - | MD | [48] |
| Dai, 2000 | 39 M | H, GS | SSS | - | LMWH | / | NA | - | - | - | NA | - | CR | [49] |
| Elbers, 2014 | 50 F | Ap, RVFD | LSS, SS | - | LMWH | - | NA | - | - | - | + | - | MD | [50] |
| Maes, 2002 | 39 F | S, H, confusion | LTS, LIJV | Increased | UH | + | - | + | - | - | + | - | CR | [51] |
| Madan, 2018 | 28 F | H, right vision loss | LSS, LTS | NA | LMWH | - | NA | - | - | - | + | - | CR | [52] |
| Silburn, 1996 | 18 F | H, confusion, fever, neglect | DCV, ISS | NA | NS | + | - | - | - | - | + | - | NA | [53] |
| Situmeang, 2022 | 37 M | H, fever | SSS, RTS, RSS | NA | LMWH | / | - | - | - | COVID-19 | + | - | CR | [54] |
| Strada,2008 | 29 M | H, S, LHe | SSS, RTS | NA | UH | / | - | + | - | - | + | Hyperhomocisteinemia | CR | [55] |
| Tashiro, 2023 | 38 F | H, bilateral VI palsy, papilledema, diplopia | SSS, RTS, RSS | Increased | UH | - | - | - | - | - | + | - | CR | [56] |
| Usami, 2009 | 34 F | H, V, diplopia, LHe | SSS, RTS, SS, DCV | Increased | UH, plasma exchanges | - | - | + | - | - | + | - | SD | [57] |
| Van Eimeren, 2012 | 8 F | V, H | Massive CVT | Increased | LMWH | / | / | + | - | - | + | Dehydration | MD | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raho, E.M.; Antonioni, A.; Cotta Ramusino, N.; Jubea, D.; Gragnaniello, D.; Franceschetti, P.; Penitenti, F.; Daniele, A.; Zatelli, M.C.; Naccarato, M.; et al. Cerebral Venous Thrombosis during Thyrotoxicosis: Case Report and Literature Update. J. Pers. Med. 2023, 13, 1557. https://doi.org/10.3390/jpm13111557
Raho EM, Antonioni A, Cotta Ramusino N, Jubea D, Gragnaniello D, Franceschetti P, Penitenti F, Daniele A, Zatelli MC, Naccarato M, et al. Cerebral Venous Thrombosis during Thyrotoxicosis: Case Report and Literature Update. Journal of Personalized Medicine. 2023; 13(11):1557. https://doi.org/10.3390/jpm13111557
Chicago/Turabian StyleRaho, Emanuela Maria, Annibale Antonioni, Niccolò Cotta Ramusino, Dina Jubea, Daniela Gragnaniello, Paola Franceschetti, Francesco Penitenti, Andrea Daniele, Maria Chiara Zatelli, Maurizio Naccarato, and et al. 2023. "Cerebral Venous Thrombosis during Thyrotoxicosis: Case Report and Literature Update" Journal of Personalized Medicine 13, no. 11: 1557. https://doi.org/10.3390/jpm13111557
APA StyleRaho, E. M., Antonioni, A., Cotta Ramusino, N., Jubea, D., Gragnaniello, D., Franceschetti, P., Penitenti, F., Daniele, A., Zatelli, M. C., Naccarato, M., Traluci, I., Pugliatti, M., & Padroni, M. (2023). Cerebral Venous Thrombosis during Thyrotoxicosis: Case Report and Literature Update. Journal of Personalized Medicine, 13(11), 1557. https://doi.org/10.3390/jpm13111557








