Nocturnal Glucose Patterns with and without Hypoglycemia in People with Type 1 Diabetes Managed with Multiple Daily Insulin Injections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
2.2. CGM Data Cleaning and Preprocessing
2.3. Clustering Procedure
2.4. Assessment of Clustering Quality
2.5. Calculation of Quantitative Parameters of Clusters
2.6. Coding
2.7. Other Statistical Procedures
3. Results
3.1. Clinical Characteristics of Patients
3.2. Glucose Clusters without NH Episodes
3.3. Glucose Clusters with NH
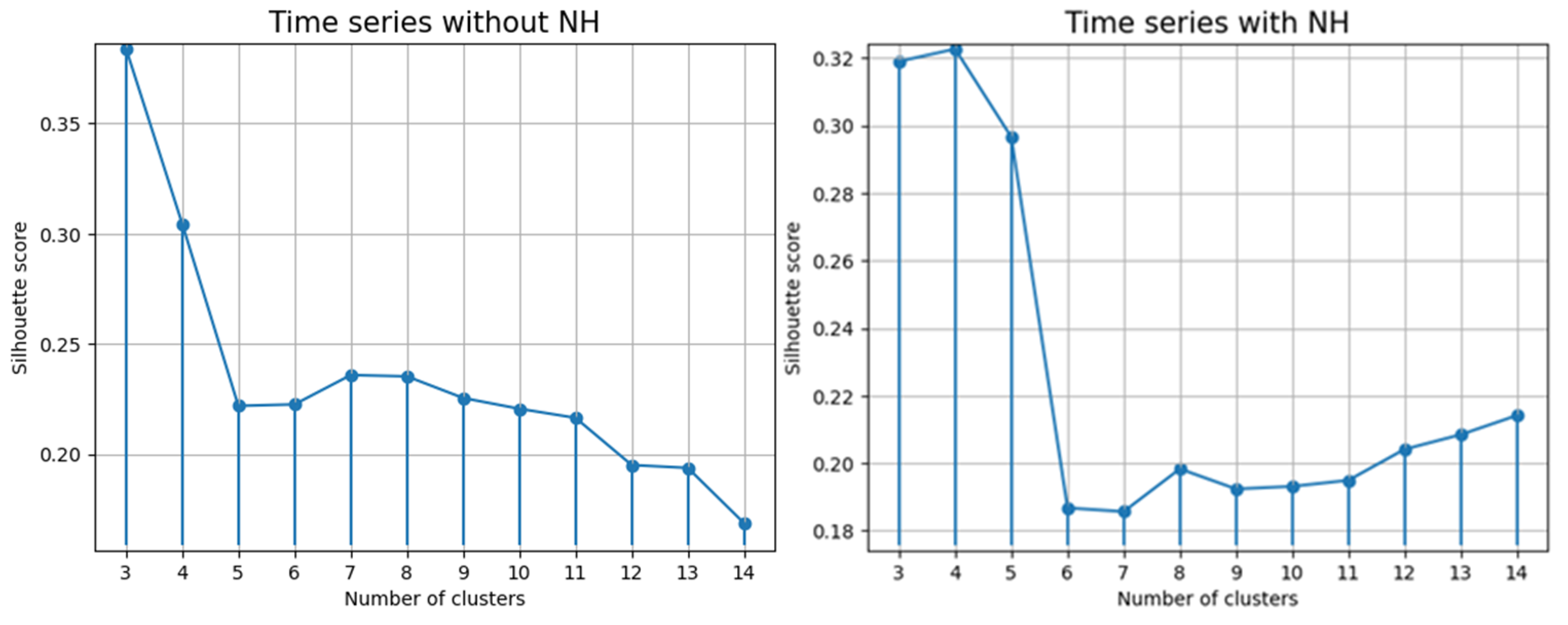
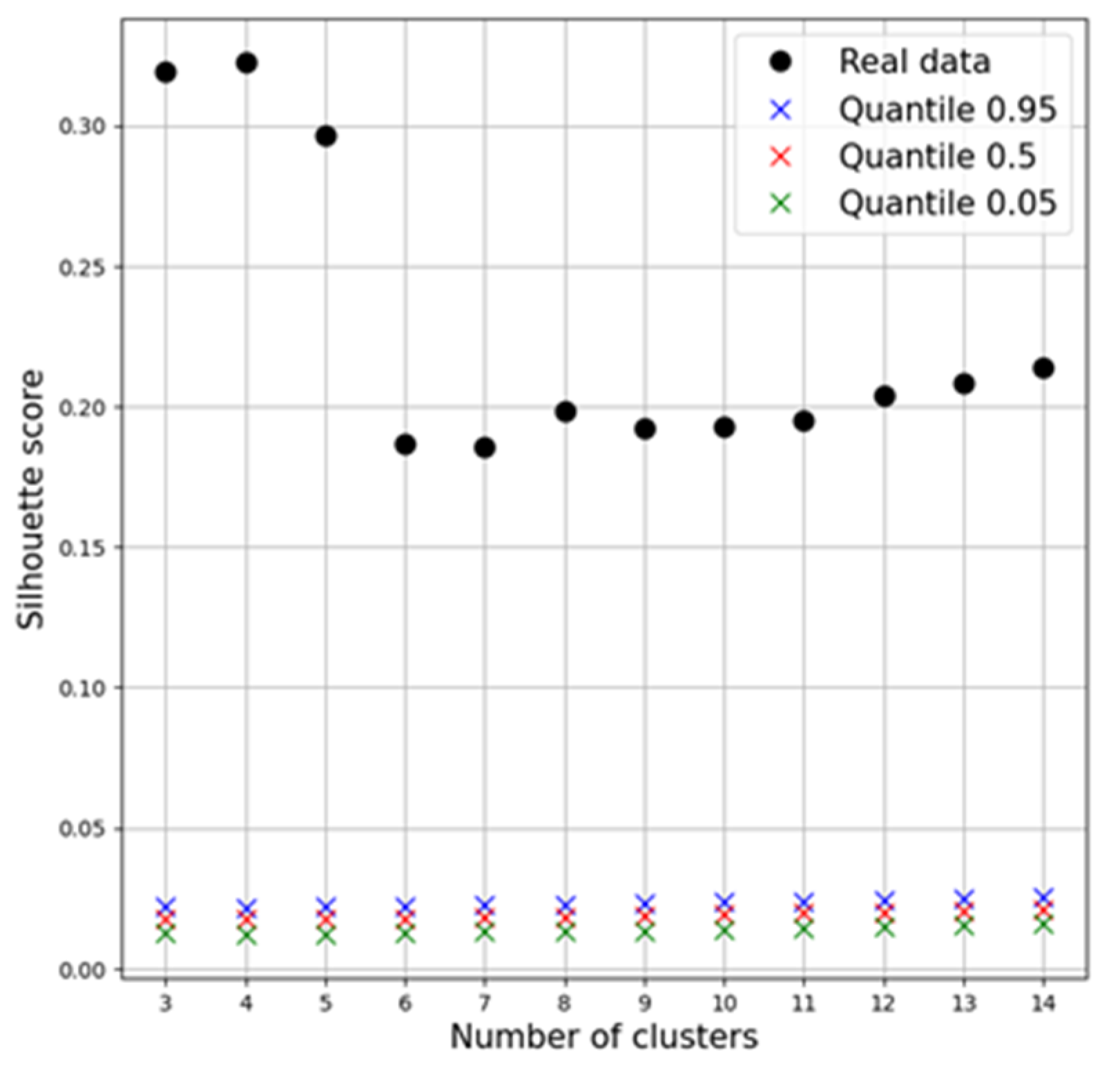
3.4. Clustering Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CGM | continuous glucose monitoring |
| HbA1c | glycated hemoglobin A1c |
| NH | nocturnal hypoglycemia |
| SS | Silhouette Score |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
References
- Type 1 Diabetes Estimates in Children and Adults. IDF Atlas Reports 2022. Available online: https://diabetesatlas.org/idfawp/resource-files/2022/12/IDF-T1D-Index-Report.pdf (accessed on 18 September 2023).
- Prigge, R.; McKnight, J.A.; Wild, S.H.; Haynes, A.; Jones, T.W.; Davis, E.A.; Rami-Merhar, B.; Fritsch, M.; Prchla, C.; Lavens, A.; et al. International comparison of glycaemic control in people with type 1 diabetes: An update and extension. Diabet. Med. 2022, 39, e14766. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E.; Rioles, N.; Liu, J.; Chapman, K.; Wolf, W.A.; Nguyen, H.; Basina, M.; Akturk, H.K.; Ebekozien, O.; Perez-Nieves, M.; et al. Prevalence of fear of hypoglycemia in adults with type 1 diabetes using a newly developed screener and clinician’s perspective on its implementation. BMJ Open Diabetes Res. Care 2023, 11, e003394. [Google Scholar] [CrossRef] [PubMed]
- Emral, R.; Pathan, F.; Cortés, C.A.Y.; El-Hefnawy, M.H.; Goh, S.Y.; Gómez, A.M.; Murphy, A.; Abusnana, S.; Rudijanto, A.; Jain, A.; et al. Self-reported hypoglycemia in insulin-treated patients with diabetes: Results from an international survey on 7289 patients from nine countries. Diabetes Res. Clin. Pract. 2017, 134, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Saik, O.V.; Klimontov, V.V. Hypoglycemia, Vascular Disease and Cognitive Dysfunction in Diabetes: Insights from Text Mining-Based Reconstruction and Bioinformatics Analysis of the Gene Networks. Int. J. Mol. Sci. 2021, 22, 12419. [Google Scholar] [CrossRef]
- Nakhleh, A.; Shehadeh, N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J. Diabetes 2021, 12, 2036–2049. [Google Scholar] [CrossRef]
- Schultes, B.; Jauch-Chara, K.; Gais, S.; Hallschmid, M.; Reiprich, E.; Kern, W.; Oltmanns, K.M.; Peters, A.; Fehm, H.L.; Born, J. Defective awakening response to nocturnal hypoglycemia in patients with type 1 diabetes mellitus. PLoS Med. 2007, 4, e69. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- Siamashvili, M.; Davis, H.A.; Davis, S.N. Nocturnal hypoglycemia in type 1 and type 2 diabetes: An update on prevalence, prevention, pathophysiology and patient awareness. Expert Rev. Endocrinol. Metab. 2021, 16, 281–293. [Google Scholar] [CrossRef]
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010, 33, 1004–1008. [Google Scholar] [CrossRef]
- Henriksen, M.M.; Andersen, H.U.; Thorsteinsson, B.; Pedersen-Bjergaard, U. Asymptomatic hypoglycaemia in Type 1 diabetes: Incidence and risk factors. Diabet. Med. 2019, 36, 62–69. [Google Scholar] [CrossRef]
- Guelho, D.; Paiva, I.; Batista, C.; Barros, L.; Carrilho, F. A1c, glucose variability and hypoglycemia risk in patients with type 1 diabetes. Minerva Endocrinol. 2014, 39, 127–133. [Google Scholar]
- Wilson, D.M.; Calhoun, P.M.; Maahs, D.M.; Chase, H.P.; Messer, L.; Buckingham, B.A.; Aye, T.; Clinton, P.K.; Hramiak, I.; Kollman, C.; et al. In Home Closed Loop Study Group. Factors associated with nocturnal hypoglycemia in at-risk adolescents and young adults with type 1 diabetes. Diabetes Technol. Ther. 2015, 17, 385–391. [Google Scholar] [CrossRef]
- McLachlan, G.J. Cluster analysis and related techniques in medical research. Stat. Methods Med. Res. 1992, 1, 27–48. [Google Scholar] [CrossRef]
- Sarría-Santamera, A.; Orazumbekova, B.; Maulenkul, T.; Gaipov, A.; Atageldiyeva, K. The Identification of Diabetes Mellitus Subtypes Applying Cluster Analysis Techniques: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9523. [Google Scholar] [CrossRef]
- Kahkoska, A.R.; Adair, L.A.; Aiello, A.E.; Burger, K.S.; Buse, J.B.; Crandell, J.; Maahs, D.M.; Nguyen, C.T.; Kosorok, M.R.; Mayer-Davis, E.J. Identification of clinically relevant dysglycemia phenotypes based on continuous glucose monitoring data from youth with type 1 diabetes and elevated hemoglobin A1c. Pediatr. Diabetes 2019, 20, 556–566. [Google Scholar] [CrossRef]
- Schroder, C.; Diez, J.L.; Laguna, A.J.; Bondia, J.; Tarin, C. Classification of postprandial glycemic patterns in type 1 diabetes subjects under closed-loop control: An in silico study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 5443–5446. [Google Scholar] [CrossRef]
- Tao, R.; Yu, X.; Lu, J.; Shen, Y.; Lu, W.; Zhu, W.; Bao, Y.; Li, H.; Zhou, J. Multilevel clustering approach driven by continuous glucose monitoring data for further classification of type 2 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e001869. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Ruan, L.; Song, Q. Time-Series Analysis of Continuous Glucose Monitoring Data to Predict Treatment Efficacy in Patients with T2DM. J. Clin. Endocrinol. Metab. 2021, 106, 2187–2197. [Google Scholar] [CrossRef]
- Varghese, J.S.; Ho, J.C.; Anjana, R.M.; Pradeepa, R.; Patel, S.A.; Jebarani, S.; Baskar, V.; Narayan, K.M.V.; Mohan, V. Profiles of Intraday Glucose in Type 2 Diabetes and Their Association with Complications: An Analysis of Continuous Glucose Monitoring Data. Diabetes Technol. Ther. 2021, 23, 555–564. [Google Scholar] [CrossRef]
- Inayama, Y.; Yamanoi, K.; Shitanaka, S.; Ogura, J.; Ohara, T.; Sakai, M.; Suzuki, H.; Kishimoto, I.; Tsunenari, T.; Suginami, K. A novel classification of glucose profile in pregnancy based on continuous glucose monitoring data. J. Obstet. Gynaecol. Res. 2021, 47, 1281–1291. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Cassisi, C.; Montaldo, P.; Aliotta, M.; Cannata, A.; Pulvirenti, A. Similarity Measures and Dimensionality Reduction Techniques for Time Series Data Mining. In Advances in Data Mining Knowledge Discovery and Applications; Karahoca, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 71–96. [Google Scholar] [CrossRef]
- Rousseeuw, P. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Kirilyuk, I.L.; Senko, O.V. Assessing the validity of clustering of panel data by Monte Carlo methods (using as example the data of the Russian regional economy). Comput. Res. Model. 2020, 12, e1501–e1513. [Google Scholar] [CrossRef]
- Ono, M.; Katsuki, T.; Makino, M.; Haida, K.; Suzuki, A. Interpretation Method for Continuous Glucose Monitoring with Subsequence Time-Series Clustering. Stud. Health Technol. Inform. 2020, 270, 277–281. [Google Scholar] [CrossRef]
- Klimontov, V.V.; Myakina, N.E. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab. Syndr. 2017, 11, 119–124. [Google Scholar] [CrossRef]
- Owens, D.R.; Bailey, T.S.; Fanelli, C.G.; Yale, J.F.; Bolli, G.B. Clinical relevance of pharmacokinetic and pharmacodynamic profiles of insulin degludec (100, 200 U/mL) and insulin glargine (100, 300 U/mL) – a review of evidence and clinical interpretation. Diabetes Metab. 2019, 45, 330–340. [Google Scholar] [CrossRef]
- Díez-Fernández, A.; Cavero-Redondo, I.; Moreno-Fernández, J.; Pozuelo-Carrascosa, D.P.; Garrido-Miguel, M.; Martínez-Vizcaíno, V. Effectiveness of insulin glargine U-300 versus insulin glargine U-100 on nocturnal hypoglycemia and glycemic control in type 1 and type 2 diabetes: A systematic review and meta-analysis. Acta Diabetol. 2019, 56, 355–364. [Google Scholar] [CrossRef]
- Brøsen, J.M.B.; Agesen, R.M.; Alibegovic, A.C.; Ullits Andersen, H.; Beck-Nielsen, H.; Gustenhoff, P.; Krarup Hansen, T.; Hedetoft, C.G.R.; Jensen, T.J.; Stolberg, C.R.; et al. Continuous Glucose Monitoring-Recorded Hypoglycemia with Insulin Degludec or Insulin Glargine U100 in People with Type 1 Diabetes Prone to Nocturnal Severe Hypoglycemia. Diabetes Technol. Ther. 2022, 24, 643–654. [Google Scholar] [CrossRef]
- Forlenza, G.P.; Li, Z.; Buckingham, B.A.; Pinsker, J.E.; Cengiz, E.; Wadwa, R.P.; Ekhlaspour, L.; Church, M.M.; Weinzimer, S.A.; Jost, E.; et al. Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children With Type 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Diabetes Care 2018, 41, 2155–2161. [Google Scholar] [CrossRef]
- Chen, E.; King, F.; Kohn, M.A.; Spanakis, E.K.; Breton, M.; Klonoff, D.C. A Review of Predictive Low Glucose Suspend and Its Effectiveness in Preventing Nocturnal Hypoglycemia. Diabetes Technol. Ther. 2019, 21, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Beck, R.W.; Raghinaru, D.; Buckingham, B.A.; Laffel, L.M.; Wadwa, R.P.; Kudva, Y.C.; Levy, C.J.; Pinsker, J.E.; Dassau, E.; et al. iDCL Trial Research Group. Glycemic Outcomes of Use of CLC Versus PLGS in Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020, 43, 1822–1828. [Google Scholar] [CrossRef] [PubMed]

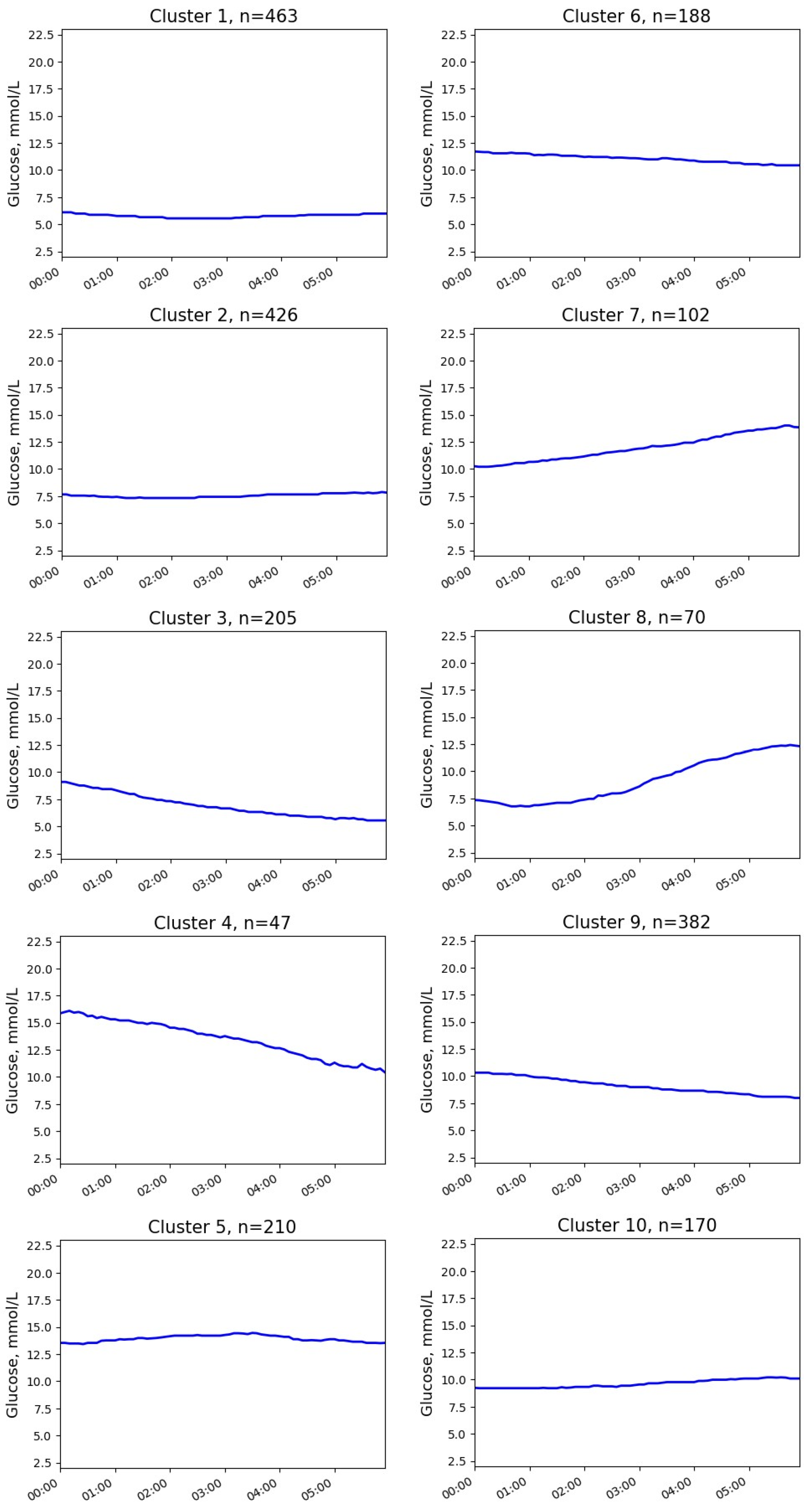
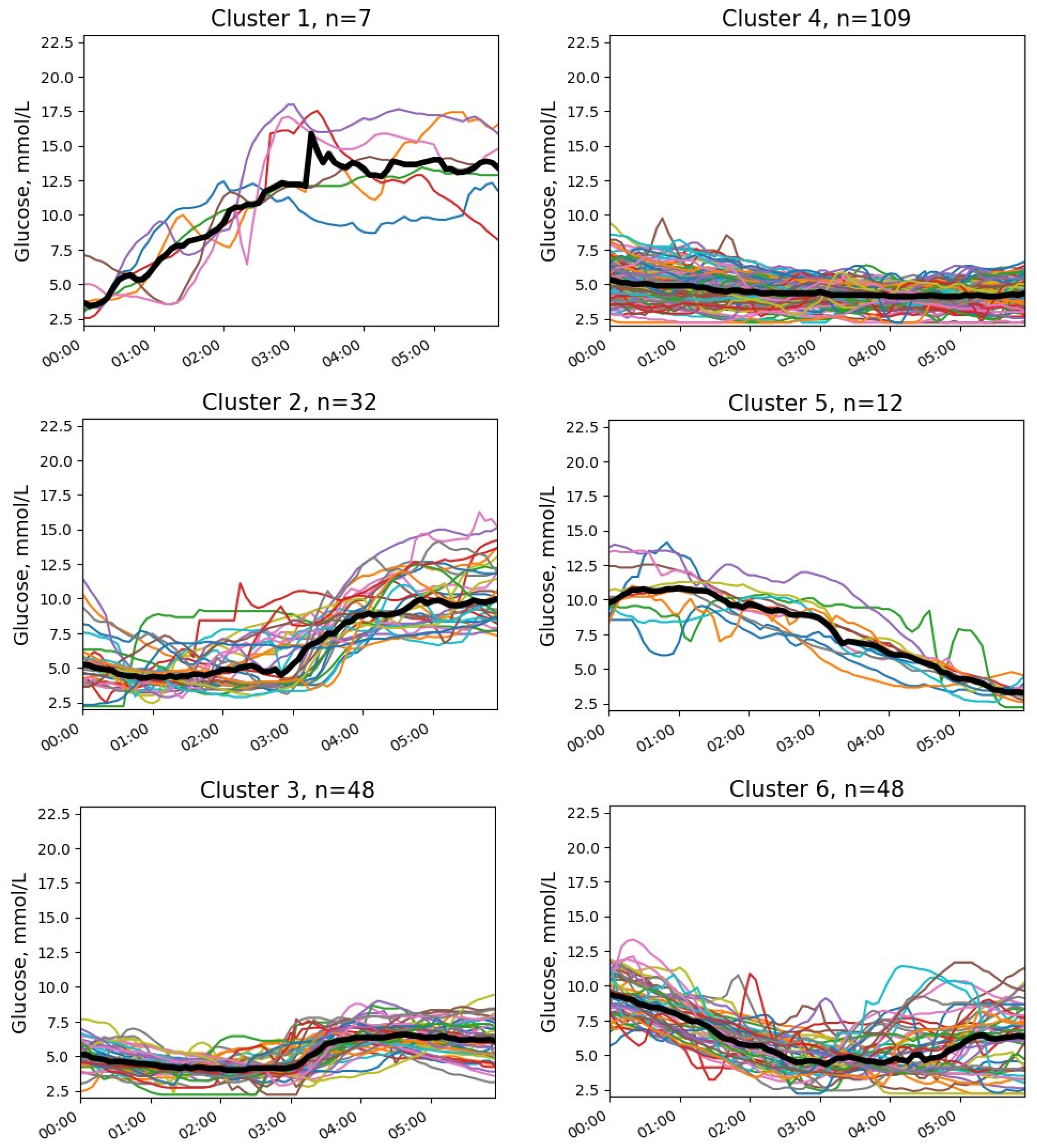

| Cluster | Parameter | Median (25; 75 Percentile) | Min–Max |
|---|---|---|---|
| 1 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 6.1 (5.4; 6.8) 6.0 (5.2; 6.8) −0.2 (−1.1; 0.7) | 3.8–9.0 3.9–10.8 −3.3–4.8 |
| 2 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 7.7 (6.7; 8.4) 7.8 (7.1; 8.8) 0.3 (−0.8; 1.6) | 3.8–10.9 4.9–11.5 −4.0–5.8 |
| 3 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 9.1 (8.0; 10.4) 5.6 (5.0; 6.2) −3.4 (−4.7; −2.4) | 5.0–16.4 4.0–9.2 −11.9–0.5 |
| 4 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 15.8 (14.9; 17.4) 10.7 (9.4; 11.4) −5.6 (−7.4; −4.0) | 11.9–21.0 7.5–13.7 −12.7–−1.0 |
| 5 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 13.4 (12.2; 15.1) 13.5 (12.1; 15.1) 0.0 (−1.7; 1.6) | 8.7–21.6 9.0–19.5 −7.5–7.0 |
| 6 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 11.7 (10.4; 12.9) 10.4 (9.8; 11.1) −1.0 (−2.6; 0.4) | 7.3–16.7 6.9–14.1 −8.0–5.9 |
| 7 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 10.2 (9.5; 11.1) 14.0 (12.8; 15.3) 3.4 (2.4; 5.4) | 8.2–13.7 11.3–21.2 −0.5–12.4 |
| 8 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 7.4 (5.9; 8.4) 12.4 (11.4; 13.4) 5.4 (3.8; 6.5) | 4.2–10.0 9.0–15.9 1.8–9.9 |
| 9 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 10.3 (9.3; 11.5) 8.0 (7.3; 8.8) −2.2 (−3.7; −1.0) | 5.1–19.3 4.2–13.0 −12.4–3.8 |
| 10 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L | 9.2 (8.2; 10.0) 10.1 (9.5; 10.8) 1.2 (−0.1; 2.2) | 5.2–12.5 7.6–13.4 −3.2–5.2 |
| Cluster | Parameter | Median (25; 75 Percentile) | Min–Max |
|---|---|---|---|
| 1 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 3.4 (3.2; 4.1) 14.1 (12.7; 15.3) 9.6 (8.3; 12.4) 0 (0; 0) | 2.6–7.0 8.5–16.4 5.9–12.7 0–1.1 |
| 2 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 5.2 (4.1; 5.8) 9.8 (8.5; 12.2) 4.6 (3.3; 6.6) 0.8 (0.2; 1.6) | 2.2–10.8 4.7–15.5 −2.0–11.4 0.0–3.2 |
| 3 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 5.1 (4.6; 5.6) 6.3 (5.2; 7.2) 1.3 (0.1; 2.6) 1.3 (0.8; 2.1) | 2.6–7.6 3.1–10.0 −2.7–5.3 0.0–5.7 |
| 4 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 5.2 (4.4; 6.1) 4.3 (3.8; 4.9) −0.8 (−2.0; 0.2) 2.1 (0.6; 3.9) | 2.3–9.1 2.2–7.4 −5.3–2.3 0.0–5.7 |
| 5 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 10.0 (9.5; 10.6) 3.4 (3.2; 3.6) −6.8 (−7.4; −5.3) 5.2 (4.4; 5.5) | 7.7–13.9 2.2–7.4 −5.3–2.3 0.0–5.7 |
| 6 | Initial glucose, mmol/L Final glucose, mmol/L Glucose change, mmol/L Start time of NH episode | 9.4 (8.2; 10.6) 6.4 (4.0; 7.9) −2.8 (−5.4; −0.4) 2.9 (2.5; 4.0) | 7.7–13.9 2.2–11.1 −9.1–2.7 1.2–5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladov, D.E.; Berikov, V.B.; Semenova, J.F.; Klimontov, V.V. Nocturnal Glucose Patterns with and without Hypoglycemia in People with Type 1 Diabetes Managed with Multiple Daily Insulin Injections. J. Pers. Med. 2023, 13, 1454. https://doi.org/10.3390/jpm13101454
Kladov DE, Berikov VB, Semenova JF, Klimontov VV. Nocturnal Glucose Patterns with and without Hypoglycemia in People with Type 1 Diabetes Managed with Multiple Daily Insulin Injections. Journal of Personalized Medicine. 2023; 13(10):1454. https://doi.org/10.3390/jpm13101454
Chicago/Turabian StyleKladov, Danil E., Vladimir B. Berikov, Julia F. Semenova, and Vadim V. Klimontov. 2023. "Nocturnal Glucose Patterns with and without Hypoglycemia in People with Type 1 Diabetes Managed with Multiple Daily Insulin Injections" Journal of Personalized Medicine 13, no. 10: 1454. https://doi.org/10.3390/jpm13101454
APA StyleKladov, D. E., Berikov, V. B., Semenova, J. F., & Klimontov, V. V. (2023). Nocturnal Glucose Patterns with and without Hypoglycemia in People with Type 1 Diabetes Managed with Multiple Daily Insulin Injections. Journal of Personalized Medicine, 13(10), 1454. https://doi.org/10.3390/jpm13101454







