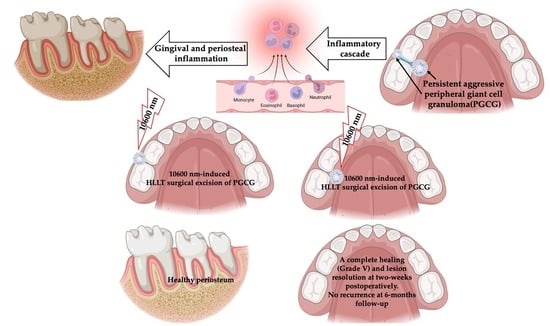
10,600 nm High Level-Laser Therapy Dosimetry in Management of Unresponsive Persistent Peripheral Giant Cell Granuloma to Standard Surgical Approach: A Case Report with 6-Month Follow-Up
Abstract
1. Introduction
PGCG Excision with Surgical Lasers vs. Conventional Surgical Approach
2. Materials and Methods
2.1. Study Design
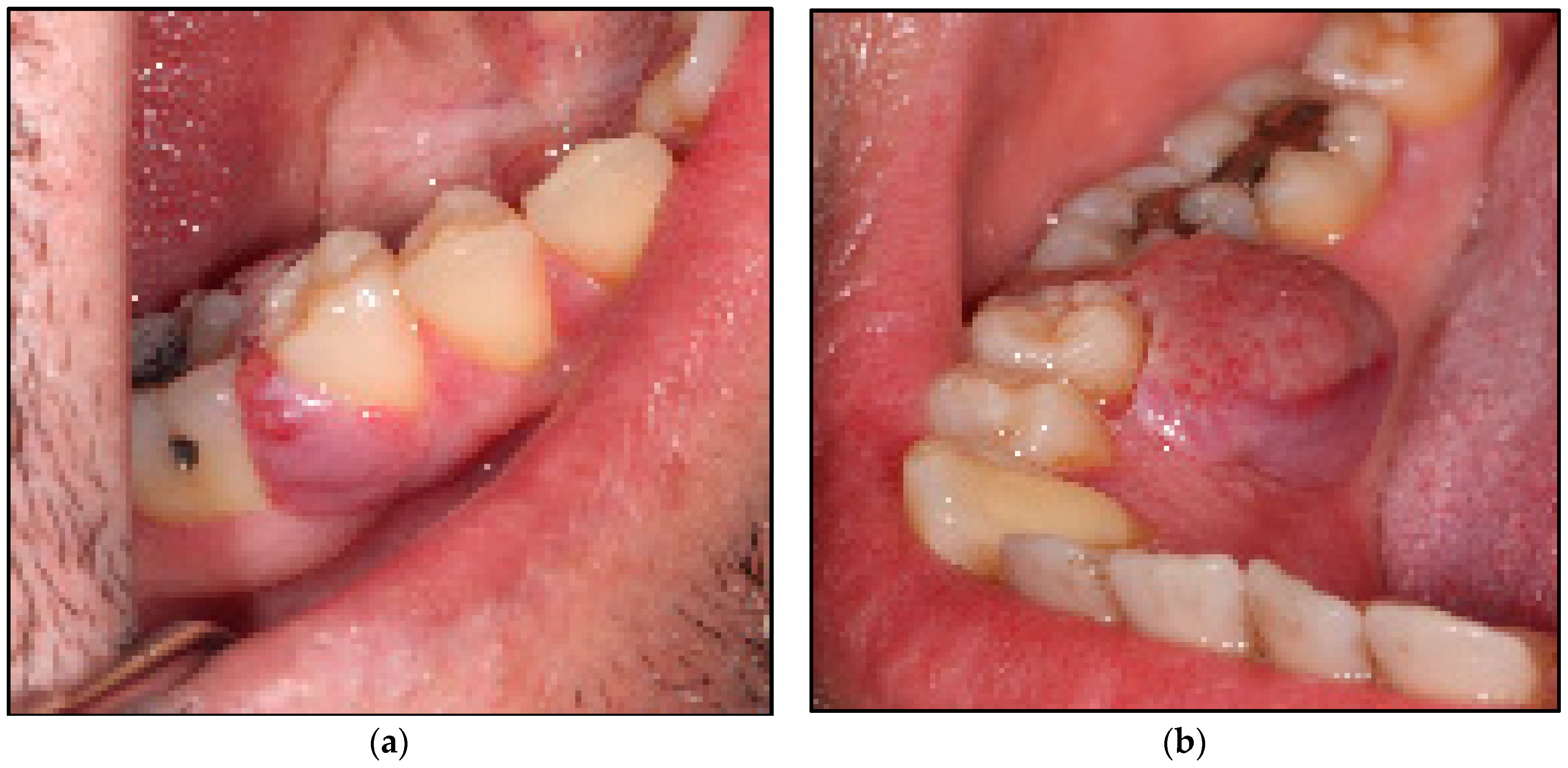
2.2. Study Participant and Case Description
2.2.1. Clinical Examination
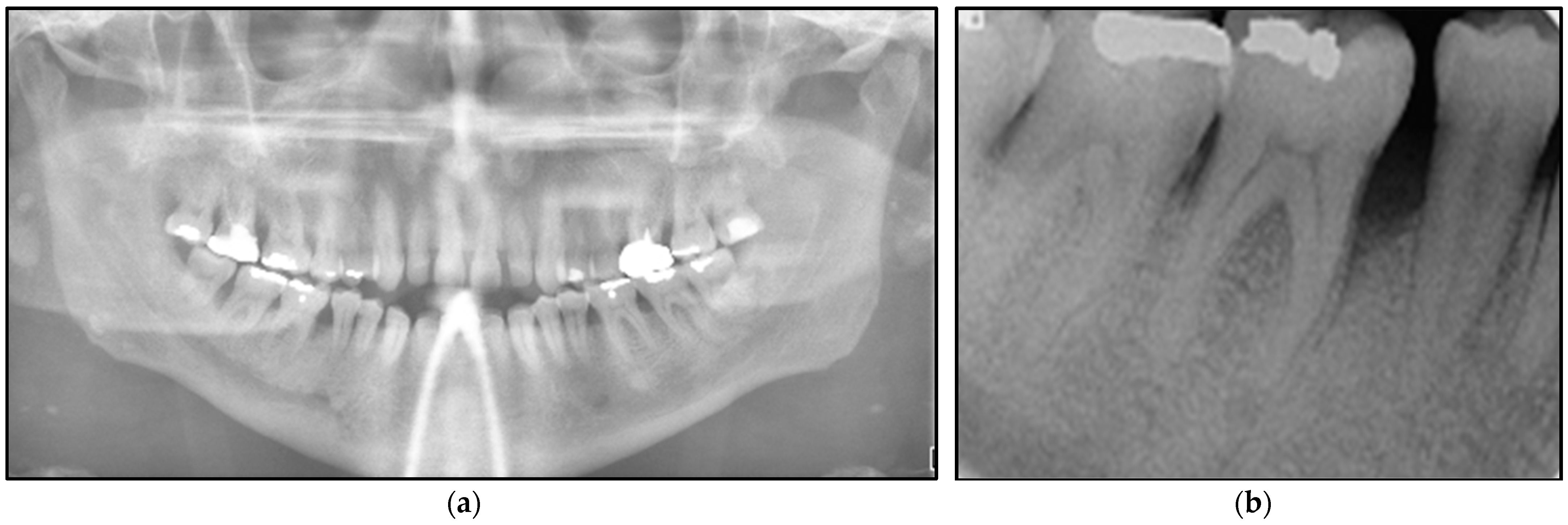
2.2.2. Investigations
2.2.3. Differential Diagnosis
2.3. Interventions
Carbon Dioxide (λ 10,600 nm) Surgical Laser
2.4. Dosimetry of λ 10,600 nm Surgical Laser
2.5. Description of Laser-Assisted Surgical Approach
2.6. Laser Nurse Checklist of Variables One-Day Post-Operatively
2.7. Outcome Assessment Measures
2.7.1. Visual Analogue Scale (VAS)
2.7.2. Wound Healing
2.7.3. Haemostasis Assessment Tool
2.7.4. Inflammation, Oedema and Infection Assessment
2.7.5. Patient Satisfaction Assessment
3. Results
3.1. Pain, Swelling, Infection and Patient’s Satisfaction
3.2. Haemostasis Assessment
3.3. Histopathological Interpretation
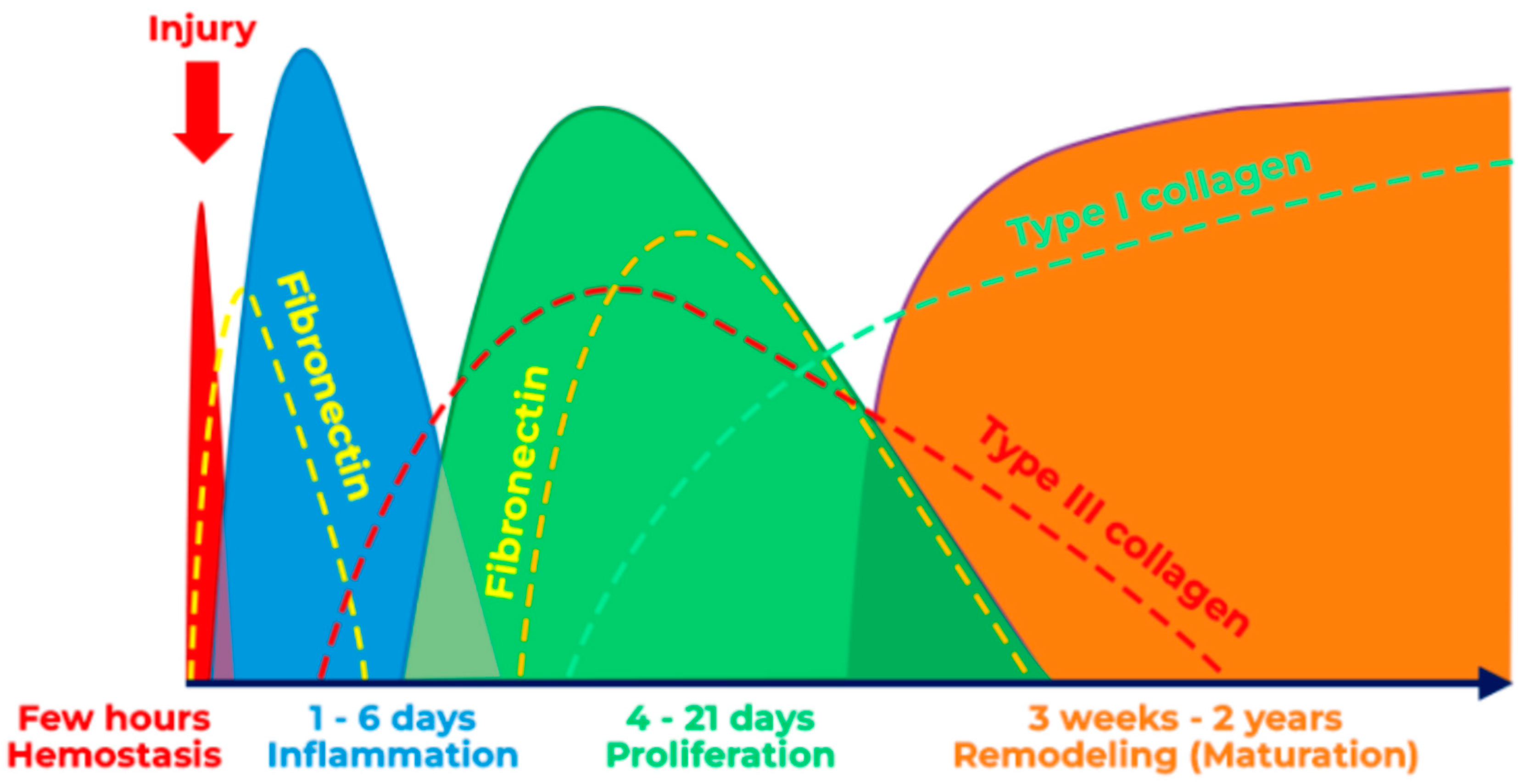
3.4. Wound Healing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fligelstone, S.; Ashworth, D. Peripheral giant cell granuloma: A case series and brief review. Ann. R. Coll. Surg. Engl. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Triantafillidou, K.; Venetis, G.; Karakinaris, G.; Iordanidis, F. Central giant cell granuloma of the jaws: A clinical study of 17 cases and a review of the literature. Ann. Otol. Rhinol. Laryngol. 2011, 120, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hamama, M.; Kheder, K.; Haidar, O. Central giant cell granuloma formation in an edentulous area in the posterior portion of mandible: A case report. Int. J. Surg. Case Rep. 2023, 112, 108971. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Gomes, C.C.; Gomez, R.S. Peripheral giant cell granuloma: An updated analysis of 2824 cases reported in the literature. J. Oral Pathol. Med. 2018, 47, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, C.; Mortellaro, C.; Limongelli, L.; Tempesta, A.; Favia, G. Peripheral giant cell granuloma associated with dental implants. J. Craniofac. Surg. 2018, 29, e196–e199. [Google Scholar] [CrossRef] [PubMed]
- Jané-Salas, E.; Albuquerque, R.; Font-Muñoz, A.; González-Navarro, B.; EstrugoDevesa, A.; López-López, J. Pyogenic granuloma/peripheral giant cell granuloma associated with implants. Int. J. Dent. 2015, 2015, 839032. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Gomes, C.C.; Gomez, R.S. Peripheral giant cell granuloma associated with dental implants: A systematic review. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, A.; Eshghi, A.; Jafarnejadi, P.; Enshaei, Z. A review and report of peripheral giant cell granuloma in a 4- year-old child. Case Rep. Dent. 2016, 2016, 7536304. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, D.; Bugone, E.; da Silva, J.L.; de Quevedo, A.S.; Visioli, F.; Puricelli, E. Intraoral peripheral giant cell granuloma. Res. Soc. Dev. 2022, 11, e330111032954. [Google Scholar] [CrossRef]
- Abofoul, S.; Hurvitz, A.Z.; Grienstein, O.K.; Shuster, A.; Vered, M.; Edel, J.; Kaplan, I. Peripheral giant cell granuloma associated with dental implants: Case-series. Clin. Implant. Dent. Relat. Res. 2022, 24, 133–137. [Google Scholar] [CrossRef]
- Babu, B.; Hallikeri, K. Reactive lesions of oral cavity: A retrospective study of 659 cases. J. Indian. Soc. Periodontol. 2017, 21, 258–263. [Google Scholar]
- Tchernev, G.; Kandathil, L.J.; Oliveira, N. Giant cell epulis. Wien. Med. Wochenschr. 2023, 173, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Ogbureke, K.U.; Salha, W.; Nwizu, N.N. Peripheral Giant Cell Granuloma. Tex. Dent. J. 2016, 133, 178–179. [Google Scholar] [PubMed]
- Asrani, S.; Reddy, P.B.; Dhirawani, R.B.; Jain, S.; Pathak, S.; Asati, P. Cryosurgery: A Simple Tool to Address Oral Lesions. Contemp. Clin. Dent. 2018, 9, S17–S22. [Google Scholar] [CrossRef]
- Alekhya, K.L.N.V.; Kadakampally, D. Recurrent peripheral giant cell granuloma: A case report. Dent. Med. Probl. 2017, 54, 97–100. [Google Scholar]
- Asnaashari, M.; Mehdipour, M.; Moradi-Abbasabadi, F.; Azari-Marhabi, S. Expedited removal of pyogenic granuloma by diode laser in a pediatric patient. J. Lasers Med. Sci. 2015, 6, 40–44. [Google Scholar] [PubMed]
- Hasanoglu Erbasar, G.N.; Senguven, B.; Gultekin, S.E.; Cetiner, S. Management of a Recurrent Pyogenic Granuloma of the Hard Palate with Diode Laser: A Case Report. J. Lasers Med. Sci. 2016, 7, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Ugurlu, F.; Basel, B.; Sener, C.B. Oral pyogenic granuloma associated with a dental implant treated with an Er:YAG laser: A case report. J. Oral. Implantol. 2015, 41, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Truschnegg, A.; Acham, S.; Kqiku, L.; Beham, A.; Jakse, N. CO2 Laser Excision of a Pyogenic Granuloma Associated with Dental Implants: A Case Report and Review of the Literature. Photomed. Laser Surg. 2016, 34, 425–431. [Google Scholar] [CrossRef]
- Chandel, V.; Jangra, B.; Khurana, N.; Garg, A. Venous malformations management by Er,Cr:YSGG laser: An Alternative approach. Laser Ther. 2017, 26, 305–310. [Google Scholar] [CrossRef]
- Sufiawati, I.; Siregar, F.D.; Wahyuni, I.S.; Syamsudin, E. Evaluation of diode laser efficacy in treating benign oral soft tissue masses: A case series. Int. J. Surg. Case Rep. 2023, 114, 109075. [Google Scholar] [CrossRef]
- Eroglu, C.N.; Tunç, S.K.; Elasan, S. Removal of epulis fissuratum by Er,Cr:YSGG laser in comparison with the conventional method. Photomed. Laser Surg. 2015, 33, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Power, S.; Thickett, E. Application of the diode laser for soft-tissue surgery in orthodontics: Case series. J. Orthod. 2021, 48, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Sobouti, F.; Torabi, S.; Bakhshandehfard, A.; Amirian, A.; Shariati, M.; Morshedi, E.; Barati, M. Comparison of Carbon Dioxide Laser with Surgical Blade for Removal of Epulis Fissuratum. A Randomized Clinical Trial. J. Lasers Med. Sci. 2016, 7, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Zeba, J.; Ahmed, N.; Shukla, D. Diode laser for treatment of peripheral giant cell granuloma. J. Dent. Lasers 2015, 9, 107–109. [Google Scholar] [CrossRef]
- Gökmenoğlu, C.; Sadık, E.; Yavuz, M.C.; Çanakçı, V.; Topaloğlu, M.; Kara, C. Treatment of Different Oral Soft Tissue Lesions with Surgical Neodymium: Yttrium-Aluminum-Garnet Laser: Case Series. Clin. Adv. Periodontics 2016, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef]
- Román-Quesada, N.; González-Navarro, B.; Izquierdo-Gómez, K.; Jané-Salas, E.; Marí-Roig, A.; Estrugo-Devesa, A.; López-López, J. An analysis of the prevalence of peripheral giant cell granuloma and pyogenic granuloma in relation to a dental implant. BMC Oral. Health 2021, 21, 204. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lee, B.S.; Jhang, Y.T.; Ma, K.S.-K.; Huang, C.-P.; Fu, K.-L.; Lai, C.-H.; Tseng, W.-Y.; Kuo, M.Y.-P.; Chen, Y.-W. Er:YAG laser irradiation enhances bacterial and lipopolysaccharide clearance and human gingival fibroblast adhesion on titanium discs. Sci. Rep. 2021, 11, 23954. [Google Scholar] [CrossRef]
- Raggio, B.; Chheda, N. Inflammatory myofibroblastic tumor of the epiglottis excised with a carbon dioxide laser: Case report and literature review. Ear Nose Throat J. 2018, 97, E31–E33. [Google Scholar] [CrossRef]
- Fukuoka, H.; Fukuoka, N.; Daigo, Y.; Daigo, E.; Ishikawa, M.; Kibe, T. Healing of open upper lip vermillion wounds irradiated with CO2 laser immediately after injury. Photobiomodul Photomed. Laser Surg. 2021, 39, 612–616. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Parker, S. The advantages of carbon dioxide laser applications in paediatric oral surgery. A prospective cohort study. Lasers Med. Sci. 2016, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Amaroli, A.; Signore, A.; Benedicenti, S. Utilization of carbon dioxide laser therapy in the management of denture induced hyperplasia and vestibuloplasty in a medically compromised patient: A case report. Int. J. Prosthodont. 2019, 32, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Fujino, T. Laser Applications in Plastic and Reconstructive Surgery. Keio J. Med. 1993, 24, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Calderhead, R.G. Low Level Laser Therapy: A Practical Introduction; John Wiley and Sons: Chichester, UK, 1988. [Google Scholar]
- Hanna, R.; Pawelczyk-Madalińska, M.; Sălăgean, T.; Nap, M.E.; Bordea, I.R.; Benedicenti, S. A Novel Concept of Combined High-Level-Laser Treatment and Transcutaneous Photobiomodulation Therapy Utilisation in Orthodontic Periodontal Interface Management. Sensors 2022, 22, 2263. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P.; Pinheiro, A.L.; Mester, A.R.; Franke, R.P.; Whelan, H.T. Biostimulatory windows in low-intensity laser activation: Lasers, scanners, and NASA’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Benedicenti, S. Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials. Antioxidants 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Bensadoun, R.J.; Beken, S.V.; Burton, P.; Carroll, J.; Benedicenti, S. Outpatient Oral Neuropathic Pain Management with Photobiomodulation Therapy: A Prospective Analgesic Pharmacotherapy-Paralleled Feasibility Trial. Antioxidants 2022, 11, 533. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Tomov, G.; Hopper, C.; Rebaudi, F.; Rebaudi, A.L.; Bensadoun, J.R. Emerging potential of phototherapy in management of symptomatic oral lichen planus: A systematic review of randomised controlled clinical trials. J. Biophotonics 2023, 16, e202300046. [Google Scholar] [CrossRef]
- Calderhead, R.G. Photobiological Basics of Photomedicine: A Work of Art Still in Progress. Med. Lasers 2017, 6, 45–57. [Google Scholar] [CrossRef][Green Version]
- Woodfield, J.C.; Jamil, W.; Sgar, P.M. Incidence and significance of postoperative complications occurring between discharge and 30 days: A prospective cohort study. J. Surg. Res. 2016, 206, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sirintawat, N.; Sawang, K.; Chaiyasamut, T.; Wongsirichat, N. Pain measurement in oral and maxillofacial surgery. J. Dent. Anesth. Pain. Med. 2017, 17, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Atisook, R.; Euasobhon, P.; Saengsanon, A.; Jensen, M.P. Validity and Utility of Four Pain Intensity Measures for Use in International Research. J. Pain. Res. 2021, 14, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Pié-Sánchez, J.; España-Tost, A.J.; Arnabat-Domínguez, J.; Gay-Escoda, C. Comparative study of upper lip frenectomy with the CO2 laser versus the Er, Cr:YSGG laser. Med. Oral. Patol. Oral. Cir. Bucal. 2012, 17, e228–e232. [Google Scholar] [CrossRef] [PubMed]
- Benedicenti, A.; Benedicenti, S. Atlas of Laser Therapy (the State of the Art), 4th ed.; Teamwork Media Srl: Villa Carcina, Italy, 2016. [Google Scholar]
- Laser Institute of America. American National Standard for Safe Use of Lasers. ANSI Z 136.1. 2014. Available online: https://www.lia.org/store/product/ansi-z1361-1014-safe-use-lasers-electronic-version (accessed on 8 December 2023).
- Billig, J.I.; Sears, E.D.; Travis, B.N.; Waljee, J.F. Patient-Reported Outcomes: Understanding Surgical Efficacy and Quality from the Patient’s Perspective. Ann. Surg. Oncol. 2020, 27, 56–64. [Google Scholar] [CrossRef]
- Åström, M.; Thet Lwin, Z.M.; Teni, F.S.; Burström, K.; Berg, J. Use of the visual analogue scale for health state valuation: A scoping review. Qual. Life Res. 2023, 32, 2719–2729. [Google Scholar] [CrossRef]
- Li, L.; Wu, S.; Wang, J.; Wang, C.; Zuo, W.; Yu, L.; Song, J. Development of the Emoji Faces Pain Scale and Its Validation on Mobile Devices in Adult Surgery Patients: Longitudinal Observational Study. J. Med. Internet Res. 2023, 17, e41189. [Google Scholar] [CrossRef]
- Gorad, K.; Rahate, V.; Shinde, G.; Taralekar, G.; Prabhu, V.; Singh, L. Use of Southampton Scoring for Wound Healing in Post-surgical Patients: Our Experience in Semi-urban Setup. Arch. Clin. Biomed. Res. 2021, 5, 36–41. [Google Scholar] [CrossRef]
- Fasulo, M.R.; Biguzzi, E.; Abbattista, M.; Stufano, F.; Pagliari, M.T.; Mancini, I.; Gorski, M.M.; Cannavò, A.; Corgiolu, M.; Peyvandi, F.; et al. The ISTH Bleeding Assessment Tool and the risk of future bleeding. J. Thromb. Haemost. 2018, 16, 125–130. [Google Scholar] [CrossRef]
- Yamasaki, A.; Ito, H.; Yusa, J.; Sakurai, Y.; Okuyama, N.; Ozawa, R. Expression of heat shock proteins, Hsp70 and Hsp25, in the rat gingiva after irradiation with a CO2 laser in coagulation mode. J. Periodontal Res. 2010, 45, 323–330. [Google Scholar] [CrossRef]
- Singh, C.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Raber-Durlacher, J.E.; Benedicenti, S. Role of Photobiomodulation Therapy in Neurological Primary Burning Mouth Syndrome. A Systematic Review and Meta-Analysis of Human Randomised Controlled Clinical Trials. Pharmaceutics 2021, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, J.R.; Nair, R.J. Low-Level Laser Therapy in the Management of Mucositis and Dermatitis Induced by Cancer Therapy. Photomed. Laser Surg. 2015, 33, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Das, N. Fundamentals and Laser-Tissue Interaction Physics in Dentistry. Int. J. Curr. Pharm. Res. 2023, 9, 344–351. [Google Scholar]
- Amaral, M.B.; de Avila, J.M.; Abreu, M.H.; Mesquita, R.A. Diode laser surgery versus scalpel surgery in the treatment of fibrous hyperplasia: A randomized clinical trial. Int. J. Oral. Maxillofac. Surg. 2015, 4, 1383–1389. [Google Scholar] [CrossRef]

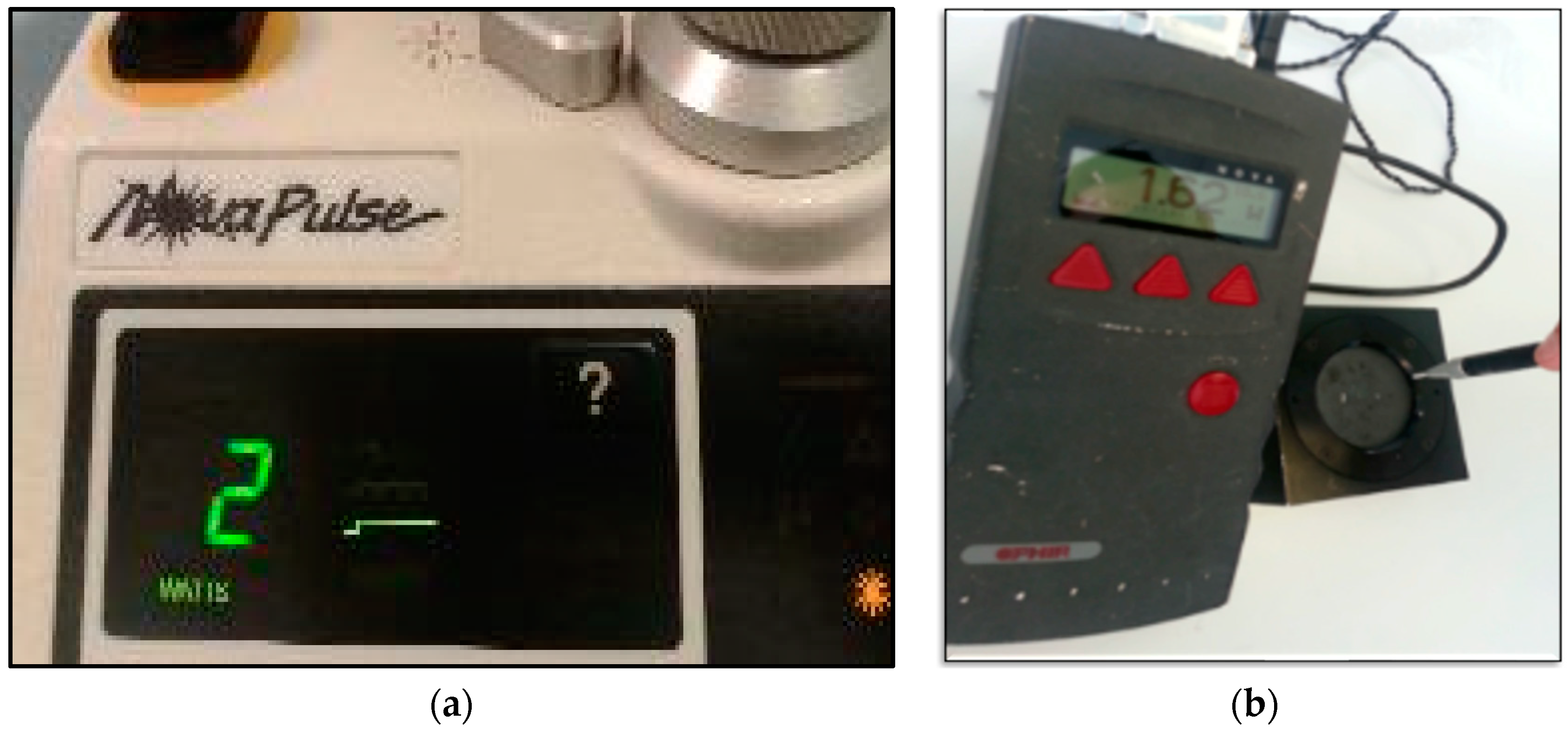
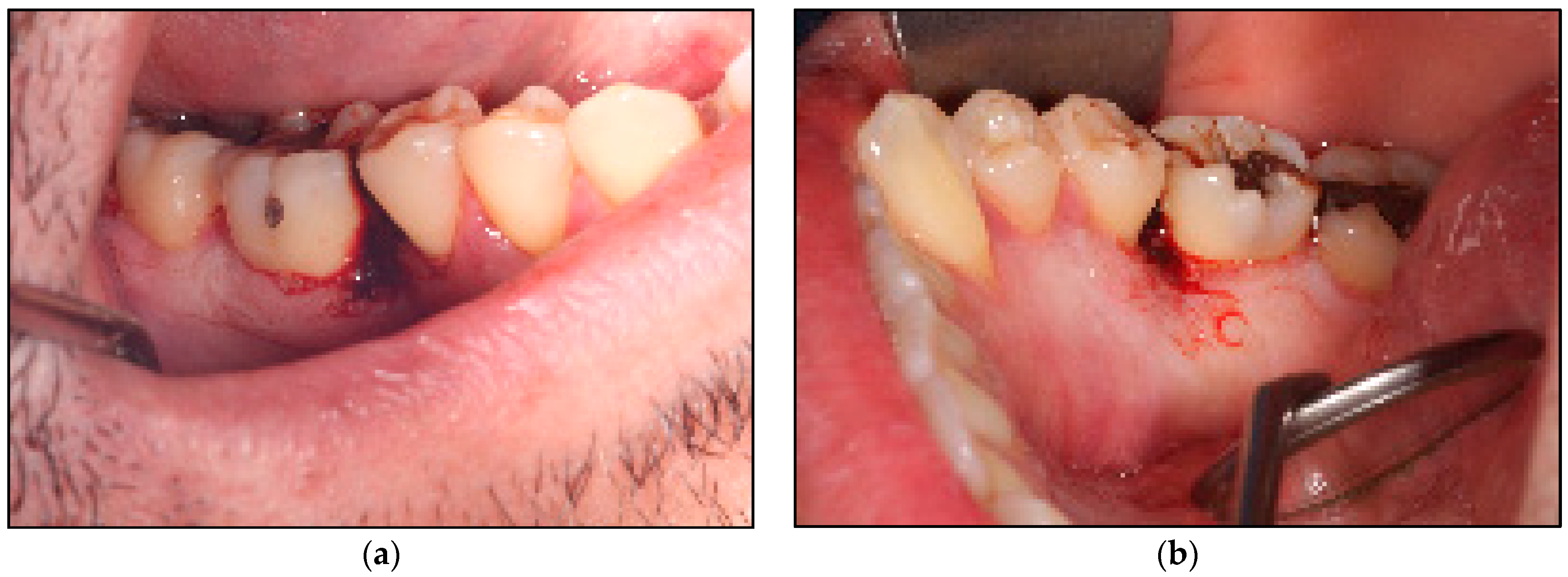
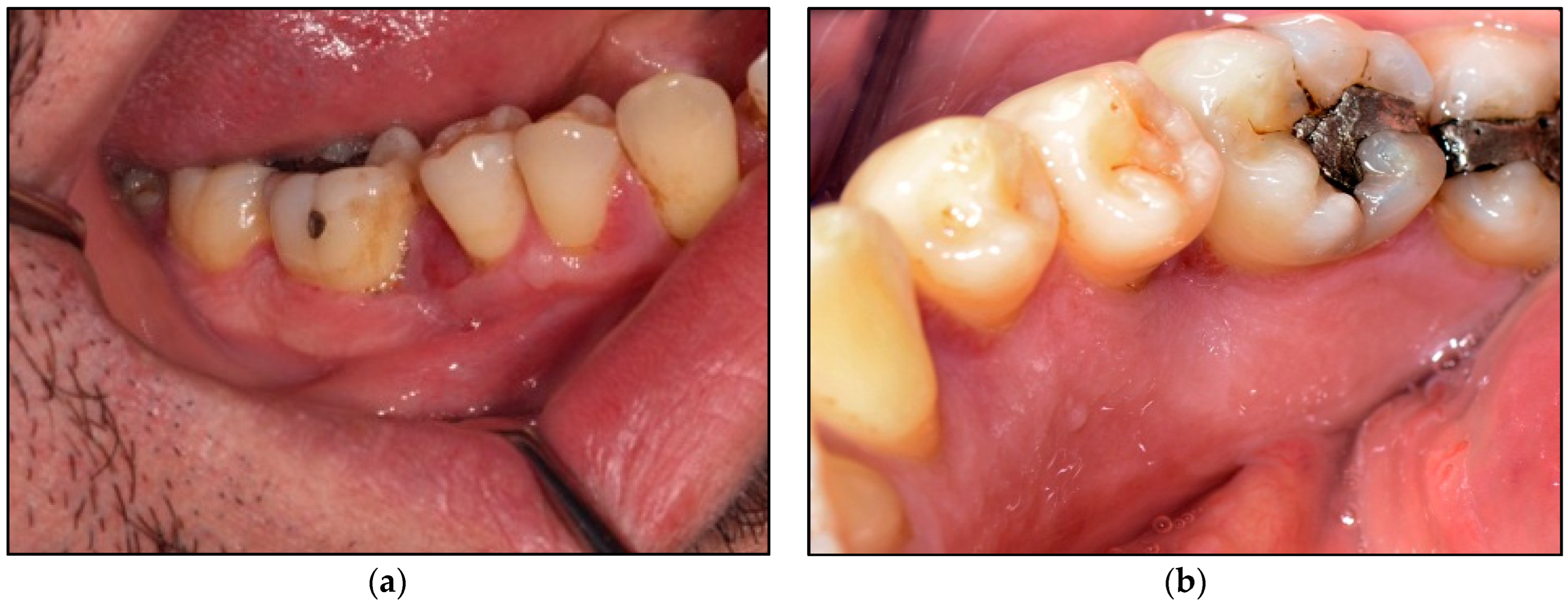
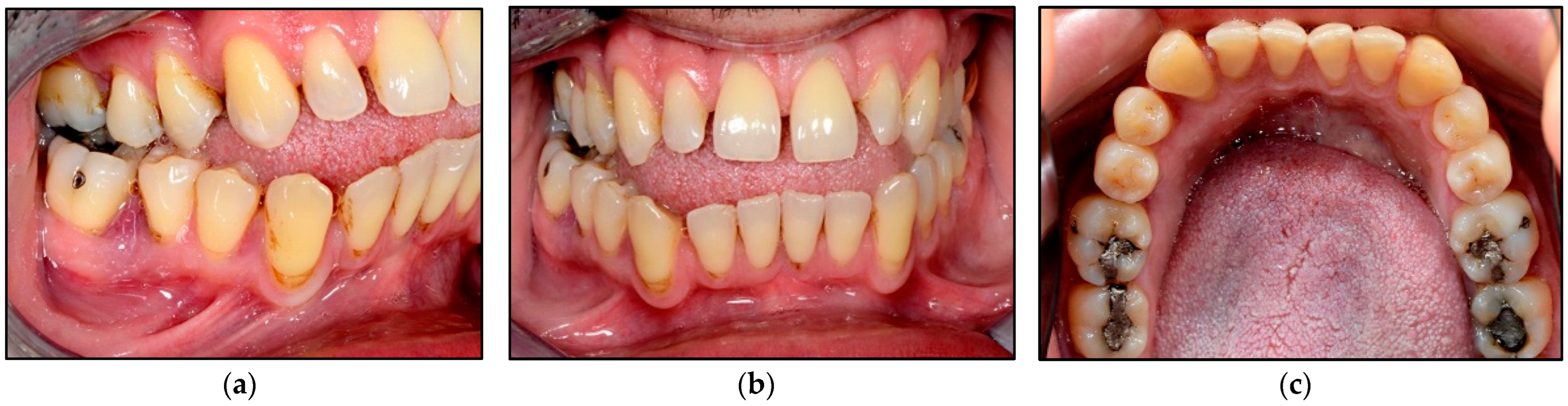
| Device Specifications | Manufacturer | LX-20SP Nova Pulse, Lumenis, Israel |
| Wavelength (nm) | 10,600 | |
| Emission mode | Gated continuous wave | |
| Duty cycle (%) | 50% duty cycle, 5 s on/5 s off | |
| Delivery system | Fibre optic | |
| Energy distribution | Gaussian | |
| Beam divergence | 8 degrees | |
| Irradiation Parameters | Peak power output (W) | 1.62 |
| Average power output (W) | 0.81 | |
| Spot diameter (mm) | 0.8 | |
| Spot diameter at focus (cm2) | 0.005 | |
| Spot diameter at tissue (cm) | 0.1081 | |
| Laser-to-tissue distance (mm) | <1 | |
| Spot area at tissue (cm2) | 0.0092 | |
| Total energy (J) | 73 | |
| Peak fluence (J/cm2) | 7934.78 | |
| Peak irradiance at spot area (W/cm2) | 324.0 | |
| Peak irradiance at tissue (W/cm2) | 176.08 | |
| Average irradiance at spot area (W/cm2) | 162 | |
| Average irradiance at tissue (W/cm2) | 88.04 | |
| Speed of the movement (mm/s) | ~2 | |
| Treatment Protocol | Total treatment duration (s) | 90 |
| Water irrigation (mL/min) | 12 | |
| Air (mL/min) | 20–25 (Manufacturer instructions) | |
| Aspirating airflow (mL/min) | 300 (Manufacturer instructions) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, R.; Benedicenti, S. 10,600 nm High Level-Laser Therapy Dosimetry in Management of Unresponsive Persistent Peripheral Giant Cell Granuloma to Standard Surgical Approach: A Case Report with 6-Month Follow-Up. J. Pers. Med. 2024, 14, 26. https://doi.org/10.3390/jpm14010026
Hanna R, Benedicenti S. 10,600 nm High Level-Laser Therapy Dosimetry in Management of Unresponsive Persistent Peripheral Giant Cell Granuloma to Standard Surgical Approach: A Case Report with 6-Month Follow-Up. Journal of Personalized Medicine. 2024; 14(1):26. https://doi.org/10.3390/jpm14010026
Chicago/Turabian StyleHanna, Reem, and Stefano Benedicenti. 2024. "10,600 nm High Level-Laser Therapy Dosimetry in Management of Unresponsive Persistent Peripheral Giant Cell Granuloma to Standard Surgical Approach: A Case Report with 6-Month Follow-Up" Journal of Personalized Medicine 14, no. 1: 26. https://doi.org/10.3390/jpm14010026
APA StyleHanna, R., & Benedicenti, S. (2024). 10,600 nm High Level-Laser Therapy Dosimetry in Management of Unresponsive Persistent Peripheral Giant Cell Granuloma to Standard Surgical Approach: A Case Report with 6-Month Follow-Up. Journal of Personalized Medicine, 14(1), 26. https://doi.org/10.3390/jpm14010026