1. Introduction
Micronutrients, including vitamins, trace elements, and cofactors, are widely acknowledged as vital for cell and tissue homeostasis, integral to human nutrition [
1]. Imbalances, either in deficiency or excess, can lead to oral mucosal and dental hard tissue pathologies [
1,
2].
These intricate constituents play pivotal roles in enzymatic reactions, cellular metabolism, and oxidative defence mechanisms [
1,
3]. Nevertheless, an imbalance in trace elements can result in toxicity, emphasizing the importance of maintaining precise levels in the body [
2]. Recognizing their diverse functions is essential for preventing pathologies arising from deficiencies or excessive intake, highlighting the necessity for a balanced dietary approach.
These vitamins and their synonyms or related terms that are included in the search string are listed in
Supplementary Table S1.
Trace elements are chemical micronutrients that play an important role in maintaining the integrity of physiological and metabolic processes within living tissues [
2]. Nonetheless, trace elements can also exert toxicity if they are present in excess in the body, highlighting the importance of a homeostatic status of the trace elements [
2]. After the initial search, the study selection criteria were narrowed to exclude trace elements due to the large number of studies identified. Thus, this report will focus on vitamins only.
Oral cancer (OC) includes malignant neoplasms originating from oral tissue sites including the inner lip, tongue, gingiva, floor of mouth, and palate [
4]. Oral cancer constitutes 3% of all clinically diagnosed cancers with an age-standardised rate of 12.8 per 100,000 person-years nationally in Australia [
5]. Despite several malignant neoplasms with greater prevalence, oral cancer screening by oral examination remains a vital element in patient prognosis due to the disease’s high mortality and morbidity [
5]. The 5-year survival of oral cancer is approximately 50% and disability-adjusted life years (DALYs) have increased by 87.1% from 1990 to 2017 [
6]. These are exacerbated by the risk factors including tobacco and alcohol consumption, and HPV infection.
Oral potentially malignant disorders (OPMDs) are dysplastic conditions of the lip or oral cavity with implicated increased risk of malignant transformation [
7]. Based on the consensus from the 2020 workshop organised by the World Health Organisation (WHO) Collaborating Centre for Oral Cancer, the list of OPMDs and their definitions are included in
Supplementary Table S2. Notably, OPMDs share several risk factors with OC including smoking and alcohol; given the increased risk, timely screening, early identification, and diagnosis by oral health practitioners of suspicious lesions are important for timely care and patient prognosis, particularly in high-risk populations [
7].
Previous research has identified several potential mechanisms for the anticancer properties of vitamins at a cellular level in preclinical studies, ranging from upregulating inhibitory growth signalling pathways and antioxidant properties to angiogenesis inhibition [
8]. However, the translational benefits of using vitamins as interventions in clinical cancer studies show mixed evidence regarding their therapeutic benefit, efficacy, reproducibility, and tendency to produce adverse outcomes [
8]. Furthermore, previous reviews have highlighted the impact of vitamins on oral health, highlighting mixed therapeutic benefits on dental diseases such as gingivitis and periodontitis [
9].
Despite numerous past primary studies investigating the effects of specific vitamins on OPMDs and OCs, no study to date has systematically reviewed the effects and implications of vitamins in OPMDs and OC. In our study, we have explored the current evidence base to investigate which vitamins could potentially be beneficial for each specific OPMD and OC. We also sought to understand if essential vitamin supplementation offers therapeutic benefits for OPMDs and OC. The review has shown that vitamins C and K are understudied, while studies focused on oral lichen planus (OLP) and oral leukoplakia (OL), in addition to oral cancer. Overall, there was generally mixed to promising evidence for the therapeutic effects of vitamins on OPMDs and oral cancer.
2. Materials and Methods
The screening and data collection in this systematic review adhered to the 2020 version of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [
10]. A comprehensive search was conducted on 16 May 2023 on the following databases:
The inclusion criteria specified studies that assessed the effect of essential vitamins and trace elements as interventions on:
Relevant OPMDs were designated by Warnakulasuriya et al. [
7] as shown in
Supplementary Table S2. No restrictions were placed on publication date, geographical location, patient age, or gender. All articles released after 16 May 2023, were excluded. Meta-analyses, systematic reviews, other reviews, non-peer-reviewed articles, expert opinions, published extracts and letters to editors, non-English language articles, retracted articles, and studies using only self-reported measures were manually excluded.
The initial aim of the project was to assess, if any, the therapeutic role of essential vitamins and trace elements in OPMDs and OC. The search strategy revolved around 3 core concepts:
Vitamins
Trace elements
OPMDs and OC
As such, the search string was implemented as per
Supplementary Table S3. These records were imported into Covidence (Veritas Health Innovation, 2023, Australia) where duplicates were automatically removed.
Following the PRISMA protocol, two separate reviewers were chosen to conduct title and abstract screening independently in Covidence, according to the inclusion and exclusion criteria above. Pilot screenings of 30 and 50 articles were conducted, with conflicts discussed to ensure inter-examiner consistency prior to screening all records. The full-text screening conflicts were resolved by consensus or by a third group member if no consensus was achieved. Cohen’s kappa coefficient of inter-rater reliability was calculated at each step of screening (
Supplementary Table S4).
The number of articles remaining for full-text review was deemed unachievable for the time frame of this assessment; hence a new round of title/abstract screening was conducted to remove trace elements. These articles were set aside for a future comprehensive systematic review.
The vitamin-only articles were sent for full-text retrieval, with those unretrievable being excluded. Full-text review was performed based on independent teams of reviewers.
Each group member extracted records into a pre-determined data extraction table in Excel (Microsoft, 2023, Redmond, WA, USA). The domains captured included the following: study type, vitamin of intervention, presence of additional therapy, route of administration, dose, frequency of administration, and duration. Information specific to in vitro studies included cell origin, type, and organ. In vivo animal studies included animal strain, origin, gender, age and weight, and route of administration. Finally, in vivo human studies included country, sex, sample size, age, comorbidities, risk factors, and route of administration.
A quality assessment of the included articles was carried out using criteria adapted from the OHAT Risk of Bias Rating Tool for Human and Animal Studies (National Toxicology Program, 2015). For this study, five domains (selection, selective reporting, performance, attrition/exclusion, and detection) were assessed using eight questions, with modifications made to ensure relevance to in vitro studies. For each question, bias was assigned as: “definitely low risk”, “probably low risk”, “probably high risk”, or “definitely high risk”. The assessment questions are supplied in
Supplementary Table S8.
The evidence compiled from the final studies included was presented in data extraction tables (
Supplementary Tables S5–S7). Each domain was then sorted to analyse trends.
3. Results
3.1. Data Collection
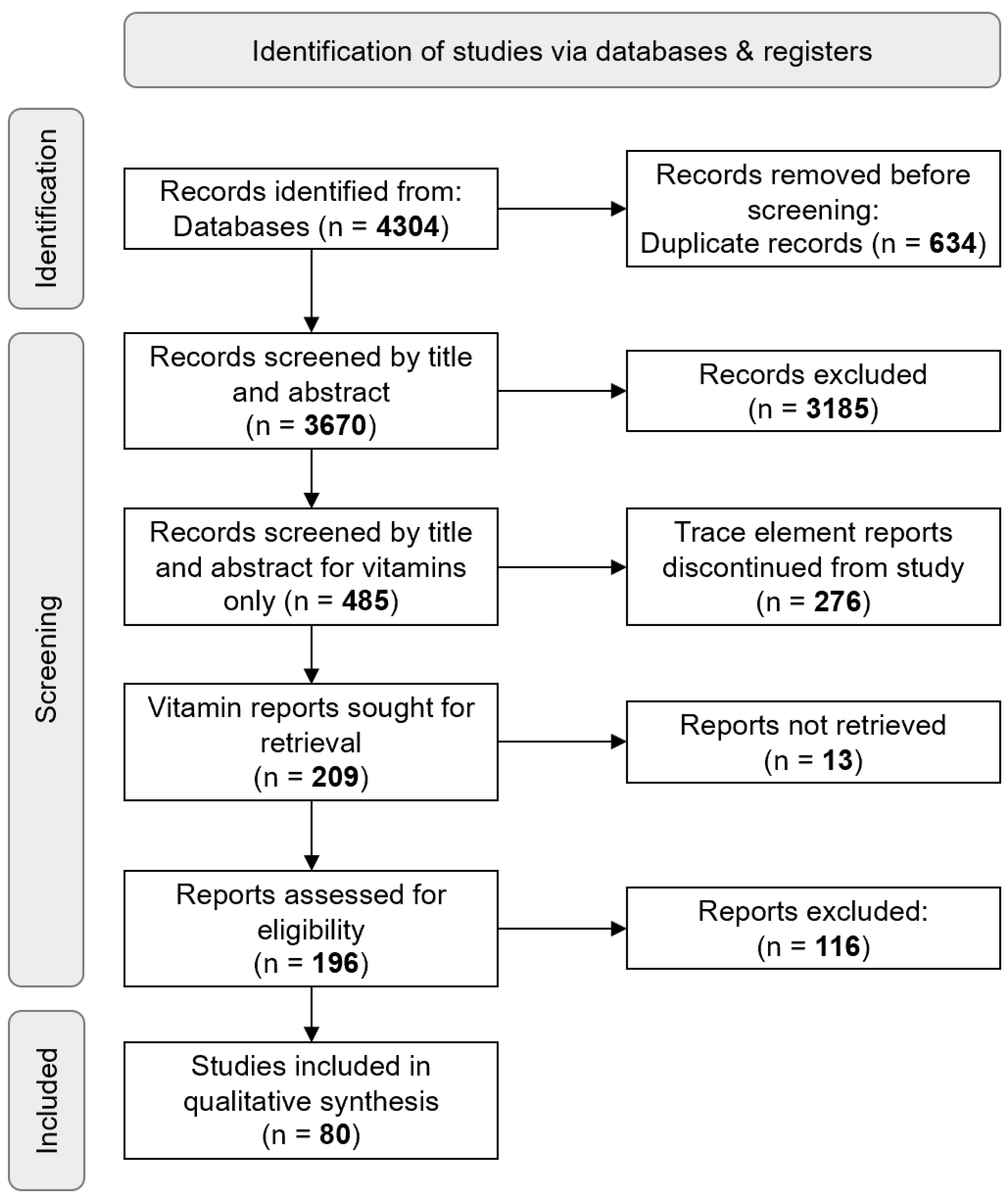
A total of 4304 records were retrieved collectively from Medline, Embase, and Web of Science, with 634 duplicates automatically removed. After screening 3670 articles by title and abstract, 485 studies were included; Cohen’s kappa coefficients are described in
Supplementary Table S4. From the remaining 209 vitamin-only full-text records, 80 records were analysed (
Figure 1).
3.2. Quality Assessment of Studies (OHAT)
Two independent reviewers assessed all included studies using OHAT for risk of bias. An initial risk of assessment was performed upon a small selection of papers and conflicting bias allocations were resolved by discussion before subsequent assessment of all remaining papers. A “definitely low risk of bias” was observed in the categories of ‘selection’, ‘selective reporting’, ‘performance’, ‘attrition/exclusion’, and ‘detection’ (8.125%, 77.500%, 16.875%, 18.750%, and 4.375%, respectively). “Probably low risk” was observed in ‘selection’, ‘selective reporting’, ‘performance’, ‘attrition/exclusion’, and ‘detection’ (11.875%, 16.250%, 6.875%, 6.250%, and 8.750%, respectively). “Probably high risk” of bias was found in ‘selection’, ‘selective reporting’, ‘performance’, ‘attrition/exclusion’, and ‘detection’ (61.250%, 5.000%, 45.625%, 56.250%, and 86.875%, respectively). A bias rating of “definitely high risk” was observed in the ‘selective reporting’ and ‘performance’ domains (1.250% in both domains). The complete assessment of studies can be found in the
Supplementary file.
3.3. Characteristics of Included Studies
The 80 studies included were published from 1959 to 2022, with the majority being produced between 1980 and 1990. Studies were contributed by 19 countries, with the USA having the most publications (
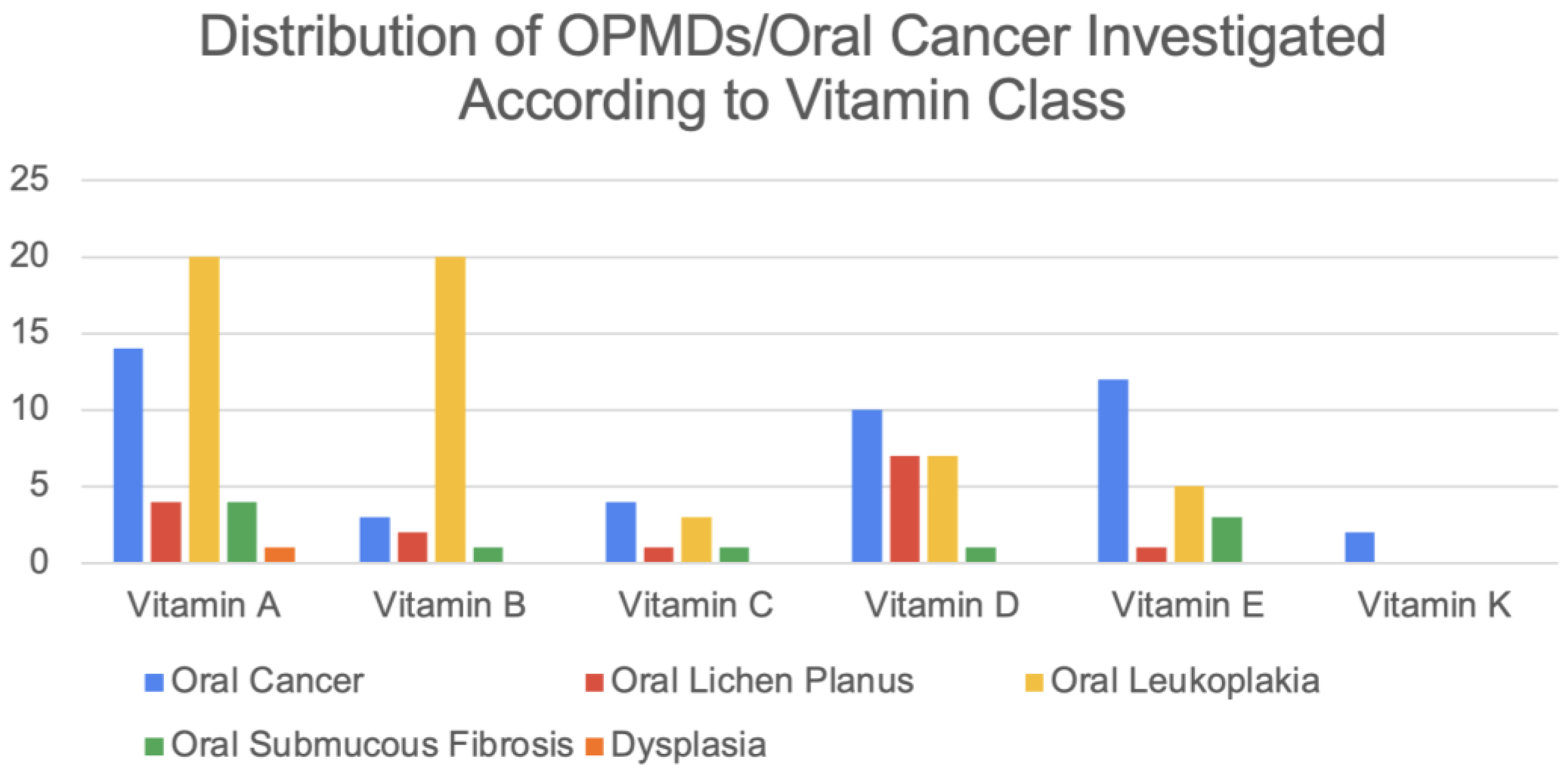
Figure S1). The distribution of OPMDs and OC investigated according to each vitamin class is shown in
Figure 2. The B complex vitamins have been collated as “Vitamin B” studies. A total of 21 studies have included vitamins as an adjunct. The distribution of study types is shown in
Supplementary Figure S2. Hybrid studies were defined as those incorporating multiple study designs.
3.3.1. In Vivo Human Studies
A total of 35 in vivo human studies were identified and are summarised in
Supplementary Table S5. The studies were conducted from 1962 to 2022 with the majority being published within the last 30 years. The included in vivo human studies explored OL, OLP, SF, DYS, and OC.
Vitamin A was investigated in 25 studies either as a monotherapy or therapeutic adjunct. Vitamin A was delivered topically, orally, or systemically via intramuscular injections. Primarily studied in patients with OL (
Figure 2), vitamin A reduced the number and size of OL lesions and decreased the level of perceived pain [
11,
12,
13,
14,
15,
16,
17] (Four studies reporting vitamin A’s effects on lesion regression showed complete remission [
18,
19,
20,
21], while two studies did not observe a significant clinical remission [
22,
23]. Mixed evidence from vitamin A-treated OLP lesions was shown in two studies reporting reduced lesion number or improved clinical symptoms [
24,
25]; one study reported no change in lesion count [
26]. Vitamin A was found to increase retinoic acid-binding protein expression in OC cells [
27]; however, two other publications reported no statistically significant effect on the recurrence and death rate of OC [
28,
29]. Studies investigating vitamin A supplementation on SF and DYS reported improved burning mouth symptoms and improved mouth opening [
30].
One study showed that vitamin B12 as a monotherapy and in conjunction with levamisole had reduced pain in patients and exerted a therapeutic effect on the ulcerative lesions in OLP [
31]. Two studies including vitamin B with other vitamins also showed therapeutic effects on clinical symptoms of OLP and SF [
1,
26].
Vitamin C was used in three studies involving OLP, SF, and OL. The studies used vitamin C as part of their multi-therapy regimen. Oral administration of vitamins A and C found no significant results in the remission or malignant transformation of OL lesions to OC [
23].
Three studies found that oral or topical vitamin D exerted a therapeutic effect on OLP through reduction in lesion number, decreased lesion severity, or improvements to clinical burning symptoms [
32]. The role of vitamin D in SF was part of a multi-vitamin regimen and saw clinical improvement in SF symptoms [
1].
Vitamin E was explored in seven studies looking at OLP, OL, OC, and SF. One study found that the topical application of vitamin E reduced the lesion size, without any therapeutic effects on the clinical symptoms of OLP. Two of three OL studies found that vitamin E applied orally or topically reduced lesion sizes, and histological changes were also observed [
33,
34]. One SF study utilized topical vitamin E with a corticosteroid (Betonil) and found that vitamin E enhanced the therapeutic effect of the corticosteroid [
35]. In OC, the use of vitamin E with radiotherapy saw a reduction in markers correlating with malignant transformations [
36]. There were no in vivo human studies concerning vitamin K.
3.3.2. In Vivo Animal Studies
A total of 29 in vivo animal studies were identified, conducted between 1960 and 2020 (
Supplementary Table S6). Among these 29 publications, 22 studies utilised the golden hamster animal model. A total of 26 studies focused on OC; the rest focused on oral lichen planus and oral leukoplakia.
Ten studies on OC showed that vitamin A supplementation, oral or injected, significantly reduced the lesion size progression and tumour count, with signs of total inhibition of carcinogenesis in the hamster cheek pouch model [
37]. Notably, one study reported that topical vitamin A treatment resulted in oral leukoplakia progressing to OSCC in the hamster buccal pouch model [
38].
Furthermore, four studies described that vitamin C applied orally or topically restricted carcinoma growth and invasion into the sub-epithelium, and decreased the incidence of OL lesions [
39,
40,
41]. However, one study reported that vitamin C supplementation resulted in significantly larger tumours and a slightly increased number of gross tumours [
42].
Three of the six studies investigating vitamin D employed the administration of vitamin D via intraperitoneal injection, while the other studies administered vitamin D orally or topically. These studies reported that vitamin D supplementation significantly reduced the degree of dysplasia, and suppressed OC tumour growth in both mice and hamster study models [
43].
Seven studies concluded that vitamin E applied orally or topically in the hamster buccal pouch model inhibited and prevented carcinogenic action in OC. The sole vitamin B study found that a low-vitamin B1 diet in hamster models resulted in malignant neoplasms arising in a significantly shorter period compared to hamsters with adequate vitamin B1 [
44]. There were no in vivo animal studies concerning vitamin K.
3.3.3. In Vitro Studies
A total of 23 in vitro studies were identified (
Supplementary Table S7). They encompassed a variety of samples, including primary specimens from commercial cell lines and human and animal biopsies. Both cancer cells and dysplastic cells were commonly used. Five studies showed that vitamin A did not influence cell proliferation but may affect oral cancer progression through increased retinoic acid-binding protein expression, clonogenic activity, as well as potential protection against genotoxic damage. Concerning vitamin E, low-dose physiological concentrations were shown to support OSCC growth, while high-dose pharmacological concentrations inhibited cell growth, even when used as a single therapy [
45]. Vitamin E was also shown to enhance the cancer-inhibitory effect of 13-cis-retinoic acid, interferon-alpha2A, and cisplatin [
46].
One study discovered that vitamin B increased OSCC proliferation in a dose-dependent manner, and another concluded that vitamin B9 antimetabolites can be used as an alternative therapy for OCs that are resistant to other therapies.
Ten studies explored the effect of vitamin D on OC; generally, vitamin D administration was found to inhibit SCC growth and OLP development in a dose-dependent manner. When used as an adjunct along with soy isoflavone or cisplatin, vitamin D was shown to enhance the anti-tumour effect of other additional therapies.
The cytotoxic and antioxidant properties of vitamin K3 were highlighted in two papers, which were attributed to its anti-neoplastic and anti-migratory effects on precancerous cells, preventing progression to oral cancer. There were no in vitro studies concerning vitamin C.
4. Discussion
This review identified many studies that attempted to elucidate the role of vitamins in preventing or mitigating the effects of OPMDs and OC. Our review showed that the majority of the evidence involved specific vitamins (A, D, and E) and their role in select OPMDs (OL and OLP) and OC. In animal models, specifically hamster cheek pouches, the role of vitamins in preventing OC progression was promising [
47,
48,
49,
50]. However, the evidence of vitamins in OC upon translation into human clinical trials remains mixed: some studies found that Vitamin A did not significantly achieve a therapeutic effect [
28,
29], while supplementation of Vitamin E was shown to reduce markers of malignant transformation [
36]. Several in vivo animal studies demonstrated the therapeutic effects of vitamin A on the progression of OCs; however, few reported outcomes regarding OPMDs.
Vitamin A preclinical studies predominantly showed therapeutic properties through its inhibitory effects on tumour cell growth and differentiation [
26,
27]; however, one in vitro study demonstrated no effect on preventing the progression of OLP into OC [
48]. In clinical studies investigating OPMDs, reduction in lesion size and number, and improvement in patient symptoms in vitamin A adjunct or monotherapies were observed [
21,
51,
52].
In clinical investigations, vitamins of the B complex used as part of a multi-therapy approach have been documented to exhibit therapeutic effects on the clinical symptoms of OLP and SF [
1,
26]. Additionally, preclinical studies have shed light on the potential repercussions of vitamin B deficiency in hamsters, highlighting an increased susceptibility to malignant neoplasms [
44]. Notably, an in vitro study reported that vitamin B9 antimetabolites held promise as an alternative therapeutic option for OC that demonstrated resistance to conventional treatment modalities [
53].
Vitamin C studies in three human trials aimed at conditions like OLP, SF, and OL, utilising vitamin C in combination therapies [
1,
23,
26]. Vitamin C administration in animal studies showed positive effects on OPMDs and OC, inhibiting carcinoma growth and reducing oral leucoplakia lesions, except for one study that reported conflicting results of increased tumour size and number with vitamin C supplementation [
39,
40]. Unfortunately, no in vitro studies were included to report the effect of vitamin C on OPMDs and OC.
Several in vitro studies on vitamin D showed consistent findings supporting its potential to inhibit SCC growth and OLP development, and enhance the therapeutic effect of additional cancer therapies [
54,
55]. In vivo animal studies further supported its effects in delaying cancer progression [
43], but showed no significant effect on oral squamous cell carcinoma cell death rate. However, there was increased cell death in a combination treatment with cisplatin and erlotinib. Therefore, vitamin D may have a potential inhibitory effect on cancer cell growth but can be suggested as an adjunct in cancer therapy rather than as a single treatment. When translated to human studies, vitamin D was investigated as an intervention only for OLP. With topical or oral supplementation of ~50,000 IU, its therapeutic effects worked to reduce the severity, lesion size, and burning sensation of OLP.
Vitamin E in vivo animal studies showed some extent of delaying and inhibiting effect on OC, with the results obtained from the buccal mucosa of hamsters [
56]. One human study conducted topical vitamin E application on patients with OLP, showing decreased lesion size, which had also been identified in a study with OC patients [
51]. Interestingly, with regard to the dosage of vitamin E, there seemed to be a dual effect with treatment, promoting OC growth at a low dosage (10 µM), while having a regulatory effect at a high dosage (100 and 154 µM) [
54].
The current research landscape in the field of vitamins predominantly revolves around the exploration of the therapeutic effects and mechanisms of action associated with vitamins A, D, and E. There remains a lack of investigations on the potential therapeutic applications of vitamin K. Additionally, there is limited research examining the role of vitamins in the context of other OPMDs including AK, cGVHD, DC, EL, OLE, OLL, PVL, and PLRS. As some promising evidence is found, this may warrant further investigation of vitamins to broader types of OPMDs to enhance our understanding of their potential implications and therapeutic value to patients.
However, this review has several limitations: firstly, vitamins have diverse alternative nomenclature in the literature that our search strings have strived to incorporate. Nonetheless, due to the diversity of both synonyms and derivatives of each vitamin, active metabolites and synthetic forms, we acknowledge the possibility of relevant articles not captured in our study. Additionally, the heterogeneity in study characteristics, methods, and outcome measurements, makes direct comparisons of study findings onerous and may potentially impact the overall conclusions. Future studies may benefit from utilising a standardised guideline to report robust and comparable data surrounding this topic.
5. Conclusions
In summary, this comprehensive review elucidates the intricate roles of vitamins, particularly A, D, and E, in the context of oral potentially malignant disorders and oral cancer. The assessment of the therapeutic benefits of different vitamins can potentially guide clinicians in treating patients with OPMDs and oral cancer. While preclinical studies in animal models demonstrate promising therapeutic properties, clinical translation reveals mixed outcomes, highlighting the need for further research and nuanced approaches. Vitamin A exhibits inhibitory effects on tumour cell growth, yet its efficacy in preventing oral cancer from progressing remains debated. The vitamin B complex, when integrated into multi-therapy approaches, shows therapeutic promise in alleviating clinical symptoms of OPMDs. Vitamin C, employed in combination therapies, exhibits positive effects on OPMDs and OC in animal studies; however, conflicting results from one study warrant further investigation. Vitamin D consistently demonstrates potential in inhibiting squamous cell carcinoma growth and enhancing cancer therapy effects. Vitamin E, both in animal models and human studies, presents dual effects contingent on dosage, emphasizing the need for precise administration. Notably, the research landscape primarily centres around vitamins A, D, and E, leaving vitamin K and other OPMDs warranting further exploration. Despite limitations in nomenclature and study heterogeneity, this review provides crucial insights, emphasizing the imperative of standardized guidelines for future research in this vital area of study.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/jpm13101520/s1, Figure S1: Geographical distribution of the studies that were included in the systematic review; Figure S2: Distribution of the type of studies; Table S1: List of essential vitamins; Table S2: OPMDs based on 2020 WHO Consensus (13); Table S3: Search string including all keywords and operators. ^: Search string 6 was used in Medline and EBM databases only, as the Web of Science database does not support subject heading-based search; Table S4: Number of articles and kappa coefficients of record screening by title/abstract; Table S5: In vivo human studies; Table S6: In vivo animal studies; Table S7: In vitro studies; Table S8: OHAT risk of bias assessment criteria.
Author Contributions
Conceptualization, R.P. and A.C.; methodology, R.P. and A.C.; software, J.K.L.S., X.L. and F.C.; validation, J.K.L.S., X.L. and F.C.; formal analysis, J.K.L.S., X.L. and F.C.; investigation, J.K.L.S., X.L. and F.C.; resources, A.C.; data curation, R.P. and A.C.; writing—original draft preparation, J.K.L.S., X.L., F.C., C.M., R.P. and A.C.; writing—review and editing, M.M., F.C., C.M., T.Y., R.P. and A.C.; supervision, R.P. and A.C.; project administration, R.P. and A.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the support of the Melbourne Dental School, The University of Melbourne. Furthermore, the authors are grateful to University of Melbourne librarian Lindy Cochrane, for assisting and guiding the formation of the literature search strategy used in this systematic review. The authors also gratefully acknowledge the support provided by Xuran Wang, Kai Chen, Ciaran Ho, Christy Tsz Ching Lau, and Stephanie Nguyen during the preliminary phase of the project. All the individuals acknowledged in this article provided their consent.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maher, R.; Aga, P.; Johnson, N.W.; Sankaranarayanan, R.; Warnakulasuriya, S. Evaluation of multiple micronutrient supplementation in the management of oral submucous fibrosis in Karachi, Pakistan. Nutr. Cancer 1997, 27, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.-E.; Yan, J.-Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.L.; Feng, X.S.; Yang, J.; Li, G.H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Ellington, T.D.; Henley, S.J.; Senkomago, V.; O’Neil, M.E.; Wilson, R.J.; Singh, S.; Thomas, C.C.; Wu, M.; Richardson, L.C. Trends in Incidence of Cancers of the Oral Cavity and Pharynx—United States 2007–2016. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Ahmadian, E.; Shahi, S.; Roshangar, L.; Khan, H.; Kouhsoltani, M.; Dizaj, S.M.; Sharifi, S. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed. Pharmacother. 2019, 109, 391–401. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Wolf, T.G.; Tennert, C.; Camoni, N.; Lingström, P.; Campus, G. The Role of Vitamins in Oral Health. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 938. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 10, n71. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Ringsdorf, W.M.; Cheraskin, E. Relationship of Vitamin A and Oral Leukoplakia. Arch. Dermatol. 1963, 88, 607–612. [Google Scholar] [CrossRef]
- Stich, H.F.; Rosin, M.P.; Hornby, A.P.; Mathew, B.; Sankaranarayanan, R.; Nair, M.K. Remission of oral leukoplakias and micronuclei in tobacco/betel quid chewers treated with beta-carotene and with beta-carotene plus vitamin A. Int. J. Cancer 1988, 42, 195–199. [Google Scholar] [CrossRef]
- Garewal, H.S.; Meyskens, F.L.; Killen, D.; Reeves, D.; A Kiersch, T.; Elletson, H.; Strosberg, A.; King, D.; Steinbronn, K. Response of oral leukoplakia to beta-carotene. J. Clin. Oncol. 1990, 8, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Garewal, H.S.; Katz, R.V.; Meyskens, F.; Pitcock, J.; Morse, D.; Friedman, S.; Peng, Y.; Pendrys, D.G.; Mayne, S.; Alberts, D.; et al. Beta-carotene produces sustained remissions in patients with oral leukoplakia: Results of a multicenter prospective trial. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Toma, S.; Benso, S.; Albanese, E.; Palumbo, R.; Cantoni, E.; Nicolò, G.; Mangiante, P. Treatment of oral leukoplakia with beta-carotene. Oncology 1992, 49, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Mathew, B.; Varghese, C.; Sudhakaran, P.; Menon, V.; Jayadeep, A.; Nair, M.; Mathews, C.; Mahalingam, T.; Balaram, P.; et al. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: An assessment. Oral Oncol. 1997, 33, 231–236. [Google Scholar] [CrossRef]
- Varma, D.; Gupta, S.; Mandal, A.K. Role of p53 and bcl2 as markers of vitamin A response in premalignant lesions of the oral cavity. Indian J. Pathol. Microbiol. 2007, 50, 15–17. [Google Scholar]
- Smith, J.F. Clinical evaluation of massive buccal vitamin A dosage in oral hyperkeratosis. Oral Surg. Oral Med. Oral Pathol. 1962, 15, 282–292. [Google Scholar] [CrossRef]
- Silverman, S.; Renstrup, G.; Pindborg, J.J. Studies in Oral Leukoplakias. Vii. further Investigations on the Effects of Vitamin A on Keratinization. Acta Odontol. Scand. 1963, 21, 553–570. [Google Scholar] [CrossRef]
- Silverman, S.; Eisenberg, E.; Renstrup, G. A Study of the Effects of High Doses of Vitamin A on Oral Leukoplakia (Hyperkeratosis), including Toxicity, Liver Function and Skeletal Metabolism. J. Oral Ther. Pharmacol. 1965, 2, 9–23. [Google Scholar]
- Epstein, J.B.; Gorsky, M. Topical application of vitamin A to oral leukoplakia: A clinical case series. Cancer 1999, 86, 921–927. [Google Scholar] [CrossRef]
- Liede, K.E.; Alfthan, G.; Hietanen, J.H.; Haukka, J.K.; Saxen, L.M.; Heinonen, O.P. Beta-carotene concentration in buccal mucosal cells with and without dysplastic oral leukoplakia after long-term beta-carotene supplementation in male smokers. Eur. J. Clin. Nutr. 1998, 52, 872–876. [Google Scholar] [CrossRef]
- Nagao, T.; Warnakulasuriya, S.; Nakamura, T.; Kato, S.; Yamamoto, K.; Fukano, H.; Suzuki, K.; Shimozato, K.; Hashimoto, S. Treatment of oral leukoplakia with a low-dose of beta-carotene and vitamin C supplements: A randomized controlled trial. Int. J. Cancer 2015, 136, 1708–1717. [Google Scholar] [CrossRef]
- Günther, S.H. Vitamin A acid in treatment of oral lichen planus. Arch. Dermatol. 1973, 107, 277. [Google Scholar] [CrossRef] [PubMed]
- Buajeeb, W.; Kraivaphan, P.; Amornchat, C.; Suthamajariya, K. Reduction of micronuclei in oral lichen planus supplemented with beta-carotene. J. Oral. Sci. 2008, 50, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.; Nobile, S. Vitamin status of patients with oral lichen planus. Aust. Dent. J. 1977, 22, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.E.; Goodwin, W.J.; Jesse, R.H.; Griffin, A.C. Presence of cellular retinol and retinoic acid-binding proteins in epidermoid carcinoma of the oral cavity and oropharynx. Cancer 1982, 49, 1409–1412. [Google Scholar] [CrossRef]
- Mayne, S.T.; Cartmel, B.; Baum, M.; Shor-Posner, G.; Fallon, B.G.; Briskin, K.; Bean, J.; Zheng, T.; Cooper, D.; Friedman, C.; et al. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001, 61, 1457–1463. [Google Scholar]
- Toma, S.; Bonelli, L.; Sartoris, A.; Mira, E.; Antonelli, A.; Beatrice, F.; Giordano, C.; Benazzo, M.; Caroggio, A.; Cavalot, A.L.; et al. Beta-carotene supplementation in patients radically treated for stage I-II head and neck cancer: Results of a randomized trial. Oncol. Rep. 2003, 10, 1895–1901. [Google Scholar]
- Dhariwal, R.; Mukherjee, S.; Mohanty, S.P.; Chakraborty, A.; Ray, J.G.; Chaudhuri, K. Zinc and vitamin A can minimise the severity of oral submucous fibrosis. BMJ Case Rep. 2010, 2010, bcr1020092348. [Google Scholar] [CrossRef]
- Lin, H.-P.; Wang, Y.-P.; Chia, J.-S.; Chiang, C.-P.; Sun, A. Modulation of serum gastric parietal cell antibody level by levamisole and vitamin B12 in oral lichen planus. Oral Dis. 2011, 17, 95–101. [Google Scholar] [CrossRef]
- Nazeer, J.; Singh, S.; Jayam, C.; Singh, R.; Iqubal, M.A.; Singh, R. Assessment of the Role of Vitamin D in the Treatment of Oral Lichen Planus. J. Contemp. Dent. Pract. 2020, 21, 390–395. [Google Scholar]
- Benner, S.E.; Winn, R.J.; Lippman, S.M.; Poland, J.; Hansen, K.S.; Luna, M.A.; Hong, W.K. Regression of oral leukoplakia with alpha-tocopherol: A community clinical oncology program chemoprevention study. J. Natl. Cancer Inst. 1993, 85, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Manas, A.; Venkateswararao, C.H.; Vaid, S.; Khanna, V.D.; Muhaseena, M.; Alvi, S. Assessment of Utility of Lycopene, Selenium, and Vitamin E in Management of Oral Leukoplakia. J. Pharm. Bioallied Sci. 2022, 14, 233–235. [Google Scholar]
- Razi, A.; Iqbal, A. Topical Application of Tocopherol with Corticosteroid in Reducing the Early Signs and Symptoms of Oral Submucous Fibrosis Patients. AS Hosp. Karachi Med. 2016, 21, 100. [Google Scholar]
- Chitra, S.; Shyamala Devi, C.S. Effect of vitamin E on protein bound carbhohydrate complexes in radiation treated oral squamous cell carcinoma patients. Indian J. Clin. Biochem. 2008, 23, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Gijare, P.S.; Rao, K.V.; Bhide, S.V. Modulatory effects of snuff, retinoic acid, and beta-carotene on DMBA-induced hamster cheek pouch carcinogenesis in relation to keratin expression. Nutr. Cancer 1990, 14, 253–259. [Google Scholar] [CrossRef]
- Polliack, A.; Rwomushana, J.W.; Levij, I.S. Treatment of experimental benign hyperkeratotic lesions of the hamster cheek pouch with topical vitamin A palmitate. Pharmacol. Ther. Dent. 1971, 1, 63–70. [Google Scholar]
- Harada, T.; Enomoto, A.; Kitazawa, T.; Maita, K.; Shirasu, Y. Oral leukoplakia and costochondral hyperplasia induced by diethylnitrosamine in hamsters exposed to cigarette smoke with or without dietary vitamin C. Vet. Pathol. 1987, 24, 257–264. [Google Scholar] [CrossRef]
- Kandarkar, S.V.; Reade, P.C. The effect of topical vitamin C on palatal oral mucosal carcinogenesis using 4-nitroquinoline-1-oxide. J. Biol. Buccale 1991, 19, 199–204. [Google Scholar] [PubMed]
- Potdar, P.D.; Kandarkar, S.V.; Sirsat, S.M. Modulation by vitamin C of tumour incidence and inhibition in oral carcinogenesis. Funct. Dev. Morphol. 1992, 2, 167–172. [Google Scholar]
- Schwartz, J.; Shklar, G.; Trickler, D. Vitamin C enhances the development of carcinomas in the hamster buccal pouch experimental model. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 718–722. [Google Scholar] [CrossRef]
- Bothwell, K.D.; Shaurova, T.; Merzianu, M.; Suresh, A.; Kuriakose, M.A.; Johnson, C.S.; Hershberger, P.A.; Seshadri, M. Impact of Short-term 1,25-Dihydroxyvitamin D3 on the Chemopreventive Efficacy of Erlotinib against Oral Cancer. Cancer Prev. Res. 2015, 8, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Salley, J.J.; Eshleman, J.R.; Morgan, J.H. Effect of chronic thiamine deficiency on oral carcinogenesis. J. Dent. Res. 1962, 41, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Elattar, T.M.; Virji, A.S. Biphasic action of vitamin E on the growth of human oral squamous carcinoma cells. Anticancer Res. 1999, 19, 365–368. [Google Scholar] [PubMed]
- Zhang, X.; Chen, Z.G.; Khuri, F.R.; Shin, D.M. Induction of cell cycle arrest and apoptosis by a combined treatment with 13-cis-retinoic acid, interferon-alpha2a, and alpha-tocopherol in squamous cell carcinoma of the head and neck. Head Neck 2007, 29, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, O.; Hawach, F.; Shklar, G. Retardation of experimental oral cancer by topical vitamin E. Nutr. Cancer 1984, 6, 98–104. [Google Scholar] [CrossRef]
- Suda, D.; Schwartz, J.; Shklar, G. Inhibition of experimental oral carcinogenesis by topical beta carotene. Carcinogenesis 1986, 7, 711–715. [Google Scholar] [CrossRef]
- Schwartz, J.; Shklar, G. Regression of experimental oral carcinomas by local injection of beta-carotene and canthaxanthin. Nutr. Cancer 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Zulkapli, R.; Abdul Razak, F.; Zain, R.B. Vitamin E (α-Tocopherol) Exhibits Antitumour Activity on Oral Squamous Carcinoma Cells ORL-48. Integr. Cancer Ther. 2017, 16, 414–425. [Google Scholar] [CrossRef]
- Malaker, K.; Anderson, B.J.; Beecroft, W.A.; Hodson, D.I. Management of oral mucosal dysplasia with beta-carotene retinoic acid: A pilot cross-over study. Cancer Detect. Prev. 1991, 15, 335–340. [Google Scholar]
- Nilesh, K.; Dadhich, A.; Saluja, H.; Patil, D.; Vande, A. Treatment of Oral Submucous Fibrosis with Lycopene, Beta-Carotene, Zinc, Selenium, Copper, Alpha-Lipoic Acid, And Alpha-Tocopheryl Acetate. Ann. Dent. Spec. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Odukoya, O.; Schwartz, J.; Shklar, G. Vitamin E stimulates proliferation of experimental oral carcinoma cells in vitro. Nutr. Cancer 1986, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Dalirsani, Z.; Farajnia, S.; Javadzadeh, Y.; Mehdipour, M.; Koozegari, S. The effects of 5-fluorouracil alone and in combination with 13-cis retinoic acid and vitamin D3 on human oral squamous cell carcinoma lines. J. Contemp. Dent. Pract. 2012, 13, 345–350. [Google Scholar] [CrossRef]
- Kingsley, K.; Keiserman, M.A.; Bergman, C.J. Interactive effects of 1, 25-dihydroxyvitamin D3 and soy protein extract (SPE) on oral cancer growth in vitro: Evidence for potential functional relationships. Funct. Foods Health Dis. 2013, 3, 183. [Google Scholar] [CrossRef]
- Sawant, S.S.; Kandarkar, S.V. Role of vitamins C and E as chemopreventive agents in the hamster cheek pouch treated with the oral carcinogen-DMBA. Oral Dis. 2000, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).