Bioradiotherapy with Cetuximab May Reduce the Risk of Neck Node Relapse in Locoregionally Advanced Laryngeal Glottic Carcinoma: May HER1-Profile Be Useful in the Bioselection of Patients?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Survival Analysis According to Primary Treatments
3.2. Survival Rates According to HER1 Expression
3.3. Multivariate Analysis
3.4. Analyses of Oncological Results after Salvage Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolf, G.T.; Bellile, E.; Eisbruch, A.; Urba, S.; Bradford, C.R.; Peterson, L.; Prince, M.E.; Teknos, T.N.; Chepeha, D.B.; Hogikyan, N.D.; et al. Survival Rates Using Individualized Bioselection Treatment Methods in Patients With Advanced Laryngeal Cancer. JAMA Otolaryngol.—Head Neck Surg. 2017, 143, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Swisher-McClure, S.; Mitra, N.; Li, J.; Cohen, R.B.; Ahn, P.H.; Lukens, J.N.; Chalian, A.A.; Weinstein, G.S.; O’Malley, B.W.; et al. Total Laryngectomy Versus Larynx Preservation for T4a Larynx Cancer: Patterns of Care and Survival Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Ismaila, N.; Lewin, J.S.; Nathan, C.A.; Adelstein, D.J.; Eisbruch, A.; Fass, G.; Fisher, S.G.; Laurie, S.A.; Le, Q.-T.; et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1143–1169. [Google Scholar] [CrossRef]
- Pfister, D.G.; Laurie, S.A.; Weinstein, G.S.; Mendenhall, W.M.; Adelstein, D.J.; Ang, K.K.; Clayman, G.L.; Fisher, S.G.; Forastiere, A.A.; Harrison, L.B.; et al. American Society of Clinical Oncology Clinical Practice Guideline for the Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer. J. Clin. Oncol. 2006, 24, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.; Bourhis, J.; Domenge, C.; Designé, L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. Lancet 2000, 355, 949–955. [Google Scholar] [CrossRef]
- Budach, W.; Hehr, T.; Budach, V.; Belka, C.; Dietz, K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006, 6, 28. [Google Scholar] [CrossRef]
- Baxi, S.S.; O’Neill, C.; Sherman, E.J.; Atoria, C.L.; Lee, N.Y.; Pfister, D.G.; Elkin, E.B. Trends in chemoradiation use in elderly patients with head and neck cancer: Changing treatment patterns with cetuximab. Head Neck 2016, 38, E165–E171. [Google Scholar] [CrossRef]
- Bonner, J.; Giralt, J.; Harari, P.; Spencer, S.; Schulten, J.; Hossain, A.; Chang, S.-C.; Chin, S.; Baselga, J. Cetuximab and Radiotherapy in Laryngeal Preservation for Cancers of the Larynx and Hypopharynx: A Secondary Analysis of a Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 842–849. [Google Scholar] [CrossRef]
- Lefebvre, J.L.; Pointreau, Y.; Rolland, F.; Alfonsi, M.; Baudoux, A.; Sire, C.; de Raucourt, D.; Malard, O.; Degardin, M.; Tuchais, C.; et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. J. Clin. Oncol. 2013, 31, 853–859. [Google Scholar] [CrossRef]
- Janoray, G.; Pointreau, Y.; Alfonsi, M.; Sire, C.; Geoffrois, L.; de Raucourt, D.; Bardet, E.; Calais, M.-H.; Garaud, P.; Calais, G. Induction chemotherapy followed by cisplatin or cetuximab concomitant to radiotherapy for laryngeal/hypopharyngeal cancer: Long-term results of the TREMPLIN randomised GORTEC trial. Eur. J. Cancer 1990 2020, 133, 86–93. [Google Scholar] [CrossRef]
- Husain, Z.A.; Burtness, B.A.; Decker, R.H. Cisplatin Versus Cetuximab With Radiotherapy in Locally Advanced Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2016, 34, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Holsinger, F.C.; Colevas, A.D.; Chen, M.M.; Le, Q.T.; Beadle, B.M. Survival of patients with head and neck cancer treated with definitive radiotherapy and concurrent cisplatin or concurrent cetuximab: A Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2018, 124, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Dietz, A.; Wichmann, G.; Kuhnt, T.; Pfreundner, L.; Hagen, R.; Scheich, M.; Kölbl, O.; Hautmann, M.G.; Strutz, J.; Schreiber, F.; et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann. Oncol. 2018, 29, 2105–2114. [Google Scholar] [CrossRef]
- Gebre-Medhin, M.; Brun, E.; Engström, P.; Haugen Cange, H.; Hammarstedt-Nordenvall, L.; Reizenstein, J.; Nyman, J.; Abel, E.; Friesland, S.; Sjödin, H.; et al. ARTSCAN III: A Randomized Phase III Study Comparing Chemoradiotherapy With Cisplatin Versus Cetuximab in Patients With Locoregionally Advanced Head and Neck Squamous Cell Cancer. J. Clin. Oncol. 2021, 39, 38–47. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Riboldi, V.; Borgonovo, K.; Ghilardi, M.; Cabiddu, M.; Lonati, V.; Sarti, E.; Barni, S. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta-analysis of published studies. Oral Oncol. 2014, 50, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Barney, C.L.; Walston, S.; Zamora, P.; Healy, E.H.; Nolan, N.; Diavolitsis, V.M.; Neki, A.; Rupert, R.; Savvides, P.; Agrawal, A.; et al. Clinical outcomes and prognostic factors in cisplatin versus cetuximab chemoradiation for locally advanced p16 positive oropharyngeal carcinoma. Oral Oncol. 2018, 79, 9–14. [Google Scholar] [CrossRef]
- Maddalo, M.; Borghetti, P.; Tomasini, D.; Corvò, R.; Bonomo, P.; Petrucci, A.; Paiar, F.; Lastrucci, L.; Bonù, M.L.; Greco, D.; et al. Cetuximab and Radiation Therapy Versus Cisplatin and Radiation Therapy for Locally Advanced Head and Neck Cancer: Long-Term Survival and Toxicity Outcomes of a Randomized Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 469–477. [Google Scholar] [CrossRef]
- Caudell, J.J.; Sawrie, S.M.; Spencer, S.A.; Desmond, R.A.; Carroll, W.R.; Peters, G.E.; Nabell, L.M.; Meredith, R.F.; Bonner, J.A. Locoregionally Advanced Head and Neck Cancer Treated With Primary Radiotherapy: A Comparison of the Addition of Cetuximab or Chemotherapy and the Impact of Protocol Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Koutcher, L.; Sherman, E.; Fury, M.; Wolden, S.; Zhang, Z.; Mo, Q.; Stewart, L.; Schupak, K.; Gelblum, D.; Wong, R.; et al. Concurrent Cisplatin and Radiation Versus Cetuximab and Radiation for Locally Advanced Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.J.; Trotti, A.M.; Kish, J.; Russell, J.S.; Rao, N.G.; McCaffrey, J.; Padhya, T.A.; Otto, K.J.; Caudell, J.J. Comparison of every 3 week cisplatin or weekly cetuximab with concurrent radiotherapy for locally advanced head and neck cancer. Oral Oncol. 2015, 51, 704–708. [Google Scholar] [CrossRef]
- Levy, A.; De Felice, F.; Bellefqih, S.; Guigay, J.; Deutsch, E.; Nguyen, F.; Blanchard, P.; Tao, Y. Toxicity of concomitant cetuximab and radiotherapy with or without initial taxane-based induction chemotherapy in locally advanced head and neck cancer. Head Neck 2016, 38, E905–E910. [Google Scholar] [CrossRef]
- Tang, C.; Chan, C.; Jiang, W.; Murphy, J.D.; von Eyben, R.; Colevas, A.D.; Pinto, H.; Lee-Enriquez, N.; Kong, C.; Le, Q.-T. Concurrent cetuximab versus platinum-based chemoradiation for the definitive treatment of locoregionally advanced head and neck cancer. Head Neck 2015, 37, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Almadori, G.; Coli, A.; De Corso, E.; Mele, D.A.; Settimi, S.; Di Cintio, G.; Brigato, F.; Scannone, D.; Carey, T.E.; Paludetti, G.; et al. Nuclear HER3 expression improves the prognostic stratification of patients with HER1 positive advanced laryngeal squamous cell carcinoma. J. Transl. Med. 2021, 19, 408. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-2438-9. [Google Scholar]
- Xie, J.; Liu, C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat. Med. 2005, 24, 3089–3110. [Google Scholar] [CrossRef]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Sorgini, A.; Kim, H.A.J.; Zeng, P.Y.F.; Shaikh, M.H.; Mundi, N.; Ghasemi, F.; Di Gravio, E.; Khan, H.; MacNeil, D.; Khan, M.I.; et al. Analysis of the TCGA Dataset Reveals that Subsites of Laryngeal Squamous Cell Carcinoma Are Molecularly Distinct. Cancers 2020, 13, 105. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Lauxen, I.S.; Filho, M.S.A.; Nör, J.E. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014, 74, 2869–2881. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, L.; Jayakar, S.K.; Ow, T.J.; Segall, J.E. Mechanisms of Invasion in Head and Neck Cancer. Arch. Pathol. Lab. Med. 2015, 139, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lun, L.; Jiang, X.; Wang, Y.; Li, X.; Du, G.; Wang, J. APE1 facilitates PD-L1-mediated progression of laryngeal and hypopharyngeal squamous cell carcinoma. Int. Immunopharmacol. 2021, 97, 107675. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, M.; Almadori, G.; Ferrandina, G.; Distefano, M.; Romanini, M.E.; Cadoni, G.; Benedetti-Panici, P.; Paludetti, G.; Scambia, G.; Mancuso, S. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br. J. Cancer 1996, 74, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.R.; Carvalho, A.L.; Longatto Filho, A.; Scorsato, A.P.; López, R.V.M.; Rautava, J.; Syrjänen, S.; Syrjänen, K. Detection of human papillomavirus in laryngeal squamous cell carcinoma: Systematic review and meta-analysis. Laryngoscope 2016, 126, 885–893. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Chernock, R.D.; Wang, X.; Gao, G.; Lewis, J.S.; Zhang, Q.; Thorstad, W.L.; El-Mofty, S.K. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod. Pathol. 2013, 26, 223–231. [Google Scholar] [CrossRef]
- Scambia, G.; Panici, P.B.; Battaglia, F.; Ferrandina, G.; Almadori, G.; Paludetti, G.; Maurizi, M.; Mancuso, S. Receptors for epidermal growth factor and steroid hormones in primary laryngeal tumors. Cancer 1991, 67, 1347–1351. [Google Scholar] [CrossRef]
- Almadori, G.; Cadoni, G.; Galli, J.; Ferrandina, G.; Scambia, G.; Exarchakos, G.; Paludetti, G.; Ottaviani, F. Epidermal growth factor receptor expression in primary laryngeal cancer: An independent prognostic factor of neck node relapse. Int. J. Cancer 1999, 84, 188–191. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Zhang, W.; He, J.; Zhao, Y.; Chen, X. Prognostic role of epidermal growth factor receptor in head and neck cancer: A meta-analysis: EGFR and Head and Neck Cancer. J. Surg. Oncol. 2013, 108, 387–397. [Google Scholar] [CrossRef]
- Almadori, G.; Bussu, F.; Gessi, M.; Ferrandina, G.; Scambia, G.; Lauriola, L.; Paludetti, G.; Ranelletti, F.O. Prognostic significance and clinical relevance of the expression of the HER family of type I receptor tyrosine kinases in human laryngeal squamous cell carcinoma. Eur. J. Cancer 2010, 46, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Madoz-Gúrpide, J.; Zazo, S.; Chamizo, C.; Casado, V.; Caramés, C.; Gavín, E.; Cristóbal, I.; García-Foncillas, J.; Rojo, F. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J. Transl. Med. 2015, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Andratschke, N.H.; Milas, L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. The EGFR as a target for anticancer therapy—Focus on cetuximab. Eur. J. Cancer 2001, 37 (Suppl. 4), 16–22. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Atasoy, B.M.; Daley, F.M.; Dische, S.; Richman, P.I.; Saunders, M.I.; Trott, K.R.; Wilson, G.D. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J. Clin. Oncol. 2005, 23, 5560–5567. [Google Scholar] [CrossRef]
- Liang, K.; Ang, K.K.; Milas, L.; Hunter, N.; Fan, Z. The epidermal growth factor receptor mediates radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 246–254. [Google Scholar] [CrossRef]
- Lu, H.; Liang, K.; Lu, Y.; Fan, Z. The anti-EGFR antibody cetuximab sensitizes human head and neck squamous cell carcinoma cells to radiation in part through inhibiting radiation-induced upregulation of HIF-1α. Cancer Lett. 2012, 322, 78–85. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Do, N.; Lim, S.; Im, T. Expression of c-erbB receptors, MMPs and VEGF in squamous cell carcinoma of the head and neck. Oncol. Rep. 2004, 12, 229–237. [Google Scholar] [CrossRef]
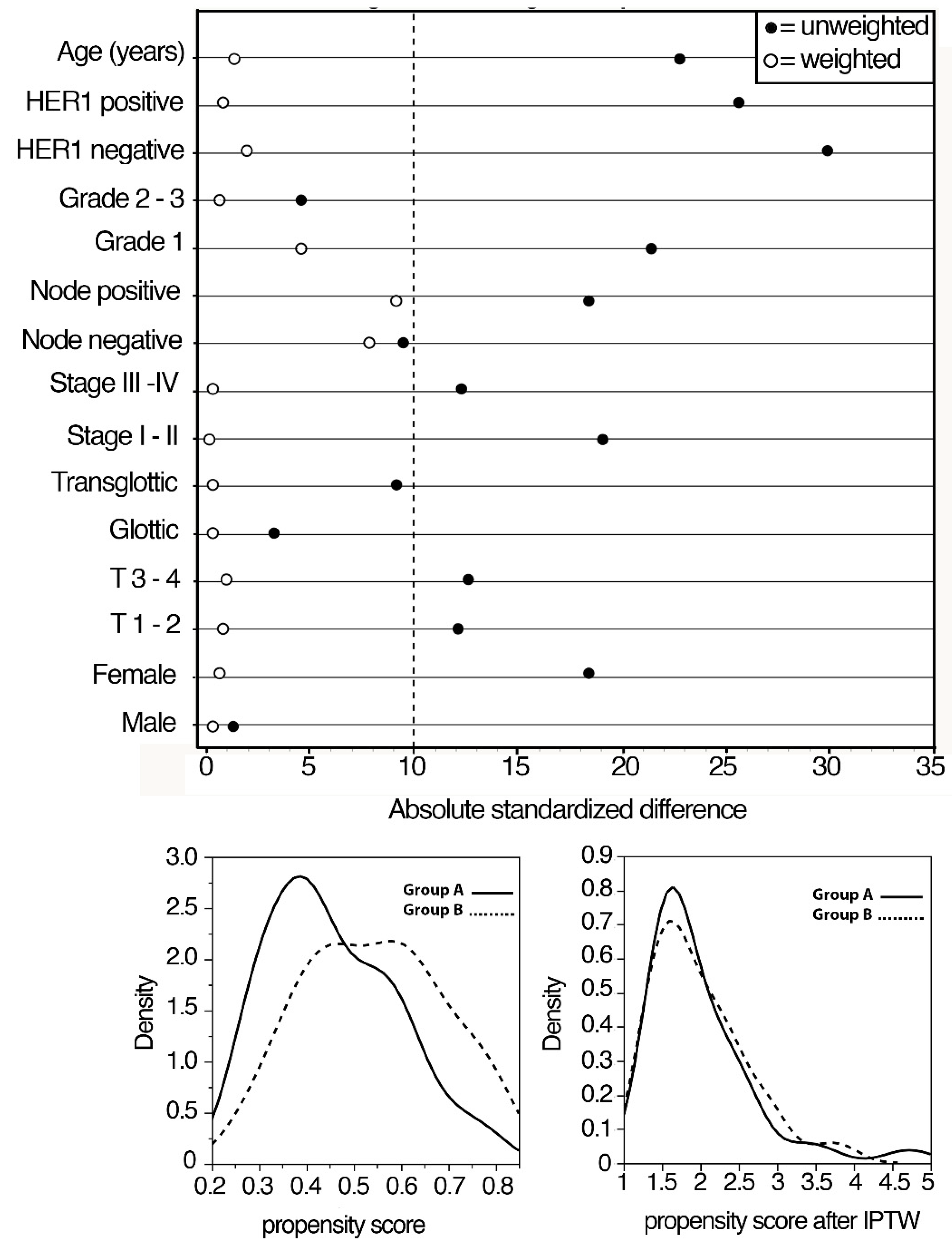
| CHARACTERISTIC | GROUP A (n = 66) | GROUP B (n = 66) | p |
|---|---|---|---|
| FOLLOW-UP (years) Median (range) | 30 (5–139) | 47 (2–91) | |
| AGE Median (Range) | 65 (40–86) | 62 (39–80) | |
| SEX: Female | 5 (8%) | 4 (6%) | |
| Male | 61 (92%) | 62 (94%) | 1.0 (1) |
| GRGRADE: 1 | 13 (20%) | 10 (15%) | |
| 2 | 22 (33%) | 18 (27%) | |
| 3 | 31 (47%) | 38 (58%) | 0.47 (2) |
| TUMOR SITE: Glottic | 47 (71%) | 49 (74%) | |
| Transglottic | 19 (29%) | 17 (26%) | 0.84 |
| T CLASSIFICATION: 2 | 36 (54%) | 31 (47%) | |
| 3 | 19 (29%) | 20 (30%) | |
| 4 | 11 (17%) | 15 (23%) | 0.60 |
| STAGE: II | 29 (44%) | 23 (35%) | |
| III | 15 (23%) | 25 (38%) | |
| IV | 22 (33%) | 18 (27%) | 0.17 |
| NODAL STATUS: 0 | 45 (68%) | 41 (62%) | |
| 1 | 5 (8%) | 15 (23%) | |
| 2 | 16 (24%) | 10 (15%) | 0.03 |
| HER1 STATUS: Negative | 25 (38%) | 36 (55%) | |
| Positive | 41 (62%) | 30 (45%) | 0.08 |
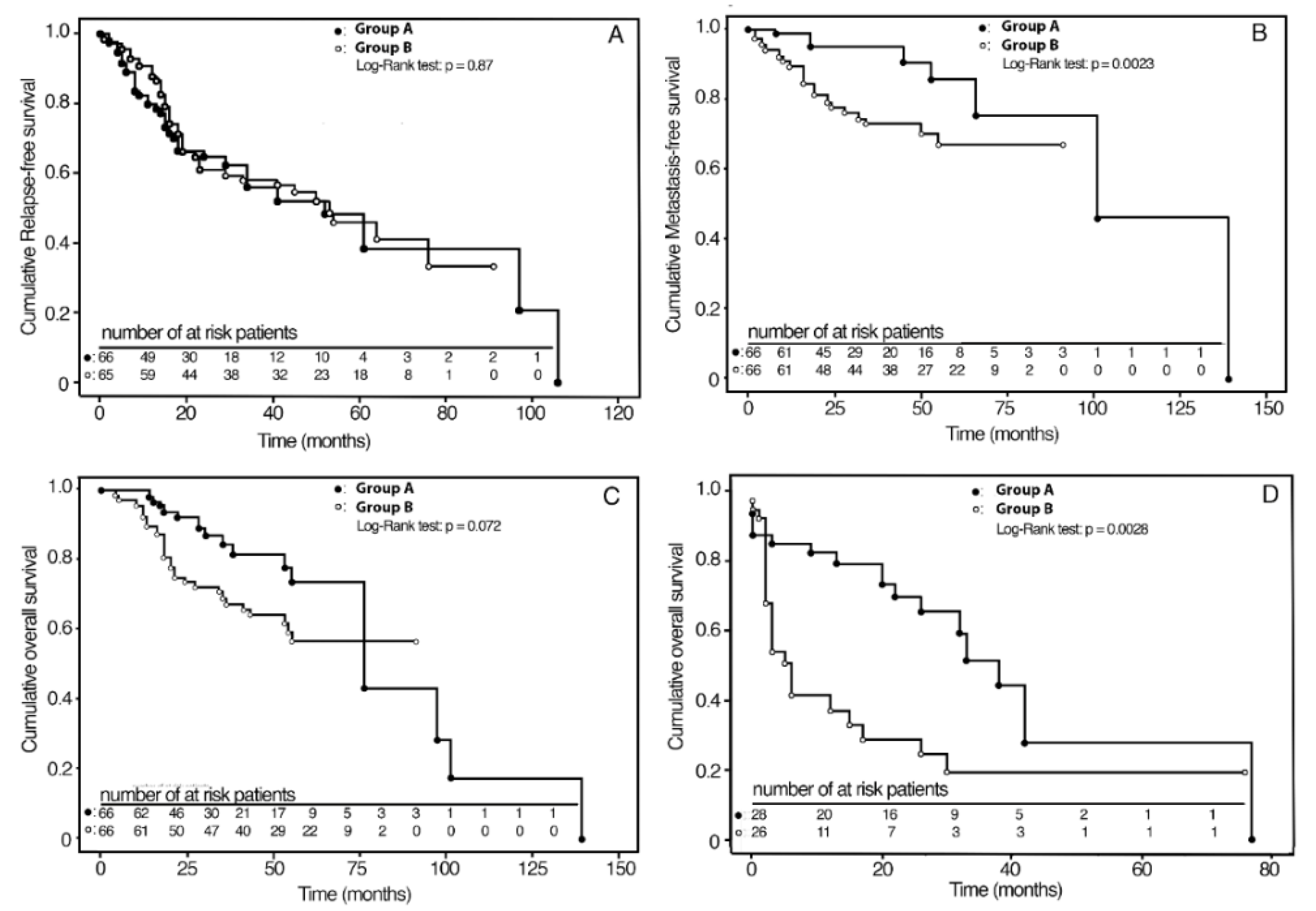
| KAPLAN–MEIER SURVIVAL ESTIMATES | UNWEIGHTED | p 1 | IPWT-ATE 2 | ABSOLUTE RISK REDUCTION | p 3 |
|---|---|---|---|---|---|
| 3-year RFS: Group A | 56% (41–70) 4 | 54% (38–70) | |||
| Group B | 63% (51–75) | 0.32 | 58% (45–71) | 4% (−2–24) | 0.72 |
| 3-year MFS: Group A | 94% (89–100) | 95% (87–99) | |||
| Group B | 74% (63–85) | 0.04 | 73% (60–83) | 22% (9–35) | 0.0008 |
| 5-year OS: Group A | 69% (52–85) | 73% (58–87) | |||
| Group B | 60% (47–74) | 0.25 | 56% (42–70) | 17% (4–36) | 0.10 |
| 3-Year RFS | 3-Year MFS | 5-Year OS | |
|---|---|---|---|
| Group A | Stage II: 62% (45–79) 1 | Stage II: 100% | Stage II: 66% (36–96) |
| Stage III–IV: 49% (26–72) | Stage III–IV: 92% (83–100) | Stage III–IV: 76% (58–93) | |
| Group B | Stage II: 59% (37–78) | Stage II: 86% (72–100) | Stage II: 61% (40–82) |
| Stage III–IV: 57% (40–75) | Stage III–IV: 63% (45–79) | Stage III–IV: 56% (39–72) | |
| Absolute probability reduction | Stage II: 3% (−37–24) | Stage II: 14% (1–28) | Stage II: 5% (−42–31) |
| Stage III–IV: 8% (−19–36) | Stage III–IV: 29% (12–48) | Stage III–IV: 20% (5–46) | |
| Adjusted log-rank test p= | Stage II: 0.82 | Stage II: 0.07 | Stage II: 0.78 |
| Stage III–IV: 0.55 | Stage III–IV: 0.002 | Stage III–IV: 0.12 |
| Kaplan–Meier Survival Estimates | HER1 Positive (n = 71) | Absolute Probability Reduction (p) | HER1 Negative (n = 61) | Absolute Probability Reduction (p) |
|---|---|---|---|---|
| 3-year MFS | Group A: 89% (76–98) 1 Group B: 59% (42–78) | 30% (6–50) p 2 = 0.01 | Group A: 99% (96–100) Group B: 87% (76–98) | 12% (2–90) p = 0.04 |
| n | RFS (RR 1-C.I. 95%-p 2) | MFS (RR- C.I. 95%-p) | OS (RR- C.I. 95%-p) | |
|---|---|---|---|---|
| Age (per year) | 132 | 0.98 (0.95.−1.00); p = 0.11 | 0.97 (0.26–1.18); p = 0.14 | 0.95 (0.92–0.99); p = 0.016 |
| Gender: Female | 9 | 1 | 1 | 1 |
| Male | 123 | 2.19 (0.51–9.45); p = 0.29 | 0.55 (0.26–1.18); p = 0.12 | 2.25 (0.49–10.4); p = 0.30 |
| Grade: 1 | 23 | 1 | 1 | 1 |
| 2–3 | 109 | 1.31 (0.60–2.90); p = 0.49 | 0.70 (0.18–2.61); p = 0.59 | 1.17 (0.34–4.0); p = 0.80 |
| Tumor site: glottic | 96 | 1 | 1 | 1 |
| transglottic | 36 | 2.39 (1.31–4.34); p = 0.043 | 0.39 (0.11–1.39); p = 0.15 | 2.10 (0.96–4.43); p = 0.064 |
| T-classification: 2 | 67 | 1 | 1 | 1 |
| 3–4 | 65 | 1.05 (0.48–2.33); p = 0.90 | 3.97 (0.67–23.5); p = 0.13 | 0.62 (0.27–1.45); p = 0.27 |
| Stage: II | 52 | 1 | 1 | 1 |
| III–IV | 80 | 0.85 (0.37–1.99); p = 0.71 | 1.41 (0.22–8.92); p = 0.71 | 2.10 (0.74–5.80); p = 0.17 |
| Nodal status: negative | 87 | 1 | 1 | 1 |
| positive | 45 | 1.38 (0.70–2.70); p = 0.35 | 1.81 (0.55–5.90); p = 0.33 | 2.92 (1.24–6.85); p = 0.014 |
| HER1 Status: negative | 61 | 1 | 1 | 1 |
| positive | 71 | 3.99 (1.97–8.09); p = 0.0001 | 6.71 (2.39–18.8); p = 0.0003 | 5.90(2.41–14.5); p = 0.0001 |
| Therapy: CTX + RT | 66 | 1 | 1 | 1 |
| Surgery+ PORT | 66 | 0.79 (0.46–1.34); p = 0.38 | 4.98 (1.46–16.9); p = 0.010 | 2.61 (1.32–5.14); p = 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almadori, G.; Coli, A.; De Corso, E.; Settimi, S.; Mele, D.A.; Brigato, F.; Scannone, D.; Galli, J.; Valentini, V.; Paludetti, G.; et al. Bioradiotherapy with Cetuximab May Reduce the Risk of Neck Node Relapse in Locoregionally Advanced Laryngeal Glottic Carcinoma: May HER1-Profile Be Useful in the Bioselection of Patients? J. Pers. Med. 2022, 12, 1489. https://doi.org/10.3390/jpm12091489
Almadori G, Coli A, De Corso E, Settimi S, Mele DA, Brigato F, Scannone D, Galli J, Valentini V, Paludetti G, et al. Bioradiotherapy with Cetuximab May Reduce the Risk of Neck Node Relapse in Locoregionally Advanced Laryngeal Glottic Carcinoma: May HER1-Profile Be Useful in the Bioselection of Patients? Journal of Personalized Medicine. 2022; 12(9):1489. https://doi.org/10.3390/jpm12091489
Chicago/Turabian StyleAlmadori, Giovanni, Antonella Coli, Eugenio De Corso, Stefano Settimi, Dario Antonio Mele, Francesca Brigato, Domenico Scannone, Jacopo Galli, Vincenzo Valentini, Gaetano Paludetti, and et al. 2022. "Bioradiotherapy with Cetuximab May Reduce the Risk of Neck Node Relapse in Locoregionally Advanced Laryngeal Glottic Carcinoma: May HER1-Profile Be Useful in the Bioselection of Patients?" Journal of Personalized Medicine 12, no. 9: 1489. https://doi.org/10.3390/jpm12091489
APA StyleAlmadori, G., Coli, A., De Corso, E., Settimi, S., Mele, D. A., Brigato, F., Scannone, D., Galli, J., Valentini, V., Paludetti, G., Lauriola, L., & Ranelletti, F. O. (2022). Bioradiotherapy with Cetuximab May Reduce the Risk of Neck Node Relapse in Locoregionally Advanced Laryngeal Glottic Carcinoma: May HER1-Profile Be Useful in the Bioselection of Patients? Journal of Personalized Medicine, 12(9), 1489. https://doi.org/10.3390/jpm12091489










