Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery
Abstract
1. Introduction
2. Materials and Methods
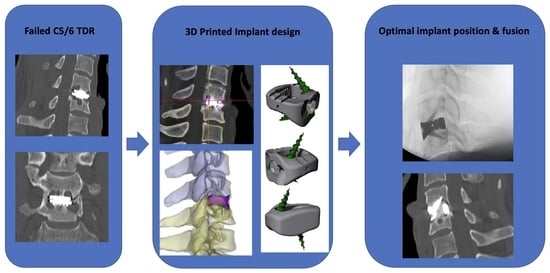
2.1. Case Presentation
2.2. Preoperative Planning
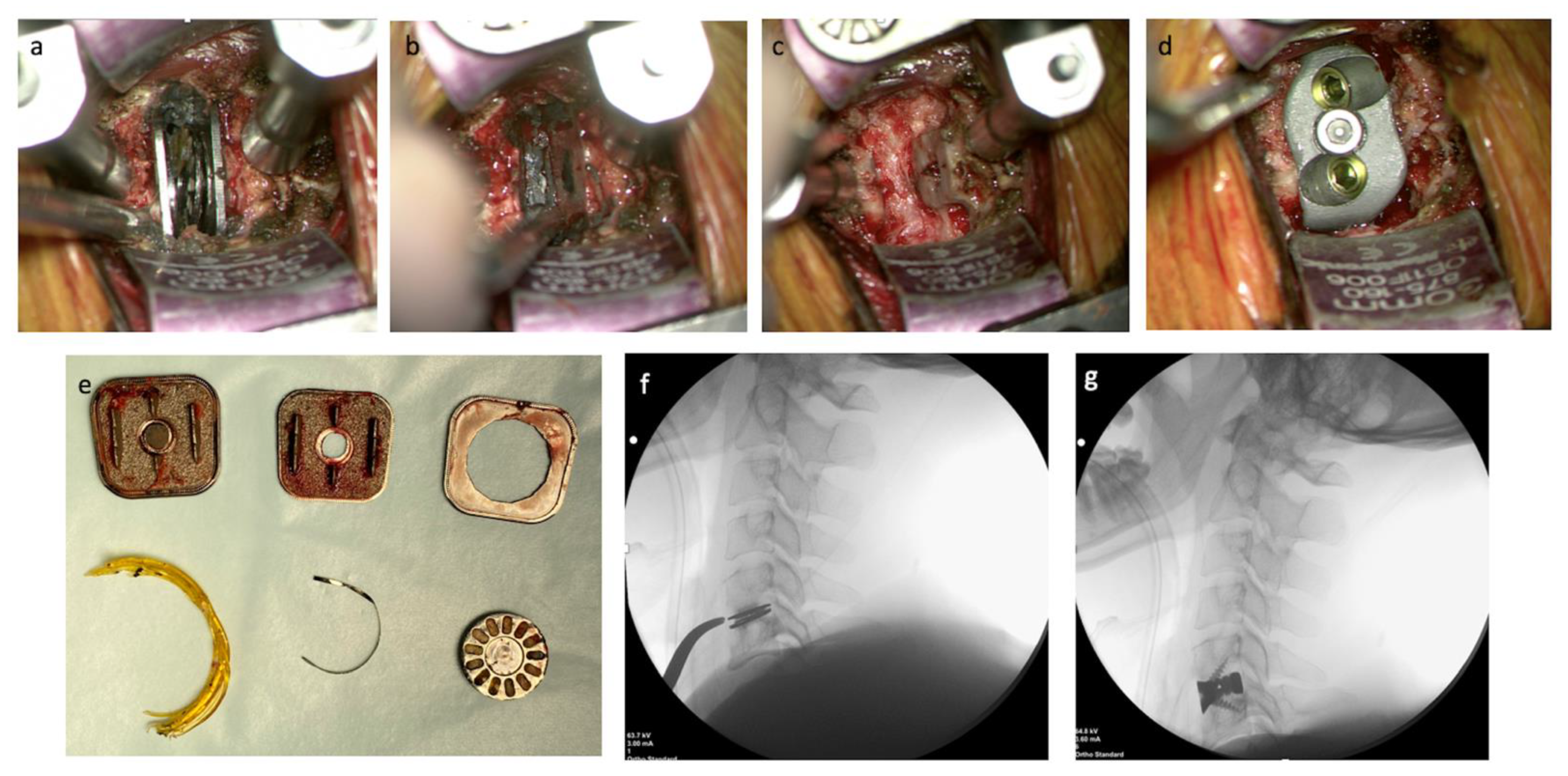
2.3. Operative Technique
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikh, S.R.; Thompson, N.R.; Benzel, E.; Steinmetz, M.; Mroz, T.; Tomic, D.; Machado, A.; Jehi, L. Can We Justify It? Trends in the Utilization of Spinal Fusions and Associated Reimbursement. Neurosurgery 2020, 86, E193–E202. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Pelletier, M.H.; Mobbs, R.J.; Walsh, W.R. The design evolution of interbody cages in anterior cervical discectomy and fusion: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Sheha, E.D.; Gandhi, S.D.; Colman, M.W. 3D printing in spine surgery. Ann. Transl. Med. 2019, 7 (Suppl. S5), S164. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Han, Y.; Jiang, Y.; Wang, J.; Zhang, X.; Miao, J. Biomechanical comparison of different prosthetic reconstructions in total en bloc spondylectomy: A finite element study. BMC Musculoskelet. Disord. 2022, 23, 955. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Parr, W.C.H.; Choy, W.J.; McEvoy, A.; Walsh, W.R.; Phan, K. Anterior Lumbar Interbody Fusion Using a Personalized Approach: Is Custom the Future of Implants for Anterior Lumbar Interbody Fusion Surgery? World Neurosurg. 2019, 123, 452–458.e1. [Google Scholar] [CrossRef]
- Choi, H.; Purushothaman, Y.; Baisden, J.L.; Rajasekaran, D.; Jebaseelan, D.; Yoganandan, N. Comparative Finite Element Modeling Study of Anterior Cervical Arthrodesis Versus Cervical Arthroplasty with Bryan Disc or Prodisc C. Mil. Med. 2021, 186 (Suppl. S1), 737–744. [Google Scholar] [CrossRef]
- Mo, Z.; Li, Q.; Jia, Z.; Yang, J.; Wong, D.W.; Fan, Y. Biomechanical consideration of prosthesis selection in hybrid surgery for bi-level cervical disc degenerative diseases. Eur. Spine J. 2017, 26, 1181–1190. [Google Scholar] [CrossRef]
- Naoum, S.; Vasiliadis, A.V.; Koutserimpas, C.; Mylonakis, N.; Kotsapas, M.; Katakalos, K. Finite Element Method for the Evaluation of the Human Spine: A Literature Overview. J. Funct. Biomater. 2021, 12, 43. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Wilcox, B.; Mobbs, R.J.; Wu, A.M.; Phan, K. Systematic review of 3D printing in spinal surgery: The current state of play. J. Spine Surg. 2017, 3, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Patralekh, M.K.; Vaish, A.; Agarwal, A.K.; Vijay, V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J. Clin. Orthop. Trauma 2018, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Choy, W.J.; Wilson, P.; McEvoy, A.; Phan, K.; Parr, W.C.H. L5 En-Bloc Vertebrectomy with Customized Reconstructive Implant: Comparison of Patient-Specific Versus Off-the-Shelf Implant. World Neurosurg. 2018, 112, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Rajaee, S.S.; Kanim, L.E.; Bae, H.W. National trends in revision spinal fusion in the USA: Patient characteristics and complications. Bone Jt. J. 2014, 96-B, 807–816. [Google Scholar] [CrossRef]
- Mok, J.M.; Cloyd, J.M.; Bradford, D.S.; Hu, S.S.; Deviren, V.; Smith, J.A.; Tay, B.; Berven, S.H. Reoperation after primary fusion for adult spinal deformity: Rate, reason, and timing. Spine 2009, 34, 832–839. [Google Scholar] [CrossRef]
- Pichelmann, M.A.; Lenke, L.G.; Bridwell, K.H.; Good, C.R.; O’Leary, P.T.; Sides, B.A. Revision rates following primary adult spinal deformity surgery: Six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine 2010, 35, 219–226. [Google Scholar] [CrossRef]
- Kelly, M.P.; Lenke, L.G.; Bridwell, K.H.; Agarwal, R.; Godzik, J.; Koester, L. Fate of the adult revision spinal deformity patient: A single institution experience. Spine 2013, 38, E1196–E1200. [Google Scholar] [CrossRef]
- Kalakoti, P.; Missios, S.; Maiti, T.; Konar, S.; Bir, S.; Bollam, P.; Nanda, A. Inpatient Outcomes and Postoperative Complications After Primary Versus Revision Lumbar Spinal Fusion Surgeries for Degenerative Lumbar Disc Disease: A National (Nationwide) Inpatient Sample Analysis, 2002–2011. World Neurosurg. 2016, 85, 114–124. [Google Scholar] [CrossRef]
- Joaquim, A.F.; Lee, N.J.; Riew, K.D. Revision Surgeries at the Index Level After Cervical Disc Arthroplasty—A Systematic Review. Neurospine 2021, 18, 34–44. [Google Scholar] [CrossRef]
- Ebinu, J.O.; Ramanathan, D.; Kurtz, S.M.; Lawandy, S.; Kim, K.D. Periprosthetic Osteolysis in Cervical Total Disc Arthroplasty: A Single Institutional Experience. Neurosurg. Open 2021, 2, okab013. [Google Scholar] [CrossRef]
- Hao, Y.-L.; Li, S.-J.; Yang, R. Biomedical titanium alloys and their additive manufacturing. Rare Met. 2016, 35, 661–671. [Google Scholar] [CrossRef]
- Mierzejewska, Ż.A.; Hudák, R.; Sidun, J. Mechanical Properties and Microstructure of DMLS Ti6Al4V Alloy Dedicated to Biomedical Applications. Materials 2019, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive manufacturing of titanium alloys in the biomedical field: Processes, properties and applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Suh, P.B.; Puttlitz, C.; Lewis, C.; Bal, B.S.; McGilvray, K. The Effect of Cervical Interbody Cage Morphology, Material Composition, and Substrate Density on Cage Subsidence. J. Am. Acad. Orthop. Surg. 2017, 25, 160–168. [Google Scholar] [CrossRef]
- Mohammad-Shahi, M.H.; Nikolaou, V.S.; Giannitsios, D.; Ouellet, J.; Jarzem, P.F. The effect of angular mismatch between vertebral endplate and vertebral body replacement endplate on implant subsidence. J. Spinal Disord. Tech. 2013, 26, 268–273. [Google Scholar] [CrossRef]
- Truumees, E.; Demetropoulos, C.K.; Yang, K.H.; Herkowitz, H.N. Failure of human cervical endplates: A cadaveric experimental model. Spine 2003, 28, 2204–2208. [Google Scholar] [CrossRef]
- Lee, N.J.; Joaquim, A.F.; Boddapati, V.; Mathew, J.; Park, P.; Kim, J.S.; Sardar, Z.M.; Lehman, R.A.; Riew, K.D. Revision Anterior Cervical Disc Arthroplasty: A National Analysis of the Associated Indications, Procedures, and Postoperative Outcomes. Glob. Spine J. 2022, 12, 1338–1344. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Parr, W.C.H.; Huang, C.; Amin, T. Rapid Personalised Virtual Planning and On-Demand Surgery for Acute Spinal Trauma Using 3D-Printing, Biomodelling and Patient-Specific Implant Manufacture. J. Pers. Med. 2022, 12, 997. [Google Scholar] [CrossRef]
- Lal, H.; Patralekh, M.K. 3D printing and its applications in orthopaedic trauma: A technological marvel. J. Clin. Orthop. Trauma 2018, 9, 260–268. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, T.; Vaish, A.; Vaish, R.; Vaishya, R.; Maini, L. Virtual preoperative planning and 3D printing are valuable for the management of complex orthopaedic trauma. Chin. J. Traumatol. 2019, 22, 350–355. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tredan, D.A.M.; Mobbs, R.J.; Maharaj, M.; Parr, W.C.H. Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery. J. Pers. Med. 2023, 13, 19. https://doi.org/10.3390/jpm13010019
Tredan DAM, Mobbs RJ, Maharaj M, Parr WCH. Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery. Journal of Personalized Medicine. 2023; 13(1):19. https://doi.org/10.3390/jpm13010019
Chicago/Turabian StyleTredan, David A. M., Ralph J. Mobbs, Monish Maharaj, and William C. H. Parr. 2023. "Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery" Journal of Personalized Medicine 13, no. 1: 19. https://doi.org/10.3390/jpm13010019
APA StyleTredan, D. A. M., Mobbs, R. J., Maharaj, M., & Parr, W. C. H. (2023). Combining Virtual Surgical Planning and Patient-Specific 3D-Printing as a Solution to Complex Spinal Revision Surgery. Journal of Personalized Medicine, 13(1), 19. https://doi.org/10.3390/jpm13010019