Application of Reactive Oxygen Species in Dental Treatment
Abstract
1. Introduction
2. Materials and Methods
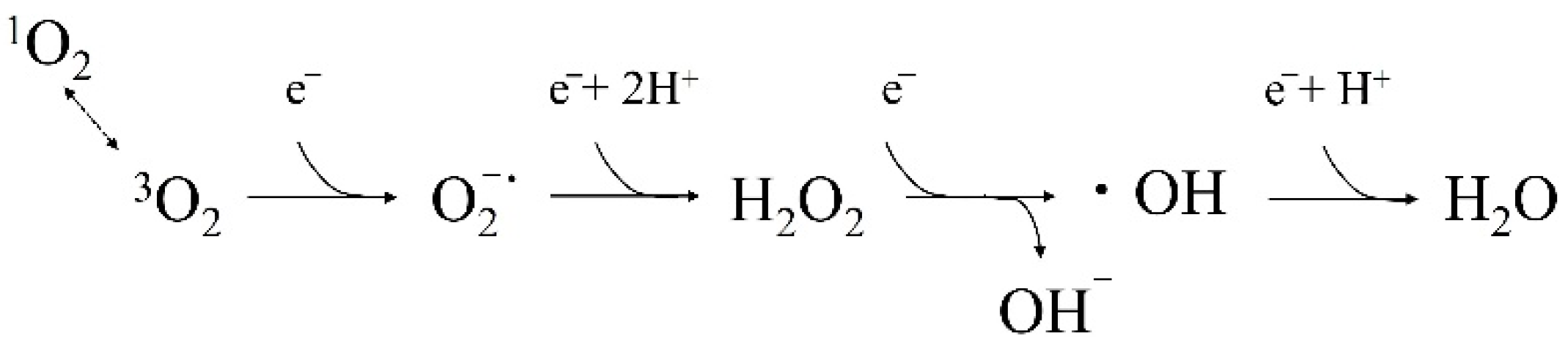
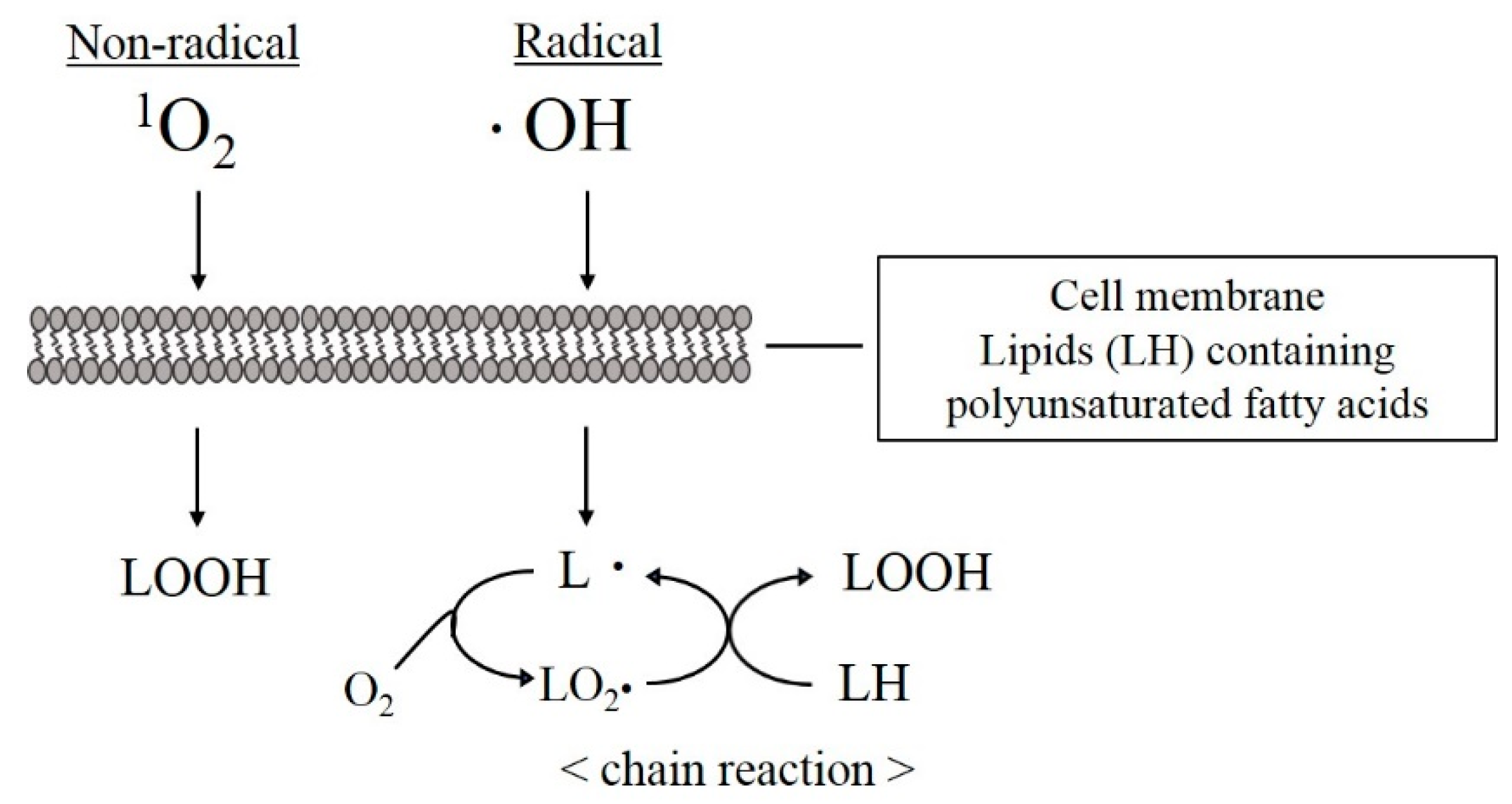
3. What Are ROS and Free Radicals?
4. Root Canal Cleaning by ROS
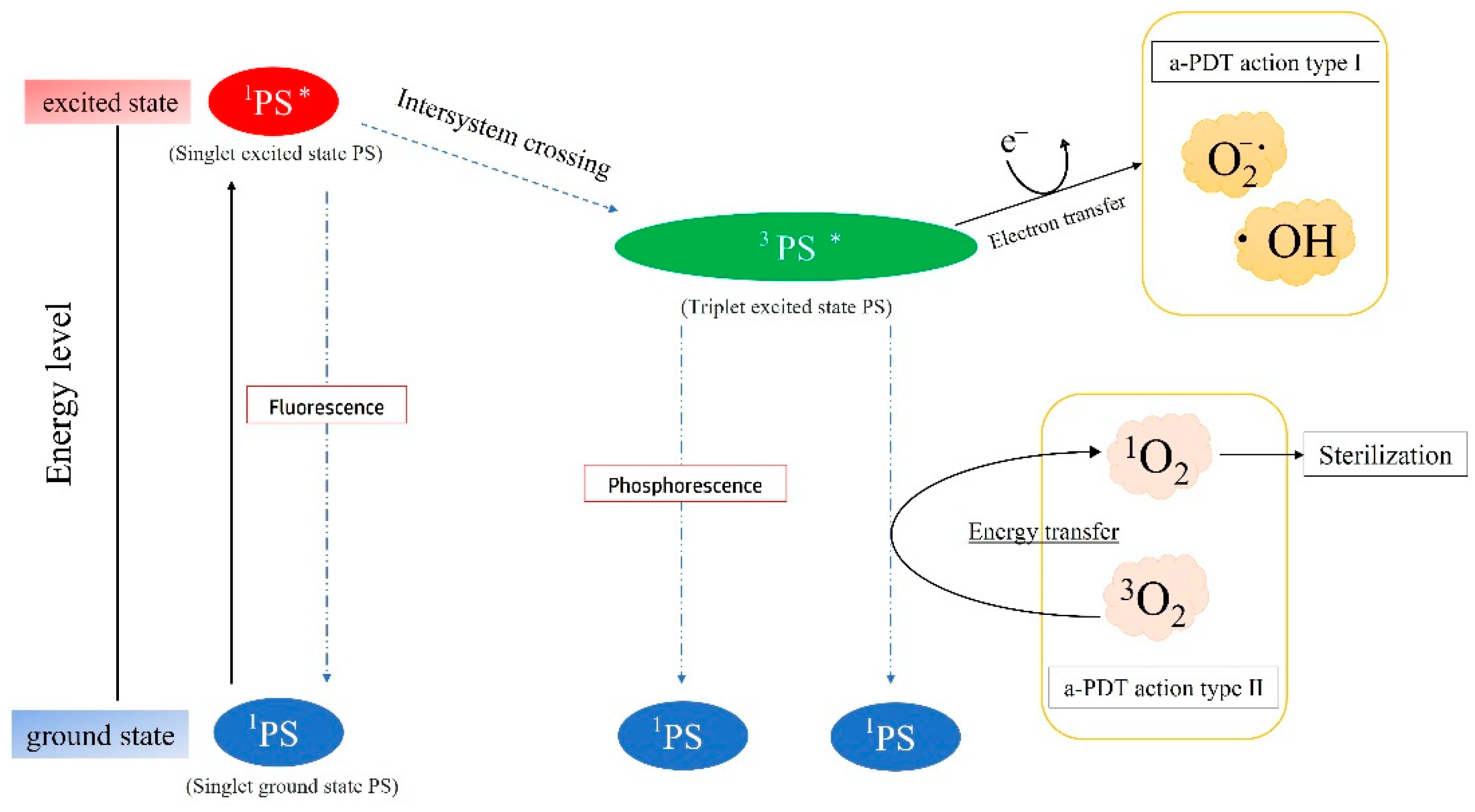
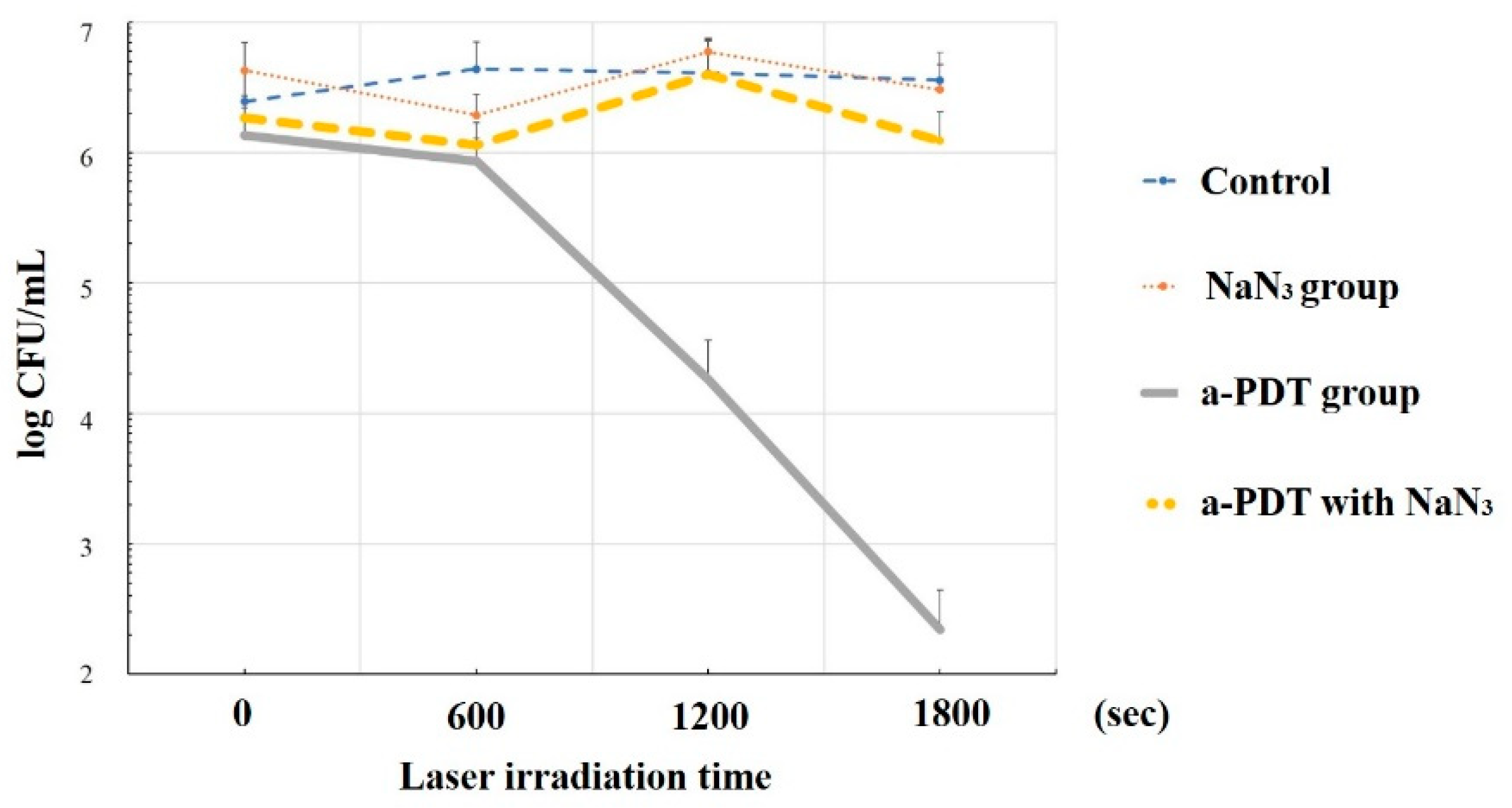
4.1. Bactericidal Efficacy for Bacteria Associated with Refractory Apical Periodontitis and the Appropriate PS Concentration for 1O2 Generation
4.2. Sterilization Mechanism of a-PDT by 1O2
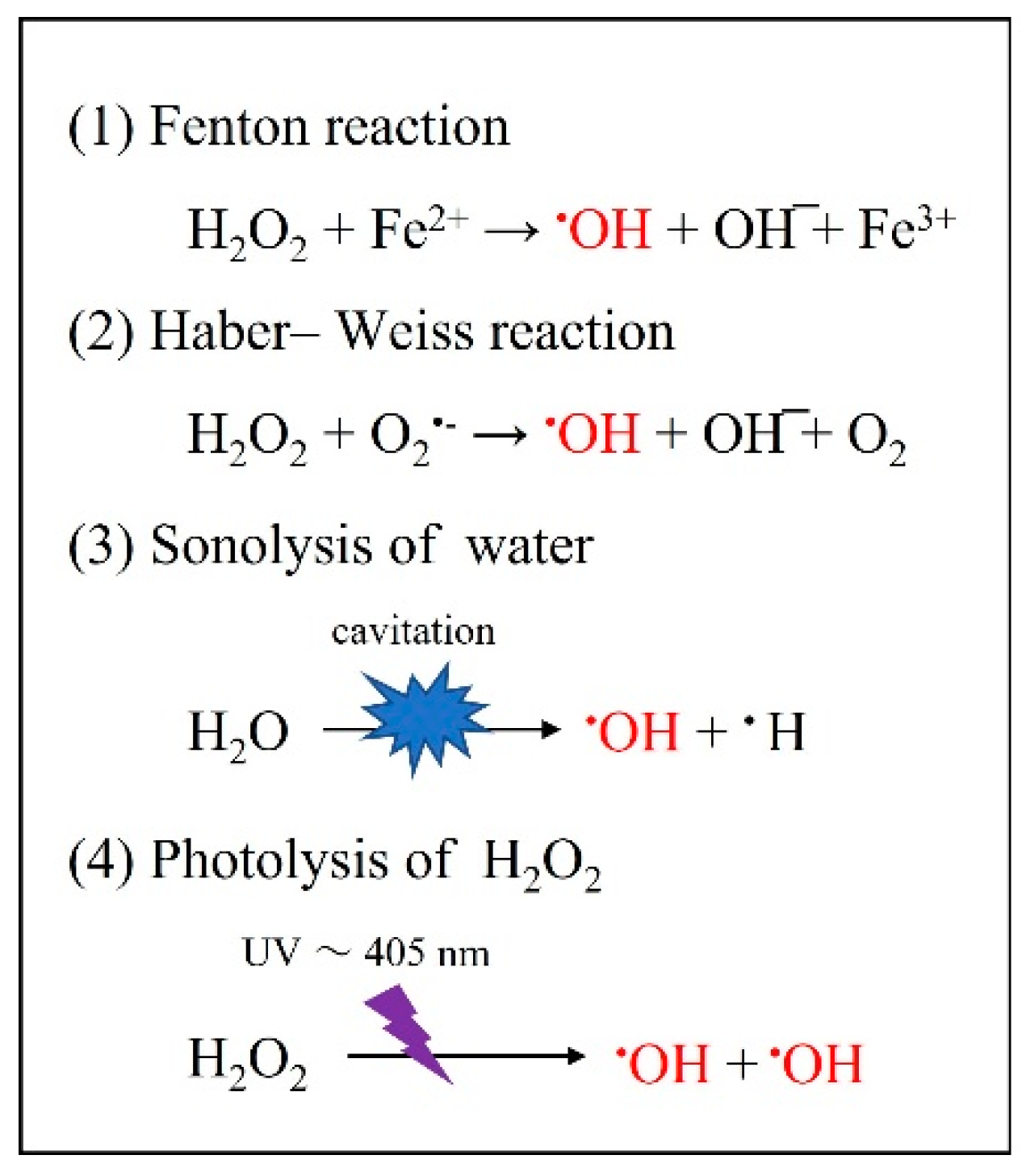
4.3. Bactericidal Effect of •OH Resulting from Other Photochemical Reactions
4.4. Effect of •OH on Smear Layer Removal
5. Discussion
6. Conclusions
- The light source and PS should be standardized, taking into account the optimal generation of ROS.
- The possible disadvantages of dental treatment after ROS exposure should be considered.
- The advantages and disadvantages to the organism induced by ROS should be familiarized.
- A-PDT should be used as an adjunct to conventional disinfection methods at this time, and when using NaClO together with a-PDT, it might be reducing the concentration of NaClO, thereby reducing harmful effects on living organisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, S.; Tang, S.-J. Reactive Oxygen Species (ROS) are Critical for Morphine Exacerbation of HIV-1 gp120-Induced Pain. J. Neuroimmune Pharmacol. 2021, 16, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palm, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Murray, T.V.A.; Ahmad, A.; Brewer, A.C. Reactive oxygen at the heart of metabolism. Trends Cardiovasc. Med. 2014, 24, 113–120. [Google Scholar] [CrossRef]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The Future Challenge of Reactive Oxygen Species (ROS) in Hypertension: From Bench to Bed Side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Chaudhary, M.; Gadbail, A.R.; Sharma, A.; Tekade, S. Oxidative and antioxidative mechanisms in oral cancer and precancer: A review. Oral Oncol. 2014, 50, 10–18. [Google Scholar] [CrossRef]
- Shilpa, B.; Ahmed, A.; Mohammed, M.; Abdulaziz, S.A.; Nassreen, H.M.A.; Apathsakayan, R.; Mazen, F.A.; Ali, R.; Asma, S.A.; Vikrant, R.P.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, M.A.B.; Khan, A.P.; Liu, H.; Siddiqui, S.; Mallidi, S.; Leon, P.; Daly, L.; Rudd, G.; Cuckov, F.; et al. Clinical evaluation of smartphone-based fluorescence imaging for guidance and monitoring of ALA-PDT treatment of early oral cancer. J. Biomed. Opt. 2020, 25, 063813. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anticancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P.; Candeias, L.P. Fenton chemistry: An introduction. Radiat. Res. 1996, 145, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Ikai, H.; Nakamura, K.; Shirato, M.; Kanno, T.; Iwasawa, A.; Sasaki, K.; Niwano, Y.; Kohno, M. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob. Agents Chemother. 2010, 54, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Nakamura, K.; Ikai, H.; Hayashi, E.; Shirato, M.; Mokudai, T.; Iwasawa, A.; Niwano, Y.; Kohno, M.; Sasaki, K. Novel denture-cleaning system based on hydroxyl radical disinfection. Int. J. Prosthodont. 2012, 25, 376–380. [Google Scholar] [PubMed]
- Sato, H.; Niwano, Y.; Nakamura, K.; Mokudai, T.; Ikai, H.; Kanno, T.; Egusa, H. Efficacy and safety of a therapeutic apparatus using hydrogen peroxide photolysis to treat dental and periodontal infectious diseases. Toxicol. Sci. 2016, 41, 793–799. [Google Scholar] [CrossRef]
- Toki, T.; Nakamura, K.; Kurauchi, M.; Kanno, T.; Katsuda, Y.; Ikai, H.; Hayashi, E.; Egusa, H.; Sasaki, K.; Niwano, Y. Synergistic interaction between wavelength of light and concentration of H2O2 in bactericidal activity of photolysis of H2O2. J. Biosci. Bioeng. 2015, 119, 358–362. [Google Scholar] [CrossRef]
- Hayashi, E.; Mokudai, T.; Yamada, Y.; Nakamura, K.; Kanno, T.; Sasaki, K.; Niwano, Y. In vitro and in vivo anti-Staphylococcus aureus activities of a new disinfection system utilizing photolysis of hydrogen peroxide. J. Biosci. Bioeng. 2012, 114, 193–197. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Ikai, H.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Photo-irradiation of proanthocyanidin as a new disinfection technique via reactive oxygen species formation. PLoS ONE 2013, 8, e60053. [Google Scholar] [CrossRef]
- Ibi, H.; Hayashi, M.; Yoshino, F.; Tamura, M.; Yoshida, A.; Kobayashi, Y.; Shimizu, K.; Lee, M.-C.; Imai, K.; Ogiso, B. Bactericidal effect of hydroxyl radicals generated by the sonolysis and photolysis of hydrogen peroxide for endodontic applications. Microb. Pathog. 2017, 103, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Tsujimoto, Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J. Endod. 2004, 30, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, G.J.; Kim, J.M.; Kil Park, J.; Lee, J.K.; Kim, G.C. Tooth bleaching with nonthermal atmospheric pressure plasma. J. Endod. 2009, 35, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Figdor, D. Apical periodontitis: A very prevalent problem. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 651–652. [Google Scholar] [CrossRef]
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Paiva, S.S.M.; Magalhães, K.M.; Guimarães-Pinto, T. Cultivable bacteria in infected root canals as identified by 16S rRNA gene sequencing. Oral Microbiol. Immunol. 2007, 22, 266–271. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Debelian, G.J.; Tronstad, L. Microbiota of Periapical Lesions Refractory to Endodontic Therapy. J. Endod. 2002, 28, 304–310. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Hube, B. Candida albicans morphology: Still in focus. Expert Rev. Anti-Infect. Ther. 2017, 15, 327–330. [Google Scholar] [CrossRef]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Guivarc’h, M.; Ordioni, U.; Ahmed, H.M.A.; Cohen, S.; Catherine, J.-H.; Bukiet, F. Sodium Hypochlorite Accident: A Systematic Review. J. Endod. 2017, 43, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Cancro, L.P.; Fischman, S.L. Hydrogen peroxide: A review of its use in dentistry. J. Periodontol. 1995, 66, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, O.; Kleier, D.J.; Averbach, R.E. Anatomy of sodium hypochlorite accidents. Compend. Contin. Educ. Dent. 2007, 28, 544–550. [Google Scholar] [PubMed]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.-Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontology 2000 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Prażmo, E.J.; Kwaśny, M.; Łapiński, M.; Mielczarek, A. Photodynamic Therapy as a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Kinoshita, J. Photodynamic Therapy in Endodontics. J. Contemp. Dent. Pract. 2017, 18, 534–538. [Google Scholar] [CrossRef]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Maisch, T.; Szeimies, R.-M.; Jori, G.; Abels, C. Antibacterial photodynamic therapy in dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef]
- Maldonado-Carmona, N.; Ouk, T.-S.; Calvete, M.J.F.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Garin, C.; Alejo, T.; Perez-Laguna, V.; Prieto, M.; Mendoza, G.; Arruebo, M.; Sebastian, V.; Rezusta, A. Chalcogenide nanoparticles and organic photosensitizers for synergetic antimicrobial photodynamic therapy. J. Mater. Chem. B 2021, 9, 6246–6259. [Google Scholar] [CrossRef] [PubMed]
- Fimple, J.L.; Fontana, C.R.; Foschi, F.; Ruggiero, K.; Song, X.; Pagonis, T.C.; Tanner, A.C.R.; Kent, R.; Doukas, A.G.; Stashenko, P.P.; et al. Photodynamic treatment of endodontic polymicrobial infection in vitro. J. Endod. 2008, 34, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Guellmar, A.; Völpel, A.; Gitter, B.; Albrecht, V.; Sigusch, B.W. Photodynamic suppression of Enterococcus faecalis using the photosensitizer mTHPC. Lasers Surg. Med. 2011, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Komine, C.; Tsujimoto, Y. A small amount of singlet oxygen generated via excited methylene blue by photodynamic therapy induces the sterilization of Enterococcus faecalis. J. Endod. 2013, 39, 411–413. [Google Scholar] [CrossRef]
- Kageyama, M.; Otsuka, I.; Komine, C. Clarification of the Sterilization Mechanism of Antimicrobial Photodynamic Therapy for Candida albicans. JSLD 2019, 29, 141–147. [Google Scholar] [CrossRef]
- Komine, C.; Konishi, Y.; Ogura, Y.; Omori, H.; Suzuki, H.; Nakajima, M.; Izawa, M.; Fuchigami, M.; Tsuzukibash, O.; Fukatsu, A.; et al. Fungicidal Effect of Antibacterial Photodynamic Therapy with Suppressing Singlet Oxygen Generation. Nihon Univ. J. Oral Sci. 2019, 45, 164–172. [Google Scholar]
- Komine, C.; Konishi, Y.; Ishii, M.; Izawa, M.; Tsujimoto, Y. The smear layer removal effect of hydroxyl radicals generated from hydrogen peroxide irradiated with 405-nm LED light. Int. J. Microdent. 2021, 12, 78–84. [Google Scholar]
- Nakanishi, M.; Konishi, Y.; Komine, C. Antifungal effect of hydroxyl radical generated from hydrogen peroxide irradiated with visible light. JJSEDP 2021, 13, 14–19. [Google Scholar] [CrossRef]
- Tanaka, M.; Kinoshita, M.; Yoshihara, Y.; Shinomiya, N.; Seki, S.; Nemoto, K.; Hirayama, T.; Dai, T.; Huang, L.; Hamblin, M.R.; et al. Optimal photosensitizers for photodynamic therapy of infections should kill bacteria but spare neutrophils. Photochem. Photobiol. 2012, 88, 227–232. [Google Scholar] [CrossRef]
- Chan, Y.; Lai, C.H. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med. Sci. 2007, 18, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C.; Stjernholm, R.L.; Steele, R.H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem. Biophys. Res. Commun. 1972, 47, 679–684. [Google Scholar] [CrossRef]
- Krinsky, N.I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science 1974, 186, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Tatsuzawa, H.; Maruyama, T.; Misawa, N.; Fujimori, K.; Hori, K.; Sano, Y.; Kambayashi, Y.; Nakano, M. Inactivation of bacterial respiratory chain enzymes by singlet oxygen. FEBS Lett. 1998, 439, 329–333. [Google Scholar] [CrossRef]
- Gonzales, F.; Maisch, T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef]
- Cló, E.; Snyder, J.W.; Ogilby, P.R.; Gothelf, K.V. Control and selectivity of photosensitized singlet oxygen production: Challenges in complex biological systems. ChemBioChem 2007, 268, 475–481. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A 1934, 147, 332–351. [Google Scholar]
- Miyaji, A.; Kohno, M.; Inoue, Y.; Baba, T. Hydroxyl radical generation by dissociation of water molecules during 1.65 MHz frequency ultrasound irradiation under aerobic conditions. Biochem. Biophys. Res. Commun. 2017, 483, 178–182. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Örtengren, U.; Lingström, P.; Niwano, Y. Antimicrobial activity of hydroxyl radicals generated by hydrogen peroxide photolysis against Streptococcus mutans biofilm. Int. J. Antimicrob. Agents 2016, 48, 373–380. [Google Scholar] [CrossRef]
- Shirato, M.; Ikai, H.; Nakamura, K.; Hayashi, E.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Synergistic effect of thermal energy on bactericidal action of photolysis of H2O2 in relation to acceleration of hydroxyl radical generation. Antimicrob. Agents Chemother. 2012, 56, 295–301. [Google Scholar] [CrossRef]
- Shirato, M.; Nakamura, K.; Tenkumo, T.; Kano, Y.; Ishiyama, K.; Kanno, T.; Sasaki, K.; Niwano, Y.; Matsuura, H. Oral mucosal irritation potential of antimicrobial chemotherapy involving hydrogen peroxide photolysis with high-power laser irradiation for the treatment of periodontitis. J. Photochem. Photobiol. B 2019, 201, 111633. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamada, Y.; Takada, Y.; Mokudai, T.; Ikai, H.; Inagaki, R.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Corrosive effect of disinfection solution containing hydroxyl radicals generated by photolysis of H(2)O(2) on dental metals. Dent. Mater. J. 2012, 31, 941–946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, K.; Shirato, M.; Tenkumo, T.; Kanno, T.; Westerlund, A.; Örtengren, U.; Sasaki, K.; Niwano, Y. Hydroxyl radicals generated by hydrogen peroxide photolysis recondition biofilm-contaminated titanium surfaces for subsequent osteoblastic cell proliferation. Sci. Rep. 2019, 9, 4688. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Harada, A.; Yamada, Y.; Odashima, Y.; Nakamura, K.; Inagaki, R.; Kanno, T.; Sasaki, K.; Niwano, Y. Influence of a new denture cleaning technique based on photolysis of H(2)O(2) the mechanical properties and color change of acrylic denture base resin. Dent. Mater. J. 2013, 32, 529–536. [Google Scholar] [CrossRef]
- Gilboe, D.B.; Svare, C.W.; Thayer, K.E.; Drennon, D.G. Dentinal smearing: An investigation of the phenomenon. J. Prosthet. Dent. 1980, 44, 310–316. [Google Scholar] [CrossRef]
- McComb, D.; Smith, D.C. A prel1m1nary scanning electron microscopic study of root canals after endodontic procedures. J. Endod. 1975, 1, 238–242. [Google Scholar] [CrossRef]
- Torabinejad, M.; Handysides, R.; Khademi, A.A.; Bakland, L.K. Clinical implications of the smear layer in endodontics: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 658–666. [Google Scholar] [CrossRef]
- Kozuka, M.; Tsujimoto, Y.; Ishizaki, T.; Aiura, S.; Nakamura, T.; Yamazaki, M. Observation of smear layer after root canal irrigation by Cu2+/H2O2—Investigation of treatment time. J. Jpn. Endod. Assoc. 1999, 20, 11–17. [Google Scholar] [CrossRef]
- Kozuka, M.; Kawamoto, K.; Tsujimoto, Y.; Yamazaki, M. Root canal treatment using active oxygen (2 nd report)—Observation of smear layer after root canal irrigation by CuCl2 solutio. J. Jpn. Endod. Assoc. 1999, 20, 87–90. [Google Scholar] [CrossRef]
- Kozuka, M.; Tsujimoto, Y.; Miura, H.; Kawamoto, K.; Ueda, I.; Yamazaki, M. The study on root canal irrigation using Cu2+/H2O2 mixed solution—Prevention effect of dye penetration by hyaloid structure. J. Jpn. Endod. Assoc. 2000, 21, 12–16. [Google Scholar]
- Tsujimoto, Y.; Kozuka, M.; Hitotsune, N.; Yamazaki, M. Removal capability of smear layer of root canal by NaClO and H2O2 mixed solution. J. Jpn. Endod. Assoc. 1997, 18, 19–24. [Google Scholar] [CrossRef]
- Izawa, M.; Tsujimoto, Y.; Matsushima, K. Effective EDTA Concentration for Removing Smear Layer and Smear Plugs After Using Ultrasonic Tips in Microendodontic Therapy. Int. J. Microdent. 2013, 4, 114–120. [Google Scholar]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2020, 36, 279–295. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell. 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Abe, R.; Endo, T.; Shimooka, S. Effects of tooth bleaching on shear bond strength of brackets rebonded with a self-etching adhesive system. Odontology 2011, 99, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kashima, M. Effects of catechins on superoxide and hydroxyl radical. Chem. Pharm. Bull. 1999, 47, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, A. Characterization of reactive oxygen species generated from the mixture of NaClO and H2O2 used as root canal irrigants. J. Endod. 2000, 26, 11–15. [Google Scholar] [CrossRef]
- Wada, Y.; Tsujimoto, Y. The dissolution effect of sodium hypochlorite and denaturation of the structure of (Pro-Pro-Gly) 10 using electron spin resonance and nuclear magnetic resonance. Int. J. Microdent. 2012, 3, 75–83. [Google Scholar] [CrossRef]
- Cuppini, M.; Leitune, V.C.B.; Souza, M.; Alves, A.K.; Samuel, S.M.W.; Collares, F.M. In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent. Mater. J. 2019, 38, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Komine, C.; Takahashi, C.; Omori, H.; Ogura, Y.; Konishi, Y.; Suzuki, H.; Issei, O.; Fuchigami, M.; Tsuzukibashi, O.; Fukatsu, A.; et al. Bactericidal Effect of Antimicrobial Photodynamic Therapy Using Visible Light-responsive Titanium Dioxide -the First Report-. Int. J. Oral-Med. Sci. 2020, 18, 155–163. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implant. 2014, 29, e95–e102. [Google Scholar] [CrossRef]
- Omori, H.; Komine, C. Development of a Photodynamic Diagnosis Method for Oral Squamous Cell Carcinoma Using 5-Aminolevulinic Acid and a Luminescence Plate Reader. Open J. Stomatol. 2021, 11, 325–340. [Google Scholar] [CrossRef]
- Komine, C.; Omori, H.; Ogura, Y.; Konishi-Takahashi, Y.; Asaka, K.; Ono, Y.; Fuchigami, M.; Tsuzukibashi, O.; Fukatsu, A.; Fukumoto, M. Establishment of the method for objective screening of oral malignant tumors using photodynamic technique by a fluorescence plate reader. JJSDEP 2022, 14, 17–26. [Google Scholar] [CrossRef]
- Asaka, K.; Fukatsu, A.; Komine, C. The Effectiveness of a Portable Fluorescence Spectrophotometer for Early Detection of Oral Cancer. Open J. Stomatol. 2022, 12, 30–31. [Google Scholar] [CrossRef]
- Gomez, J. Detection and diagnosis of the early caries lesion. BMC Oral Health 2015, 15 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komine, C.; Uchibori, S.; Tsudukibashi, O.; Tsujimoto, Y. Application of Reactive Oxygen Species in Dental Treatment. J. Pers. Med. 2022, 12, 1531. https://doi.org/10.3390/jpm12091531
Komine C, Uchibori S, Tsudukibashi O, Tsujimoto Y. Application of Reactive Oxygen Species in Dental Treatment. Journal of Personalized Medicine. 2022; 12(9):1531. https://doi.org/10.3390/jpm12091531
Chicago/Turabian StyleKomine, Chiaki, Satoshi Uchibori, Osamu Tsudukibashi, and Yasuhisa Tsujimoto. 2022. "Application of Reactive Oxygen Species in Dental Treatment" Journal of Personalized Medicine 12, no. 9: 1531. https://doi.org/10.3390/jpm12091531
APA StyleKomine, C., Uchibori, S., Tsudukibashi, O., & Tsujimoto, Y. (2022). Application of Reactive Oxygen Species in Dental Treatment. Journal of Personalized Medicine, 12(9), 1531. https://doi.org/10.3390/jpm12091531




