Left Ventricular Unloading in Acute on Chronic Heart Failure: From Statements to Clinical Practice
Abstract
:1. Introduction
2. CASE Description
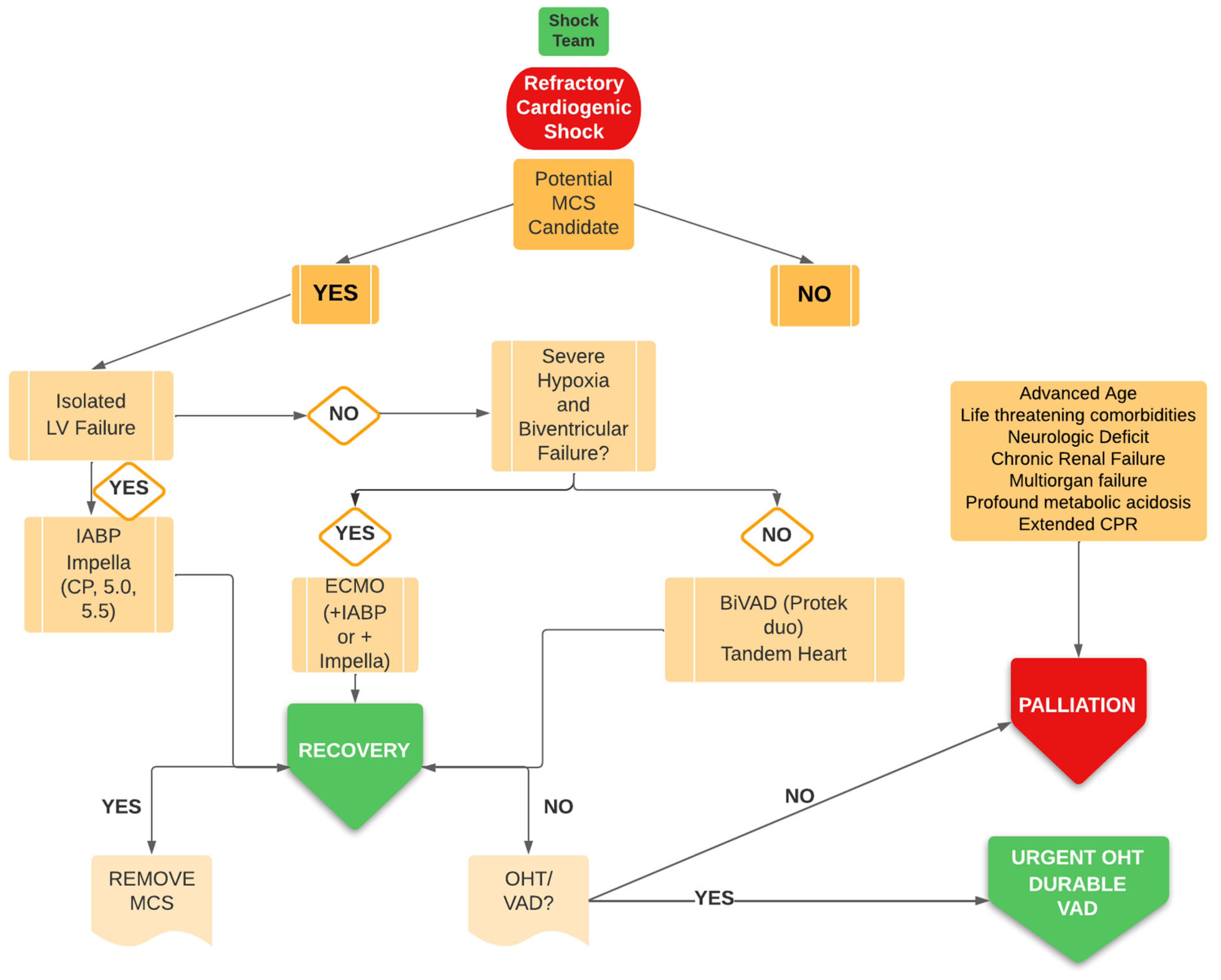
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, D.D.; Bohula, E.A.; van Diepen, S.; Katz, J.N.; Alviar, C.L.; Baird-Zars, V.M.; Barnett, C.F.; Barsness, G.W.; Burke, J.A.; Cremer, P.C.; et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005618. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Blumer, V.; Burkhoff, D.; Pahuja, M.; Sinha, S.S.; Rosner, C.; Vorovich, E.; Grafton, G.; Bagnola, A.; Hernandez-Montfort, J.A.; et al. Heart Failure-Related Cardiogenic Shock: Pathophysiology, Evaluation and Management Considerations: Review of Heart Failure-Related Cardiogenic Shock. J. Card. Fail 2021, 27, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar]
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.; Antohi, E.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Hear. Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef]
- Zweck, E. Thayer, K.L.; Helgestad, O.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Kapur, N.K. Phenotyping Cardiogenic Shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef]
- Geller, B.J.; Sinha, S.S.; Kapur, N.K.; Bakitas, M.; Balsam, L.B.; Chikwe, J.; Klein, D.G.; Kochar, A.; Masri, S.C.; Sims, D.B.; et al. Escalating and De-escalating Temporary Mechanical Circulatory Support in Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e50–e68. [Google Scholar] [CrossRef]
- Kubiak, G.; Ciarka, A.; Ceranowicz, P. Right Heart Catheterization—Background, Physiological Basics, and Clinical Implications. J. Clin. Med. 2019, 8, 1331. [Google Scholar] [CrossRef]
- Rossello, X.; Vila, M.; Rivas-Lasarte, M.; Ferrero-Gregori, A.; Sans-Roselló, J.; Duran-Cambra, A.; Sionis, A. Impact of Pulmonary Artery Catheter Use on Short- and Long-Term Mortality in Patients with Cardiogenic Shock. Cardiology 2016, 136, 61–69. [Google Scholar] [CrossRef]
- Bertaina, M.; Galluzzo, A.; Morici, N.; Sacco, A.; Oliva, F.; Valente, S.; D’Ascenzo, F.; Frea, S.; Sbarra, P.; Petitti, E.; et al. Pulmonary Artery Catheter Monitoring in Patients with Cardiogenic Shock: Time for a Reappraisal? Card Fail Rev. 2022, 8, e15. [Google Scholar] [CrossRef]
- Bertaina, M.; Galluzzo, A.; Rossello, X.; Sbarra, P.; Petitti, E.; Prever, S.B.; Boccuzzi, G.; D’Ascenzo, F.; Frea, S.; Pidello, S.; et al. Prognostic implications of pulmonary artery catheter monitoring in patients with cardiogenic shock: A systematic review and meta-analysis of observational studies. J. Crit. Care 2022, 69, 154024. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Garan, A.R.; Kapur, N.K.; O’Neill, W.W.; Lindenfeld, J.; Pinney, S.P.; Uriel, N.; Burkhoff, D.; Kern, M. Value of Hemodynamic Monitoring in Patients with Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation 2020, 141, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, L.F.; Pappalardo, F.; Lubos, E.; Grahn, H.; Rybczinski, M.; Barten, M.J.; Legros, T.; Bertoglio, L.; Schrage, B.; Westermann, D.; et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J. Crit. Care 2020, 57, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Jablonski, J.; Kras, A.; Krasney, S.; Kapur, N.K. Maximum level of mobility with axillary deployment of the Impella 5.0 is associated with improved survival. Int. J. Artif. Organs 2018, 41, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Patel, C.; Mercado-Alamo, A.; Schreiber, T.; Kaki, A. A Review of the Impella Devices. Interv. Cardiol. Rev. Res. Resour. 2022, 17, e05. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Parissis, H.; Graham, V.; Lampridis, S.; Lau, M.; Hooks, G.; Mhandu, P.C.; Parissis, H.; Graham, V.; Lampridis, S.; Lau, M.; et al. IABP: History-evolution-pathophysiology-indications: What we need to know. J. Cardiothorac. Surg. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Sjauw, K.D.; Engstrom, A.E.; Vis, M.M.; van der Schaaf, R.J.; Baan, J., Jr.; Koch, K.T.; Henriques, J.P. A systematic review and meta-analysis of intraaortic balloon pump therapy in ST-elevation myocardial infarction: Should we change the guidelines? Eur. Heart J. 2009, 30, 459–468. [Google Scholar] [CrossRef]
- Morici, N.; Oliva, F.; Ajello, S.; Stucchi, M.; Sacco, A.; Cipriani, M.; De Bonis, M.; Garascia, A.; Gagliardone, M.P.; Melisurgo, G.; et al. Management of cardiogenic shock in acute decompensated chronic heart failure: The ALTSHOCK phase II clinical trial. Am. Hear. J. 2018, 204, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Uil, C.A.D.; Van Mieghem, N.M.; Bastos, M.B.; Jewbali, L.S.; Lenzen, M.J.; Engstrom, A.E.; Bunge, J.J.; Brugts, J.J.; Manintveld, O.C.; Daemen, J.; et al. Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: A randomised trial. EuroIntervention 2019, 15, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Sheikh, F.H.; Ahmed, S.; Najjar, S.S.; Molina, E.J. Intra-Aortic Balloon Pump as a Bridge to Durable Left Ventricular Assist Device. J. Am. Hear. Assoc. 2021, 10, e019376. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Tavazzi, G.; Morici, N.; Viola, G.; Meani, P.; Oliva, F.G.; Pappalardo, F. Arterial elastance modulation by intra-aortic balloon counterpulsation in patients with acute decompensated heart failure and low-output state. J. Cardiovasc. Med. 2020, 22, 231–232. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Harasawa, H.; Li, K.S.; Zhu, D.; Santamore, W.P. Comparative significance in systolic ventricular interaction. Cardiovasc. Res. 1991, 25, 774–783. [Google Scholar] [CrossRef]
- Nagpal, A.D.; Singal, R.K.; Arora, R.C.; Lamarche, Y. Temporary Mechanical Circulatory Support in Cardiac Critical Care: A State of the Art Review and Algorithm for Device Selection. Can. J. Cardiol. 2016, 33, 110–118. [Google Scholar] [CrossRef]
- Sponga, S.; Benedetti, G.; Livi, U. Short-term mechanical circulatory support as bridge to heart transplantation: Paracorporeal ventricular assist device as alternative to extracorporeal life support. Ann. Cardiothorac. Surg. 2019, 8, 143–150. [Google Scholar] [CrossRef]
- Morici, N.; Tavazzi, G.; Pappalardo, F. SCAI B shock stage: The beginning and the end. J. Am. Coll. Cardiol. 2022, in press. [Google Scholar]
- Jain, P.; Thayer, K.L.; Abraham, J.; Everett, K.D.; Pahuja, M.; Whitehead, E.H.; Schwartz, B.P.; Lala, A.; Sinha, S.S.; Kanwar, M.K.; et al. Right Ventricular Dysfunction Is Common and Identifies Patients at Risk of Dying in Cardiogenic Shock. J. Card. Fail. 2021, 27, 1061–1072. [Google Scholar] [CrossRef]
- Tedford, R.J. Determinants of Right Ventricular Afterload (2013 Grover Conference Series). Pulm. Circ. 2014, 4, 211–219. [Google Scholar] [CrossRef]
- Lim, H.S. Gustaffson F Pulmonary artery pulsatility index: Physiological basis and clinical application. Eur. J. Heart Fail 2020, 22, 32–3829. [Google Scholar] [CrossRef] [PubMed]
- Léopold, V.; Gayat, E.; Pirracchio, R.; Spinar, J.; Parenica, J.; Tarvasmäki, T.; Lassus, J.; Harjola, V.-P.; Champion, S.; Zannad, F.; et al. Epinephrine and short-term survival in cardiogenic shock: An individual data meta-analysis of 2583 patients. Intensiv. Care Med. 2018, 44, 847–856. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.-P.; Kimmoun, A.; Cariou, A.; Lassus, J.; et al. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Combes, A.; van Diepen, S.; Hollinger, A.; Katz, J.N.; Landoni, G.; Hajjar, L.A.; Lassus, J.; Lebreton, G.; Montalescot, G.; et al. Management of cardiogenic shock complicating myocardial infarction. Intensiv. Care Med. 2018, 44, 760–773. [Google Scholar] [CrossRef]
- Morici, N.; Marini, C.; Sacco, A.; Tavazzi, G.; Cipriani, M.; Oliva, F.; Rota, M.; De Ferrari, G.M.; Campolo, J.; Frigerio, G.; et al. Early intra-aortic balloon pump in acute decompensated heart failure complicated by cardiogenic shock: Rationale and design of the randomized Altshock-2 trial. Am. Hear. J. 2020, 233, 39–47. [Google Scholar] [CrossRef]
- Pinsky, M.R. Dynamic right and left ventricular interactions in the pig. Exp. Physiol. 2020, 105, 1293–1315. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, R.H.L.; Steendijk, P.; Baan, J.; Brouwers, H.A.A.; DE Vroomen, M.; VAN Bel, F. Right Ventricular Function in Respiratory Distress Syndrome and Subsequent Partial Liquid Ventilation. Am. J. Respir. Crit. Care Med. 2000, 162, 374–379. [Google Scholar] [CrossRef]
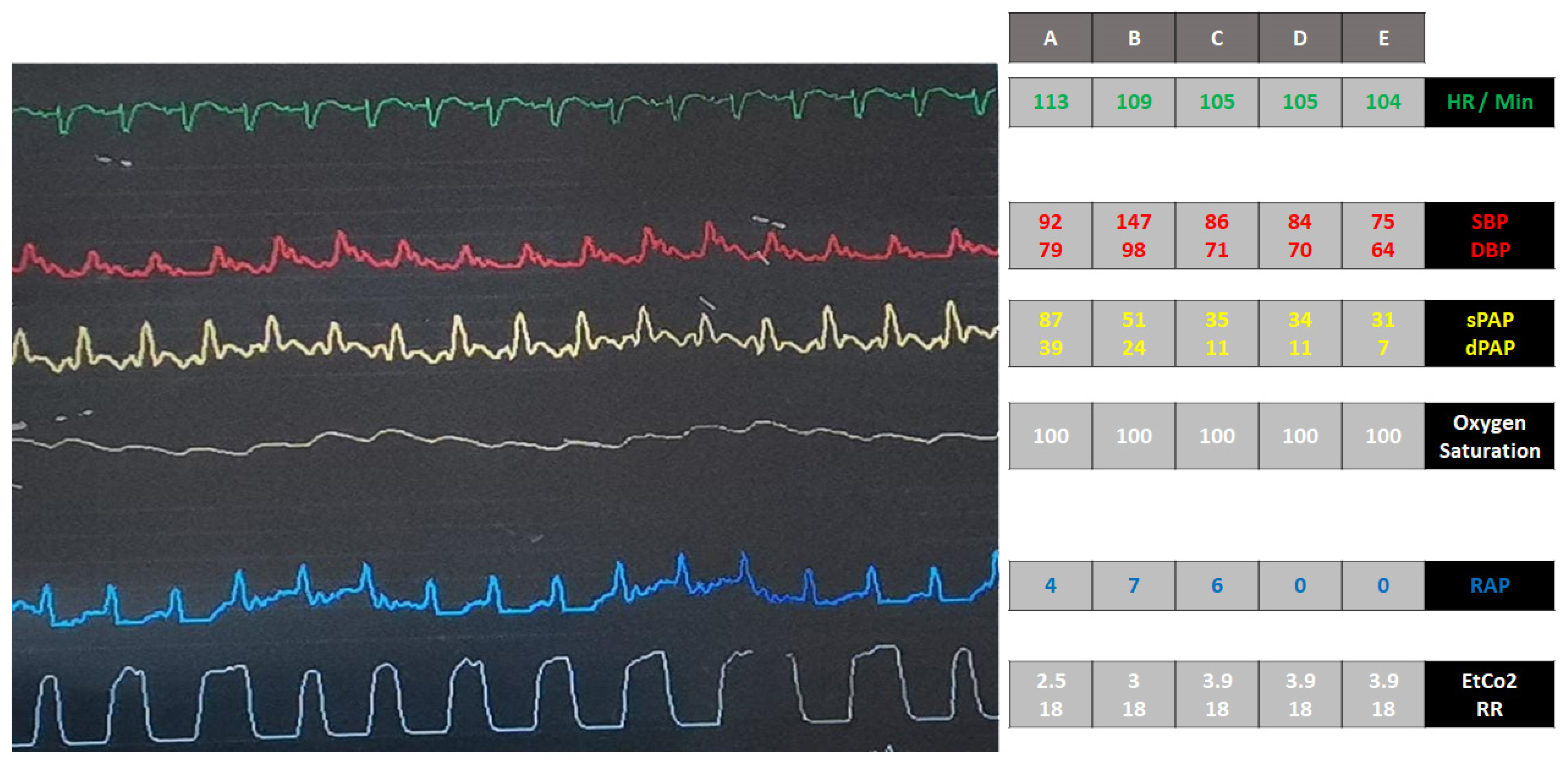
| Variables | Day 1 | Day 6 | Day 12 | Day 13 | Day 18 | Day 18 | Day 19 |
|---|---|---|---|---|---|---|---|
| SBP, (mmHg) | 110/60 | 90/50 | 110/60 | 85/40 | 70/40 | 100/60 | NA |
| MAP, mmHg | 76 | 63 | 76 | 55 | 50 | 63 | 40 |
| Lactate, mmol/L | 2.18 | 2.5 | 1.8 | 3 | 4 | 2.2 | 6.4 |
| ALT, U/L | 63 | 68 | 32 | 46 | 55 | NA | 73 |
| RAP, mmHg | 12 | 16 | 5 | 14 | 16 | 0 | 0 |
| PAWP, mmHg | NA | NA | 15 | NA | 18 | 9 | NA |
| SCAI stages | C | C | C | D | D | D | E |
| Treatment Intensity | Dopamine; SNP | Epinephrine; IABP | Epinephrine MD; weaning of IABP | Epinephrine; IABP | Epinephrine; IABP | Epinephrine; Impella CP | Epinephrine; Impella CP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, A.; Morici, N.; Oreglia, J.A.; Tavazzi, G.; Villanova, L.; Colombo, C.; Garatti, L.; Mondino, M.G.; Nava, S.; Pappalardo, F. Left Ventricular Unloading in Acute on Chronic Heart Failure: From Statements to Clinical Practice. J. Pers. Med. 2022, 12, 1463. https://doi.org/10.3390/jpm12091463
Sacco A, Morici N, Oreglia JA, Tavazzi G, Villanova L, Colombo C, Garatti L, Mondino MG, Nava S, Pappalardo F. Left Ventricular Unloading in Acute on Chronic Heart Failure: From Statements to Clinical Practice. Journal of Personalized Medicine. 2022; 12(9):1463. https://doi.org/10.3390/jpm12091463
Chicago/Turabian StyleSacco, Alice, Nuccia Morici, Jacopo Andrea Oreglia, Guido Tavazzi, Luca Villanova, Claudia Colombo, Laura Garatti, Michele Giovanni Mondino, Stefano Nava, and Federico Pappalardo. 2022. "Left Ventricular Unloading in Acute on Chronic Heart Failure: From Statements to Clinical Practice" Journal of Personalized Medicine 12, no. 9: 1463. https://doi.org/10.3390/jpm12091463
APA StyleSacco, A., Morici, N., Oreglia, J. A., Tavazzi, G., Villanova, L., Colombo, C., Garatti, L., Mondino, M. G., Nava, S., & Pappalardo, F. (2022). Left Ventricular Unloading in Acute on Chronic Heart Failure: From Statements to Clinical Practice. Journal of Personalized Medicine, 12(9), 1463. https://doi.org/10.3390/jpm12091463






