Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution
Abstract
:1. Introduction
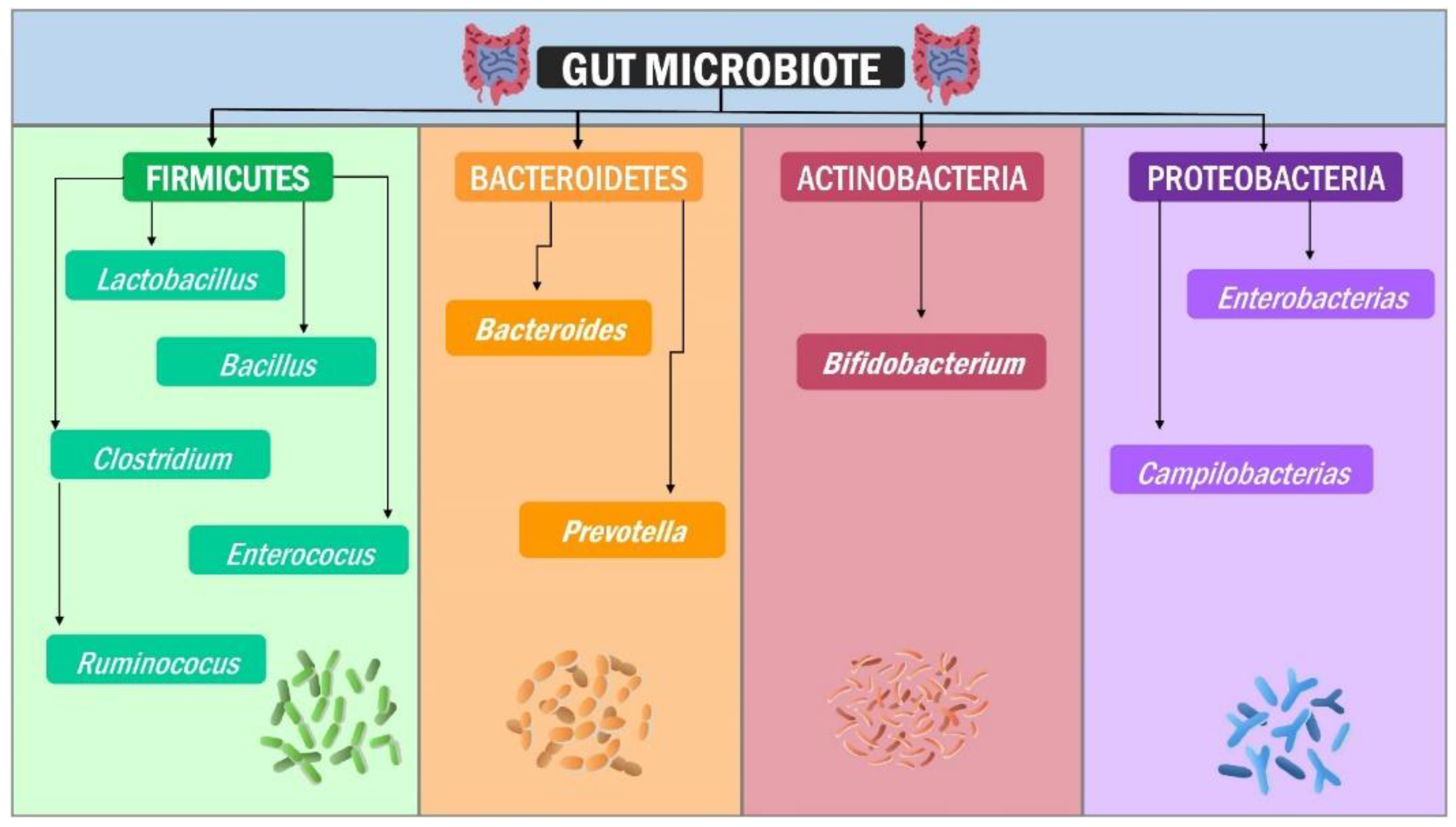
2. Structure of the Human Gut Microbiota
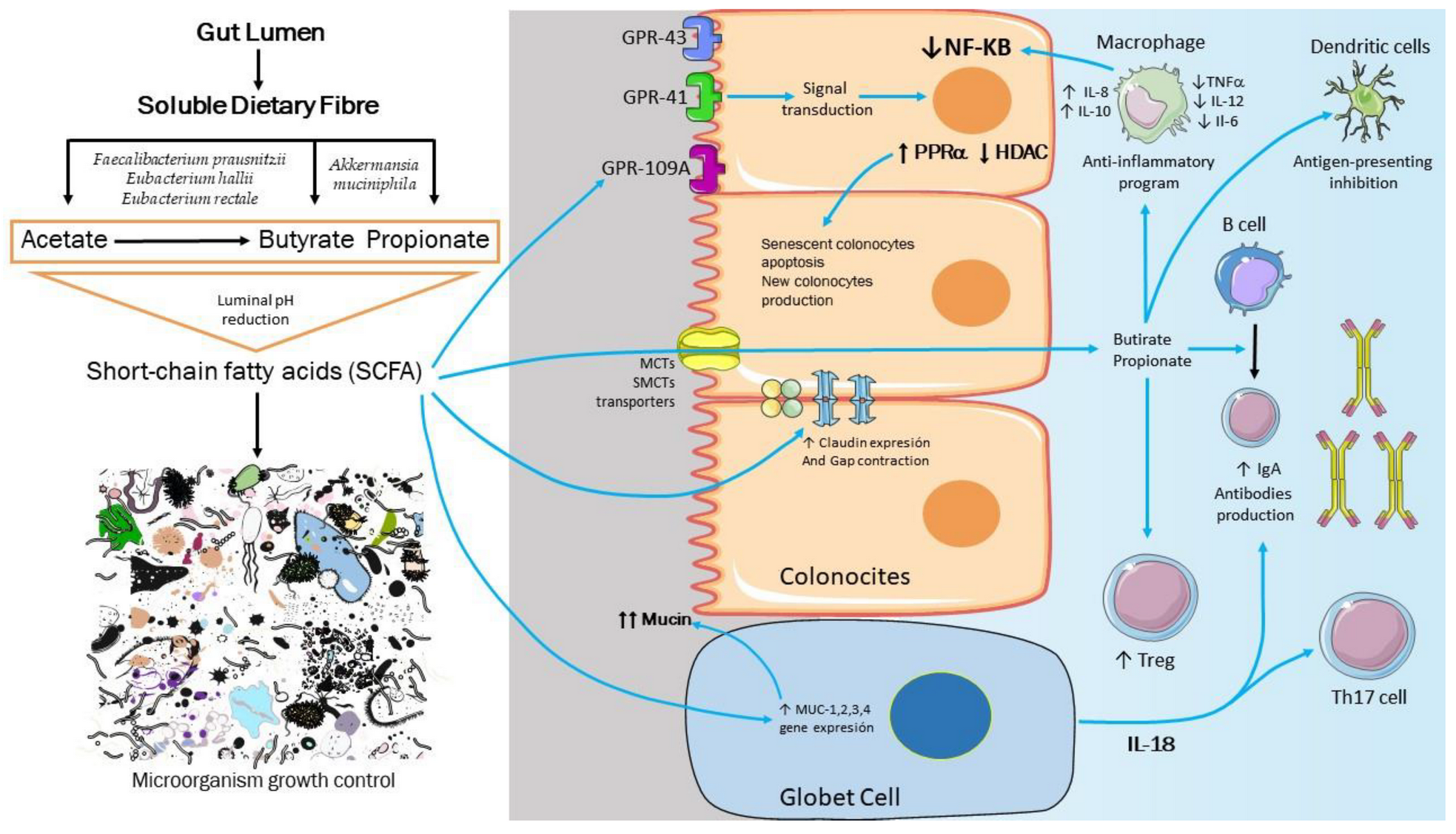
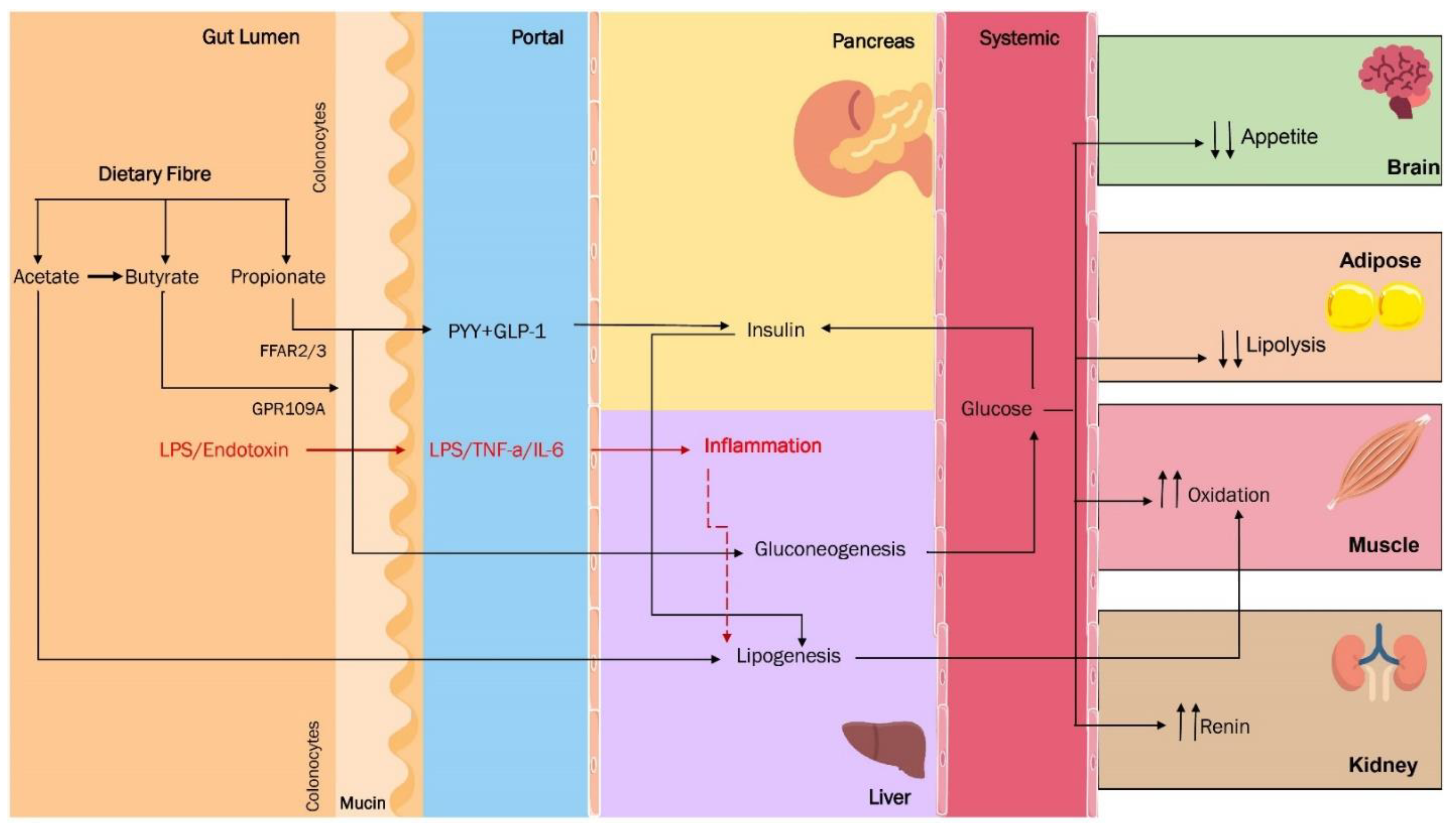
3. Are the SCFA the Missing Link between Dysbiosis and Obesity?
4. Molecular Basis of Intestinal, Immunological, and Metabolic Homeostasis Control by Gut Microbiota
5. Microbiota in Obesity: Cause, Effect, or Both?
6. Are Probiotics Effective in Endocrine-Metabolic Disease Treatment? Are These Supplements Effective in Intestinal Dysbiosis?
7. Efficacy of Probiotics in Obesity and Its Comorbidities: Fiction or Reality?
8. Prebiotics, the Cornerstone of Gut Microbiota
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CarrGómez, J.C.; Ena, J.; Lorido, J.A.; Ripoll, J.S.; Carrasco-Sánchez, F.J.; Gómez-Huelgas, R.; Soto, M.P.; Lista, J.D.; Martínez, P.P. Obesity Is a Chronic Disease. Positioning Statement of the Diabetes, Obesity and Nutrition Workgroup of the Spanish Society of internal Medicine (SEMI) for An Approach Centred on individuals with Obesity. Rev. Clín. Esp. 2021, 221, 509–516. [Google Scholar]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020, 360, 1–8. [Google Scholar]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’I, A. A Systematic Literature Review on Obesity: Understanding the Causes & Consequences of Obesity and Reviewing Various Machine Learning Approaches Used to Predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Zhang, X.; Zhang, M.; Zhao, Z.; Huang, Z.; Deng, Q.; Li, Y. Obesogenic Environmental Factors of Adult Obesity in China: A Nationally Representative Cross-Sectional Study. Environ. Res. Lett. 2020, 15, 4. [Google Scholar] [CrossRef]
- Prakash, K.; Munyanyi, M.E. Energy Poverty and Obesity. Energy Econ. 2021, 101, 105428. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, C.; Hervás Bárbara, G.; Gianzo Citores, M.; Aranceta-Bartrina, J. Prevalence of Obesity and Associated Cardiovascular Risk Factors in the Spanish Population: The ENPE Study. Rev. Esp. Cardiol. Engl. 2021, 3, 232–241. [Google Scholar] [CrossRef]
- Corazzini, R.; Morgado, F.; Gascón, T.M.; Affonso Fonseca, F.L. Evaluation of Obesity Associated with Health Risk Factors in Brazilian Public School. Obes. Med. 2020, 19, 100223. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Muñoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of Gut Microbiota in Obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M. Gut Microbiota in Patients with Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.D.; Pearson, N.M.; Seidler, K. The Link between the Gut Microbiota and Parkinson’s Disease: A Systematic Mechanism Review with Focus on α-Synuclein Transport. Brain Res. 2021, 1769, 147609. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Huang, Y.J. Microbiota–Immune interactions in Asthma Pathogenesis and Phenotype. Curr. Opin. Immunol. 2020, 66, 22–26. [Google Scholar] [CrossRef]
- Guo, L.; Yang, K.; Zhou, P.; Yong, W. Gut Microbiota in Obesity and Nonalcoholic Fatty Liver. Disease. Surg. Pract. Sci. 2021, 5, 100030. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.; Smith, G.; Ross, R. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Afrc, R.F. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]
- Gilliland, S.E. Health and Nutritional Benefits from Lactic Acid Bacteria. FEMS Microbiol. Lett. 1990, 87, 175–1788. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The Effect of Milk and Lactobacillus Feeding on Human intestinal Bacterial Enzyme Activity. Am. J. Clin. Nutr. 1984, 39, 756–761. [Google Scholar] [CrossRef]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic Acid Bacteria and their Effect-on the Immune System. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar] [PubMed]
- Vedamuthu, E.R. Starter Cultures for Yogurt and Fer-mented Milks. In RC Chandan Manufacturing Yogurt and Fermented Milks; Blackwell Publishing: Ames, IA, USA, 2006; pp. 88–115. [Google Scholar]
- Siciliano, R.A.; Mazzeo, M.F. Molecular Mechanisms of Probiotic Action: A Perspective. Curr. Opin. Microbiol. 2012, 15, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, M.; Mohammadi, G.; Mansouri, K.; Khaledian, S.; Taran, M.; Martinez, F. A Review on Anticancer, Antibacterial and Photo Catalytic Activity of Various Nanoparticles Synthesised by Probiotics. J. Biotechnol. 2022, 354, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zou, R.; Wang, L.; Chen, Y.; Qian, X.; Zhao, J. Multi-Probiotics Ameliorate Major Depressive Disorder and Accompanying Gastrointestinal Syndromes via Serotonergic System Regulation. J. Adv. Res. 2022, in press. [CrossRef]
- Wang, C.; Li, S.; Xue, P.; Yu, L.; Tian, F.; Zhao, J. The Effect of Probiotic Supplementation on Lipid Profiles in Adults with Overweight or Obesity: A Meta-Analysis of Randomised Controlled Trials. J. Funct. Foods 2021, 86, 104711. [Google Scholar] [CrossRef]
- Woźniak, D.; Cichy, W.; Przysławski, J.; Drzymała-Czyż, S. The Role of Microbiota and Enteroendocrine Cells in Maintaining Homeostasis in the Human Digestive Tract. Adv. Med. Sci. 2021, 66, 284–292. [Google Scholar] [CrossRef]
- Specter, M. Germs are Us. New Yorker, 15 October 2012; Volume 88, 32–39. [Google Scholar]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q. An integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Watanabe, M.; Kojima, H.; Fukui, M. Complete Genome Sequence and Cell Structure of Limnochorda Pilosa, a Gram-Negative Spore-former within the Phylum Firmicutes. Int. J. Syst. Evol. Microbiol. 2016, 66, 1330–1339. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem Across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Dig. Liver. Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Lapébie, P.; Lombard, V.; Drula, E.; Terrapon, N.; Henrissat, B. Bacteroidetes Use Thousands of Enzyme Combinations to Break Down Glycans. Nat. Commun. 2019, 10, 2043. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of Th17 Cells in the Mucosa of the Small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic Key Points and Immunological Tricks of Our Gut Commensals. Dig. Liver. Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides Fragilis Lipopolysaccharide and inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef] [Green Version]
- Stilling, R.M.; Van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and “Western-Lifestyle” inflammatory Diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate Modulates Bacterial Adherence on LS174T Human Colorectal Cells by Stimulating Mucin Secretion and MAPK Signaling Pathway. Nutr. Res. Pract. 2015, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic infection Through Production of Acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium Prausnitziiinfluence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, S.; Gopal, E.; Fei, Y.J.; Ganapathy, V. Functional Identification of SLC5A8, A Tumor Suppressor Down-Regulated in Colon Cancer, as a Na+-coupled Transporter for Short-chain Fatty Acids. J. Biol. Chem. 2004, 279, 13293–13296. [Google Scholar] [CrossRef] [Green Version]
- Chassard, C.; Lacroix, C. Carbohydrates and the Human Gut Microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453–460. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 Mediates Microbiota Metabolite SCFA Regulation of Antimicrobial Peptide Expression in intestinal Epithelial Cells via Activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-Chain Fatty Acids induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the mTOR–S6K Pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [Green Version]
- Larraufie, P. SCFas Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018, 74, 1–8. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute Oral Sodium Propionate Supplementation Raises Resting Energy Expenditure and Lipid Oxidation in Fasted Humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased Colonic Propionate Reduces Anticipatory Reward Responses in the Human Striatum to High-Energy Foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory Receptor Responding to Gut Microbiota-Derived Signals Plays a Role in Renin Secretion and Blood Pressure Regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial Short Chain Fatty Acid Metabolites Lower Blood Pressure via Endothelial G Protein-Coupled Receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Benítez-Páez, A.; Del Pulgar, E.M.G.; Kjølbæk, L.; Brahe, L.K.; Astrup, A.; Larsen, L.; Sanz, Y. Impact of Dietary Fiber and Fat on Gut Microbiota Re-Modeling and Metabolic Health. Trends Food Sci. Technol. 2016, 57, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and Other Health-Related Effects of Cereal-Derived Arabinoxylans, Arabinoxylan-Oligosaccharides, and Xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- McCleary, B.V. Dietary Fiber Analysis. Proc. Nutr. Soc. 2003, 62, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Ruiz-Moyano, S.; Jimenez-Espinoza, R.; Eom, H.-J.; Block, D.E.; Mills, D.A. Utilization of Galactooligosaccharides by Bifidobacterium Longum Subsp. Infantis Isolates. Food Microbiol. 2013, 33, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivière, A.; Moens, F.; Selak, M.; Maes, D.; Weckx, S.; De Vuyst, L. The Ability of Bifidobacteria to Degrade Arabinoxylan Oligosaccharide Constituents and Derived Oligosaccharides Is Strain Dependent. Appl. Environ. Microbiol. 2014, 80, 204–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; Van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Arabinoxylan-oligosaccharides (AXOS) Affect the Protein/Carbohydrate Fermentation Balance and Microbial Population Dynamics of the Simulator of Human intestinal Microbial Ecosystem: AXOS Effect on Protein/Carbohydrate Fermentation Balance. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassard, C.; Goumy, V.; Leclerc, M.; Del’homme, C.; Bernalier-Donadille, A. Characterization of the Xylan-degrading Microbial Community from Human Faeces: Xylanolytic Microbiota from Human Faeces. FEMS Microbiol. Ecol. 2007, 61, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469. [Google Scholar] [CrossRef]
- Maharshak, N.; Packey, C.D.; Ellermann, M.; Manick, S.; Siddle, J.P.; Huh, E.Y.; Plevy, S.; Sartor, R.B.; Carroll, I.M. Altered Enteric Microbiota Ecology in interleukin 10-Deficient Mice During Development and Progression of intestinal inflammation. Gut Microbes 2013, 4, 316–324. [Google Scholar] [CrossRef]
- Tseng, C.H.; Wu, C.Y. The Gut Microbiome in Obesity. J. Formos. Med. Assoc. 2019, 118, 3–9. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism and Perspective in Obesity. Gut Microbes 2018, 18, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Jáquez, J.L.; Lascurain, L.; Falcon, A.C.; Montoya, J.R. Obesidad, Disbiosis Y Trastornos Gastrointestinales Funcionales En Edades Pediátricas. Neurogastrol. LATAM Rev. 2020, 4, 4268. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its influence on Host Immunity and Disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between Gut Microbiota and Host Metabolism Predisposing to Obesity and Diabetes. Annu. Rev. Med. 2011, 62, 361–380. [Google Scholar] [CrossRef]
- Million, M.; Thuny, F.; Angelakis, E.; Casalta, J.-P.; Giorgi, R.; Habib, G.; Raoult, D. Lactobacillus Reuteri and Escherichia coli in the Human Gut Microbiota May Predict Weight Gain Associated with Vancomycin Treatment. Nutr. Diabetes 2013, 3, 87. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of Ggut Microbiota During Probiotic-Mediated Attenuation of Metabolic Syndrome in High Fat Diet-Fed Mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to High-Fat Diet-induced Obesity in Rats Is Associated with Changes in the Gut Microbiota and Gut inflammation. Am. J. Physiol.-Gastrointest. Liver. Physiol. 2010, 299, 440–448. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal Barrier: An interface between Health and Disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The Gut Microbiota in Human Energy Homeostasis and Obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Do, M.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T. Regulation of the intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, 13357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO Working Group: Geneva, Switzerland, 2002.
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualisation from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium Longum 1714 as a Translational Psychobiotic: Modulation of Sstress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and Ultraviolet-inactivated Lactobacillus Rhamnosus GG Decrease Flagellin-induced interleukin-8 Production in Caco-2 Cells. J. Nutr. 2008, 138, 2264–2268. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, S.; Ahmed, N. Probiotic Potential of Lactobacillus Strains in Human infections. Afr. J. Microbiol. Res. 2009, 3, 851–855. [Google Scholar]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.T.; Ruiz, M.A.; Morales, M.E. Microorganismos Probióticos Y Salud. Ars Pharm. 2015, 56, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- da Silva Pontes, K.S.; Guedes, M.R.; da Cunha, M.R.; de Souza Mattos, S.; Silva, M.I.B.; Neves, M.F.; Marques, B.C.A.A.; Klein, M.R.S.T. Effects of Probiotics on Body Adiposity and Cardiovascular Risk Markers in individuals with Overweight and Obesity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Clin. Nutr. 2021, 40, 4915–4931. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of Bacterial Probiotics on Obesity Diabetes and Non-Alcoholic Fatty Liver Disease Related Variables: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
| Microorganism | Location | Mechanism of Action |
|---|---|---|
| Lactobacillus plantarum | Gastrointestinal tract | Adhesion to epithelial cells Improves IL-10 production in the colon |
| Lactobacillus rhamnosus | Gastrointestinal tract and brain | Adhesion to epithelial cells, lactic acid production, Regeneration of epithelial cells, increases GABA receptors in cortical regions and decreases in the amygdala, hypothalamus, and Locus ceruleus |
| Lactobacillus salivarius | Gastrointestinal tract | Secretion of low molecular weight bacteriocins. |
| Lactobacillus reuteri | Gastrointestinal tract | Reuterin (3-hydroxypropionaldehyde) production |
| Lactobacillus acidophilus | Intestine | Cholesterol adherence |
| Lactobacillus casei | Intestine | Inhibits bacterial translocation, increases MUC gene expression, inhibits cholesterol mycelia formation, enhances NK cell activity, inhibits bacterial translocation, increases MUC gene expression, inhibits cholesterol mycelia formation, and enhances NK cell activity |
| Lactobacillus bulgaricus | Intestine | Adherence to cholesterol, inhibits the formation of cholesterol mycelia. |
| Bifidobacterium longum | Intestine | Binding to aflatoxin B1 |
| Bifidobacterium adolescentis | Intestine | Binding to aflatoxin B1 |
| Bifidobacterium animalis | Intestine | Increases intestinal motility and bile salts hydrolysis. |
| Saccharomyces boullardii | Intestine | T-cell redistribution |
| Lactobacillus plantarum | Intestine | Decreased translocation and adherence of pathogens |
| Lactobacillus paracasei | Intestine | Enhances cancer cell apoptosis |
| Saccharomyces boulardii | Intestine | Improves intestinal barrier function |
| Bifidobacterium sp. | Intestine | Secretes superoxide dismutase |
| Lactobacillus helveticus | Intestine | Secretes vitamin E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León Aguilera, X.E.; Manzano, A.; Pirela, D.; Bermúdez, V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. J. Pers. Med. 2022, 12, 1282. https://doi.org/10.3390/jpm12081282
León Aguilera XE, Manzano A, Pirela D, Bermúdez V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. Journal of Personalized Medicine. 2022; 12(8):1282. https://doi.org/10.3390/jpm12081282
Chicago/Turabian StyleLeón Aguilera, Xavier Eugenio, Alexander Manzano, Daniela Pirela, and Valmore Bermúdez. 2022. "Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution" Journal of Personalized Medicine 12, no. 8: 1282. https://doi.org/10.3390/jpm12081282
APA StyleLeón Aguilera, X. E., Manzano, A., Pirela, D., & Bermúdez, V. (2022). Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. Journal of Personalized Medicine, 12(8), 1282. https://doi.org/10.3390/jpm12081282






