Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice
Abstract
:1. Introduction
2. Methods
2.1. Animal Experiments
2.2. Serum Analysis
2.3. Analysis of Bone Mass, Microarchitecture, and Strength
2.4. Osmium Staining
2.5. Histology
2.6. RNA Isolation, RT-PCR, and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
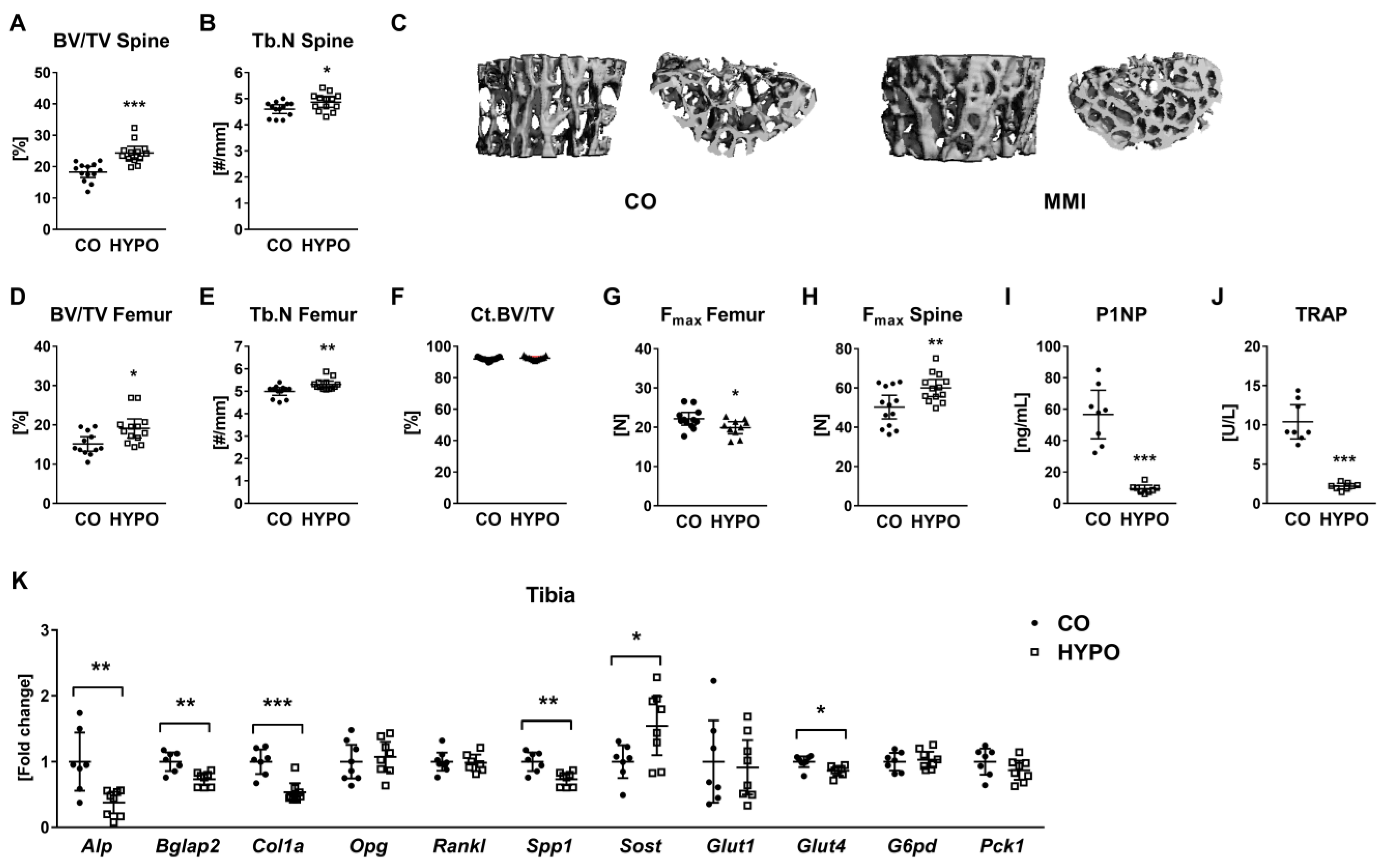
3.1. Hypothyroidism Leads to Low Bone Turnover and Increases Trabecular Bone Mass
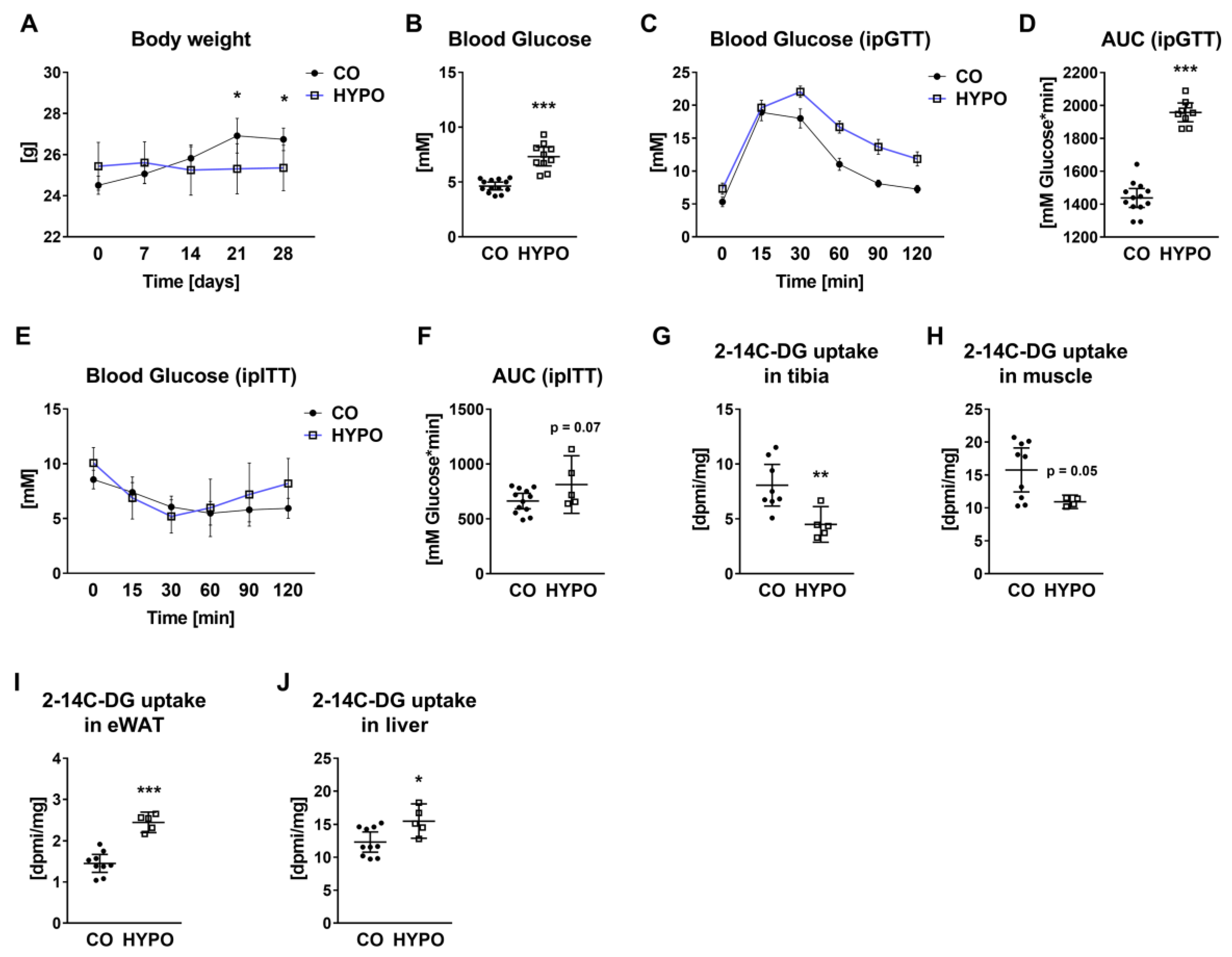
3.2. Whole-Body Glucose Metabolism and Tissue-Specific Glucose Uptake Are Altered in Hypothyroid Mice
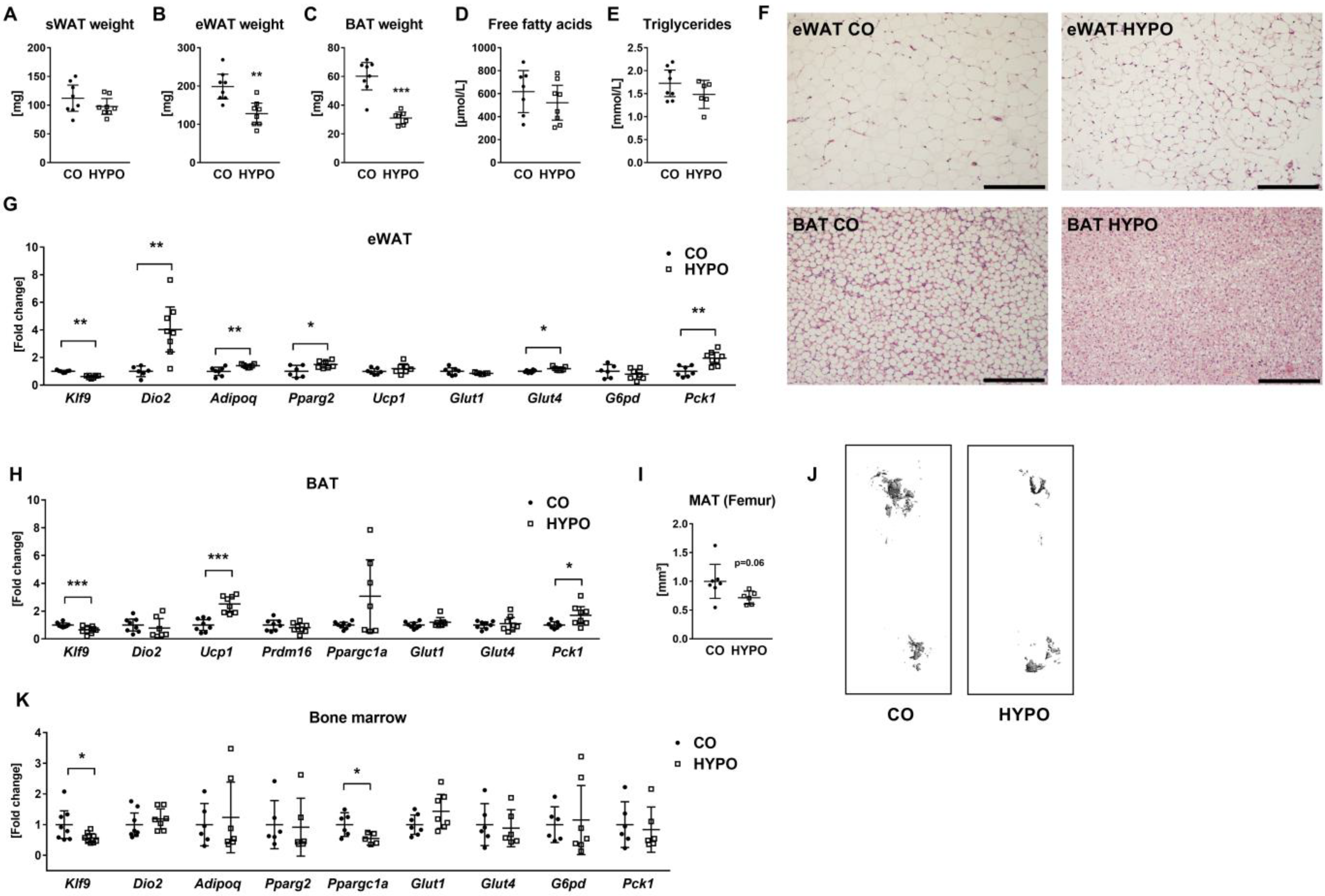
3.3. Muscle, Liver, and Adipose Tissues Are Distinctly Affected by Hypothyroidism
3.4. Bisphosphonate Treatment Impairs Bone Turnover, but Not Systemic Glucose Homeostasis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karner, C.M.; Long, F. Glucose metabolism in bone. Bone 2018, 115, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lee, W.C.; Song, C.; Ye, L.; Abel, E.D.; Long, F. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 2020, 34, 11058–11067. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Tsourdi, E.; Lademann, F.; Siggelkow, H. Impact of thyroid diseases on bone. Der Internist 2018, 59, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Mosekilde, L.; Melsen, F. Kinetics of trabecular bone resorption and formation in hypothyroidism: Evidence for a positive balance per remodeling cycle. Bone 1986, 7, 101–108. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Doria, C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020, 9, 1034. [Google Scholar] [CrossRef]
- Kaspari, R.R.; Reyna-Neyra, A.; Jung, L.; Torres-Manzo, A.P.; Hirabara, S.M.; Carrasco, N. The paradoxical lean phenotype of hypothyroid mice is marked by increased adaptive thermogenesis in the skeletal muscle. Proc. Natl. Acad. Sci. USA 2020, 117, 22544–22551. [Google Scholar] [CrossRef]
- Rakov, H.; Engels, K.; Hönes, G.S.; Brix, K.; Köhrle, J.; Moeller, L.C.; Zwanziger, D.; Führer, D. Sex-specific phenotypes of hyperthyroidism and hypothyroidism in aged mice. Biol. Sex Differ. 2017, 8, 38. [Google Scholar] [CrossRef]
- Weiner, J.; Kranz, M.; Klöting, N.; Kunath, A.; Steinhoff, K.; Rijntjes, E.; Köhrle, J.; Zeisig, V.; Hankir, M.; Gebhardt, C.; et al. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep. 2016, 6, 38124. [Google Scholar] [CrossRef] [Green Version]
- Ferrandino, G.; Kaspari, R.R.; Spadaro, O.; Reyna-Neyra, A.; Perry, R.J.; Cardone, R.; Kibbey, R.G.; Shulman, G.I.; Dixit, V.D.; Carrasco, N. Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms. Proc. Natl. Acad. Sci. USA 2017, 114, E9172–E9180. [Google Scholar] [CrossRef]
- Tsourdi, E.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Rauner, M. Hyperthyroidism and Hypothyroidism in Male Mice and Their Effects on Bone Mass, Bone Turnover, and the Wnt Inhibitors Sclerostin and Dickkopf-1. Endocrinology 2015, 156, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Lademann, F.; Ominsky, M.S.; Rijntjes, E.; Köhrle, J.; Misof, B.M.; Roschger, P.; Klaushofer, K.; Hofbauer, L.C.; Rauner, M. Sclerostin blockade and zoledronic acid improve bone mass and strength in male mice with exogenous hyperthyroidism. Endocrinology 2017, 158, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P. Bone regulation of insulin secretion and glucose homeostasis. Endocrinology 2020, 161, bqaa149. [Google Scholar] [CrossRef]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay Between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Tsourdi, E.; Tabacco, G.; Naciu, A.M.; Napoli, N.; Vescini, F.; Palermo, A. The Impact of Antiosteoporotic Drugs on Glucose Metabolism and Fracture Risk in Diabetes: Good or Bad News? J. Clin. Med. 2021, 10, 996. [Google Scholar] [CrossRef]
- Ferron, M.; McKee, M.D.; Levine, R.L.; Ducy, P.; Karsenty, G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012, 50, 568–575. [Google Scholar] [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010, 142, 309. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, F.S.; De Araújo, P.H.C.; Mota, R.F.; Carvalho, A.J.R.; De Queiroz, M.S.; De Almeida, B.B.; De Oliveira Santos Ferreira, K.C.; Metzner, R.J.M.; Ferrari, G.D.; Alberici, L.C.; et al. RANKL induces beige adipocyte differentiation in preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E866–E877. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Frey, J.L.; Li, Z.; Kushwaha, P.; Zoch, M.L.; Tomlinson, R.E.; Da, H.; Aja, S.; Noh, H.L.; Kim, J.K.; et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E11238–E11247. [Google Scholar] [CrossRef] [PubMed]
- Picke, A.K.; Gordaliza Alaguero, I.; Campbell, G.M.; Glüer, C.C.; Salbach-Hirsch, J.; Rauner, M.; Hofbauer, L.C.; Hofbauer, C. Bone defect regeneration and cortical bone parameters of type 2 diabetic rats are improved by insulin therapy. Bone 2016, 82, 108–115. [Google Scholar] [CrossRef]
- Tsourdi, E.; Colditz, J.; Lademann, F.; Rijntjes, E.; Köhrle, J.; Niehrs, C.; Hofbauer, L.C.; Rauner, M. The Role of Dickkopf-1 in Thyroid Hormone-Induced Changes of Bone Remodeling in Male Mice. Endocrinology 2019, 160, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.D.; Boyde, A.; Howell, P.G.T.; Bassett, R.H.; Galliford, T.M.; Archanco, M.; Evans, H.; Lawson, M.A.; Croucher, P.; St Germain, D.L.; et al. Optimal bone strength and mineralization requires the type 2 iodothyronine deiodinase in osteoblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 7604–7609. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Influence of Hyper- and Hypothyroidism, and the Effects of Treatment with Antithyroid Drugs and Levothyroxine on Fracture Risk. Calcif. Tissue Int. 2005, 77, 139–144. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Fractures in Patients with Hyperthyroidism and Hypothyroidism: A Nationwide Follow-Up Study in 16,249 Patients. Thyroid 2004, 12, 411–419. [Google Scholar] [CrossRef]
- Ahmad, T.; Muhammad, Z.A.; Nadeem, S. Is Hypothyroidism Associated With Outcomes in Fracture Patients? Data From a Trauma Registry. J. Surg. Res. 2021, 268, 527–531. [Google Scholar] [CrossRef]
- Maccagnano, G.; Notarnicola, A.; Pesce, V.; Mudoni, S.; Tafuri, S.; Moretti, B. The Prevalence of Fragility Fractures in a Population of a Region of Southern Italy Affected by Thyroid Disorders. BioMed Res. Int. 2016, 2016, 6017165. [Google Scholar] [CrossRef] [Green Version]
- Apostu, D.; Lucaciu, O.; Oltean-Dan, D.; Muresan, A.D.; Moisescu-Pop, C.; Maxim, A.; Benea, H. The influence of thyroid pathology on osteoporosis and fracture risk: A review. Diagnostics 2020, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Lal, A.K.; Negi, K.S. Serum Creatine Kinase Activity in Thyroid Disorders. JK Sci. 2007, 9, 25–26. [Google Scholar]
- Kornel, A.; Den Hartogh, D.J.; Klentrou, P.; Tsiani, E. Role of the Myokine Irisin on Bone Homeostasis: Review of the Current Evidence. Int. J. Mol. Sci. 2021, 22, 9136. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The effect of Irisin on bone cells in vivo and in vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Rizos, C.; Elisaf, M.; Liberopoulos, E. Effects of Thyroid Dysfunction on Lipid Profile. Open Cardiovasc. Med. J. 2011, 5, 76. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Schafer, A.L.; Grey, A.; Vittinghoff, E.; Palermo, L.; Lui, L.Y.L.; Wallace, R.B.; Cummings, S.R.; Black, D.M.; Bauer, D.C.; et al. Effects of antiresorptive therapies on glucose metabolism: Results from the FIT, HORIZON-PFT, and FREEDOM trials. J. Bone Miner. Res. 2013, 28, 1348–1354. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lademann, F.; Rauner, M.; Bonnet, N.; Hofbauer, L.C.; Tsourdi, E. Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice. J. Pers. Med. 2022, 12, 1462. https://doi.org/10.3390/jpm12091462
Lademann F, Rauner M, Bonnet N, Hofbauer LC, Tsourdi E. Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice. Journal of Personalized Medicine. 2022; 12(9):1462. https://doi.org/10.3390/jpm12091462
Chicago/Turabian StyleLademann, Franziska, Martina Rauner, Nicolas Bonnet, Lorenz C. Hofbauer, and Elena Tsourdi. 2022. "Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice" Journal of Personalized Medicine 12, no. 9: 1462. https://doi.org/10.3390/jpm12091462
APA StyleLademann, F., Rauner, M., Bonnet, N., Hofbauer, L. C., & Tsourdi, E. (2022). Low Bone Turnover Due to Hypothyroidism or Anti-Resorptive Treatment Does Not Affect Whole-Body Glucose Homeostasis in Male Mice. Journal of Personalized Medicine, 12(9), 1462. https://doi.org/10.3390/jpm12091462






