Hydrogen Sulfide-to-Thiosulfate Ratio Associated with Blood Pressure Abnormalities in Pediatric CKD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Analysis of Plasma H2S and Thiosulfate Levels
2.3. Blood Pressure Measurement and Cardiovascular Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
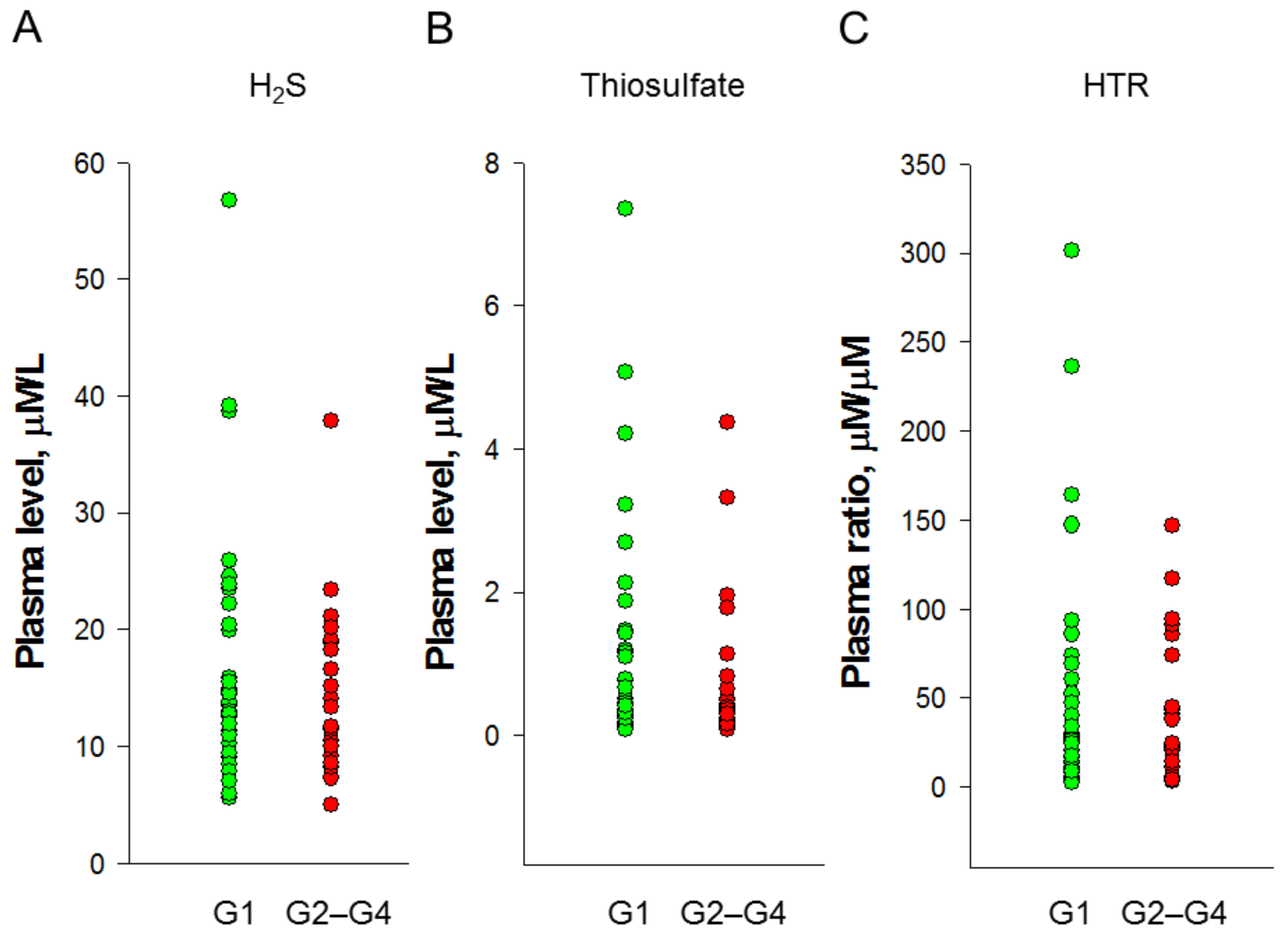
3.2. Plasma H2S and Thiosulfate Levels
3.3. Cardiovascular Assessment
3.4. Association between H2S and Cardiovascular Risk Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010, 13, 157–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef]
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharm. Rep. 2018, 70, 196–205. [Google Scholar] [CrossRef]
- Olson, K.R.; Deleon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wu, L. Trend in H2S Biology and Medicine Research—A Bibliometric Analysis. Molecules 2017, 22, 2087. [Google Scholar] [CrossRef] [Green Version]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; De Santo, N.G. Hydrogen sulphide-generating pathways in haemodialysis patients: A study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol. Dial. Transplant. 2009, 24, 3756–3763. [Google Scholar] [CrossRef] [Green Version]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307S–1315S. [Google Scholar]
- Hsu, C.N.; Lu, P.C.; Lo, M.H.; Lin, I.C.; Tain, Y.L. The association between nitric oxide pathway, blood pressure abnormalities, and cardiovascular risk profile in pediatric chronic kidney disease. Int. J. Mol. Sci. 2019, 20, 5301. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.T.; Mitsnefes, M.; Pierce, C.; Cole, S.R.; Parekh, R.S.; Furth, S.L.; Warady, B.A. Chronic Kidney Disease in Children Study Group: Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension 2008, 52, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Urbina, E.M.; Williams, R.V.; Alpert, B.S.; Collins, R.T.; Daniels, S.R.; Hayman, L.; Jacobson, M.; Mahoney, L.; Mietus-Snyder, M.; Rocchini, A.; et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research: A scientific statement from the American Heart Association. Hypertension 2009, 54, 919–950. [Google Scholar] [PubMed]
- Taal, M.W. Arterial stiffness in chronic kidney disease: An update. Curr. Opin. Nephrol. Hypertens. 2014, 23, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kupferman, J.C.; Aronson Friedman, L.; Cox, C.; Flynn, J.; Furth, S.; Warady, B.; Mitsnefes, M.; CKiD Study Group. BP control and left ventricular hypertrophy regression in children with CKD. J. Am. Soc. Nephrol. 2014, 25, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Tain, Y.L. Gasotransmitters for the Therapeutic Prevention of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2021, 22, 7808. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Sodium Thiosulfate Improves Hypertension in Rats with Adenine-Induced Chronic Kidney Disease. Antioxidants 2022, 11, 147. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; Falkner, B.; Flinn, S.K.; Gidding, S.S.; Goodwin, C. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [Green Version]
- Kollias, A.; Stergiou, W.E.; Witte, K.; Soergelm, M.; Mehls, O.; Schaefer, F.; German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and role of body dimensions. J. Hypertens. 2002, 20, 1995–2007. [Google Scholar]
- Daniels, S.R.; Kimball, T.R.; Morrison, J.A.; Khoury, P.; Meyer, R.A. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am. J. Cardiol. 1995, 76, 699–701. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Sener, A. Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6452. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. Reactive Sulfur Species: A New Redox Player in Cardiovascular Pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Pálinkás, Z.; Nagy, A.; Budai, B.; Tóth, I.; Vasas, A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim. Biophys. Acta 2014, 1840, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Xue, N.; Chen, J.; Shen, Z.; Cui, X.; Fang, Y.; Ding, X. Low Plasma Hydrogen Sulfide Is Associated with Impaired Renal Function and Cardiac Dysfunction. Am. J. Nephrol. 2018, 47, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chinali, M.; Matteucci, M.C.; Franceschini, A.; Doyon, A.; Pongiglione, G.; Rinelli, G.; Schaefer, F. Advanced Parameters of Cardiac Mechanics in Children with CKD: The 4C Study. Clin. J. Am. Soc. Nephrol. 2015, 10, 1357–1363. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, I.T.N.; Klooster, A.; Minnion, M.; Feelisch, M.; Verhaar, M.C.; van Goor, H.; Joles, J.A. Sodium thiosulfate improves renal function and oxygenation in L-NNA-induced hypertension in rats. Kidney Int. 2020, 98, 366–377. [Google Scholar] [CrossRef]
- Feng, S.J.; Li, H.; Wang, S.X. Lower Hydrogen Sulfide Is Associated with Cardiovascular Mortality, Which Involves cPKCβII/Akt Pathway in Chronic Hemodialysis Patients. Blood Purif. 2015, 40, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Azukaitis, K.; Jankauskiene, A.; Schaefer, F.; Shroff, R. Pathophysiology and consequences of arterial stiffness in children with chronic kidney disease. Pediatr. Nephrol. 2021, 36, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
| CKD Stage | G1 | G2–G4 |
|---|---|---|
| Case numbers | 35 | 21 |
| Age (years) | 10.7 (8.7–13.9) | 14.4 (10.5–16.1) * |
| Male gender (%) | 17 (42.9%) | 15 (71.4%) |
| Body height (percentile) | 50 (25–85) | 50 (25–85) |
| Body weight (percentile) | 75 (25–85) | 75 (25–91) |
| Body mass index (kg/m2) | 17.9 (15.1–20.9) | 18.6 (15.6–23.1) |
| Systolic blood pressure (mmHg) | 106 (100–112) | 120 (112–125) * |
| Diastolic blood pressure (mmHg) | 68 (63–77) | 70 (64–80) |
| CAKUT (%) | 15 (42.9%) | 14 (66.7%) |
| Hypertension (% by office BP) | 6 (20.7%) | 6 (40%) |
| Blood urea nitrogen (mg/dL) | 11 (10–13) | 16 (12.5–18.5) * |
| Creatinine (mg/dL) | 0.5 (0.45–0.55) | 0.9 (0.75–0.96) * |
| eGFR (mL/min/1.73 m2) | 120 (112–126) | 80 (70–85) * |
| Hemoglobin (g/dL) | 13.5 (13–14.1) | 14.3 (12.9–15.5) |
| Total cholesterol (mg/dL) | 158 (141–181) | 155 (133–178) |
| Low-density lipoprotein (mg/dL) | 80 (65–102) | 75 (62.5–96.5) |
| Triglyceride (mg/dL) | 61 (49–101) | 65 (50.5–103) |
| Uric acid (mg/dL) | 5.1 (4–6) | 6.4 (5.7–7.7) * |
| Glucose (mg/dL) | 87 (83–89.5) | 86 (83–91.5) |
| Sodium (mEq/L) | 141 (140–142) | 141 (140–142) |
| Potassium (mEq/L) | 4.3 (4.1–4.5) | 4.5 (4.2–4.6) |
| Calcium (mg/dL) | 9.5 (9.2–9.9) | 9.8 (9.3–10.1) |
| Phosphate (mg/dL) | 4.8 (4.5–5.2) | 4.8 (4.2–5.1) |
| Urine total protein-to-creatinine ratio (mg/g) | 52.5 (37.3–327.9) | 43.3 (30.5–225.4) |
| On antihypertensive therapy | 5 (14.3%) | 6 (28.6%) |
| CKD Stage | G1 | G2–G4 |
|---|---|---|
| 24 h ABPM | 35 | 21 |
| Abnormal ABPM profile (with any of the following abnormalities) | 22 (62.9%) | 16 (76.2%) |
| Average 24 h BP > 95th percentile | 4 (11.4%) | 7 (33.3%) * |
| Average daytime BP > 95th percentile | 2 (5.7%) | 4 (19%) |
| Average nighttime BP > 95th percentile | 9 (25.7%) | 9 (42.9%) |
| BP load ≥ 25% | 15 (42.9%) | 10 (47.6%) |
| Nocturnal decrease in BP of <10% | 21 (60%) | 12 (57.1%) |
| Left ventricular mass (g) | 86.4 (65.6–99.3) | 118 (72.2–171) * |
| Left ventricular mass index (g/m2.7) | 30.3 (25.9–36.1) | 34.2 (28.4–42.2) |
| Carotid artery intima-media thickness (mm) | 0.3 (0.3–0.4) | 0.3 (0.3–0.4) |
| Augmentation index (%) | −2.8 (−10.2–0.8) | −6 (−16.8–−4.1) |
| Pulse wave velocity (m/s) | 3.8 (3.4–4.1) | 4 (3.6–4.7) |
| Cardiovascular Markers | H2S | Thiosulfate | H2S-to-Thiosulfate Ratio | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| 24 h systolic blood pressure | 0.239 | 0.076 | −0.263 | 0.05 | 0.306 | 0.022 * |
| Daytime systolic blood pressure | 0.234 | 0.082 | −0.218 | 0.106 | 0.238 | 0.077 |
| Nighttime systolic blood pressure | 0.275 | 0.04 * | −0.27 | 0.044 * | 0.336 | 0.011 * |
| 24 h diastolic blood pressure | 0.241 | 0.074 | −0.124 | 0.362 | 0.18 | 0.184 |
| Daytime diastolic blood pressure | 0.139 | 0.307 | −0.117 | 0.39 | 0.103 | 0.449 |
| Nighttime diastolic blood pressure | 0.226 | 0.094 | −0.084 | 0.54 | 0.159 | 0.242 |
| Left ventricular mass | −0.034 | 0.804 | −0.093 | 0.495 | 0.095 | 0.484 |
| Left ventricular mass index | −0.201 | 0.137 | −0.129 | 0.343 | 0.045 | 0.742 |
| Carotid artery intima-media thickness | 0.244 | 0.07 | −0.173 | 0.201 | 0.267 | 0.047 * |
| Augmentation index | −0.127 | 0.351 | −0.115 | 0.397 | 0.076 | 0.579 |
| Pulse wave velocity | 0.004 | 0.979 | −0.039 | 0.777 | 0.054 | 0.694 |
| ABPM Profile | n | H2S | Thiosulfate | H2S-to-Thiosulfate Ratio |
|---|---|---|---|---|
| μmol/L | μmol/L | μmol/μmol | ||
| 24 h BP | ||||
| Normal | 45 | 15.5 ± 9.6 | 1.07 ± 1.44 | 39.2 ± 46.9 |
| Abnormal | 11 | 18.1 ± 8.8 | 1.02 ± 1.47 | 82.3 ± 86.7 * |
| Daytime BP | ||||
| Normal | 50 | 15.9 ± 9.8 | 1.05 ± 1.42 | 45.6 ± 59.1 |
| Abnormal | 6 | 17.1 ± 5.6 | 1.18 ± 1.65 | 64.7 ± 55.9 |
| Nighttime BP | ||||
| Normal | 38 | 14.3 ± 6.6 | 1.17 ± 1.54 | 30.3 ± 29.6 |
| Abnormal | 18 | 19.5 ± 13.1 | 0.84 ± 1.18 | 84.5 ± 84.1 * |
| BP load | ||||
| Normal | 31 | 12.9 ± 5.3 | 1.1 ± 1.52 | 28.4 ± 28.8 |
| Abnormal | 25 | 19.8 ± 11.6 * | 1.02 ± 1.35 | 71.7 ± 75.7 * |
| Night dipping | ||||
| Normal | 23 | 13.9 ± 5.4 | 0.94 ± 1.05 | 30 ± 24.9 |
| Abnormal | 33 | 17.5 ± 11.3 | 1.15 ± 1.66 | 60 ± 71.3 * |
| ABPM profile | ||||
| Normal | 18 | 13.1 ± 5.6 | 0.94 ± 1.11 | 24.7 ± 15 |
| Abnormal | 38 | 17.4 ± 10.6 | 1.12 ± 1.57 | 58.6 ± 67.9 * |
| Dependent Variable | Explanatory Variable | Adjusted a | Model | ||
|---|---|---|---|---|---|
| Beta | p Value | r | p Value | ||
| Nighttime SBP | H2S-to-thiosulfate ratio | 0.356 | 0.009 | 0.488 | 0.002 |
| 24 h DBP | Thiosulfate | 0.354 | 0.004 | 0.628 | <0.001 |
| Daytime DBP | Thiosulfate | 0.317 | 0.012 | 0.589 | <0.001 |
| Nighttime DBP | Thiosulfate | 0.32 | 0.012 | 0.574 | <0.001 |
| Left ventricular mass | H2S | −0.291 | 0.004 | 0.765 | <0.001 |
| cIMT | H2S-to-thiosulfate ratio | 0.315 | 0.021 | 0.458 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Chen, W.-L.; Liao, W.-T.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Hydrogen Sulfide-to-Thiosulfate Ratio Associated with Blood Pressure Abnormalities in Pediatric CKD. J. Pers. Med. 2022, 12, 1241. https://doi.org/10.3390/jpm12081241
Hsu C-N, Chen W-L, Liao W-T, Chang-Chien G-P, Lin S, Tain Y-L. Hydrogen Sulfide-to-Thiosulfate Ratio Associated with Blood Pressure Abnormalities in Pediatric CKD. Journal of Personalized Medicine. 2022; 12(8):1241. https://doi.org/10.3390/jpm12081241
Chicago/Turabian StyleHsu, Chien-Ning, Wei-Ling Chen, Wei-Ting Liao, Guo-Ping Chang-Chien, Sufan Lin, and You-Lin Tain. 2022. "Hydrogen Sulfide-to-Thiosulfate Ratio Associated with Blood Pressure Abnormalities in Pediatric CKD" Journal of Personalized Medicine 12, no. 8: 1241. https://doi.org/10.3390/jpm12081241
APA StyleHsu, C.-N., Chen, W.-L., Liao, W.-T., Chang-Chien, G.-P., Lin, S., & Tain, Y.-L. (2022). Hydrogen Sulfide-to-Thiosulfate Ratio Associated with Blood Pressure Abnormalities in Pediatric CKD. Journal of Personalized Medicine, 12(8), 1241. https://doi.org/10.3390/jpm12081241







