Ensemble Deep-Learning-Based Prognostic and Prediction for Recurrence of Sporadic Odontogenic Keratocysts on Hematoxylin and Eosin Stained Pathological Images of Incisional Biopsies
Abstract
:1. Introduction
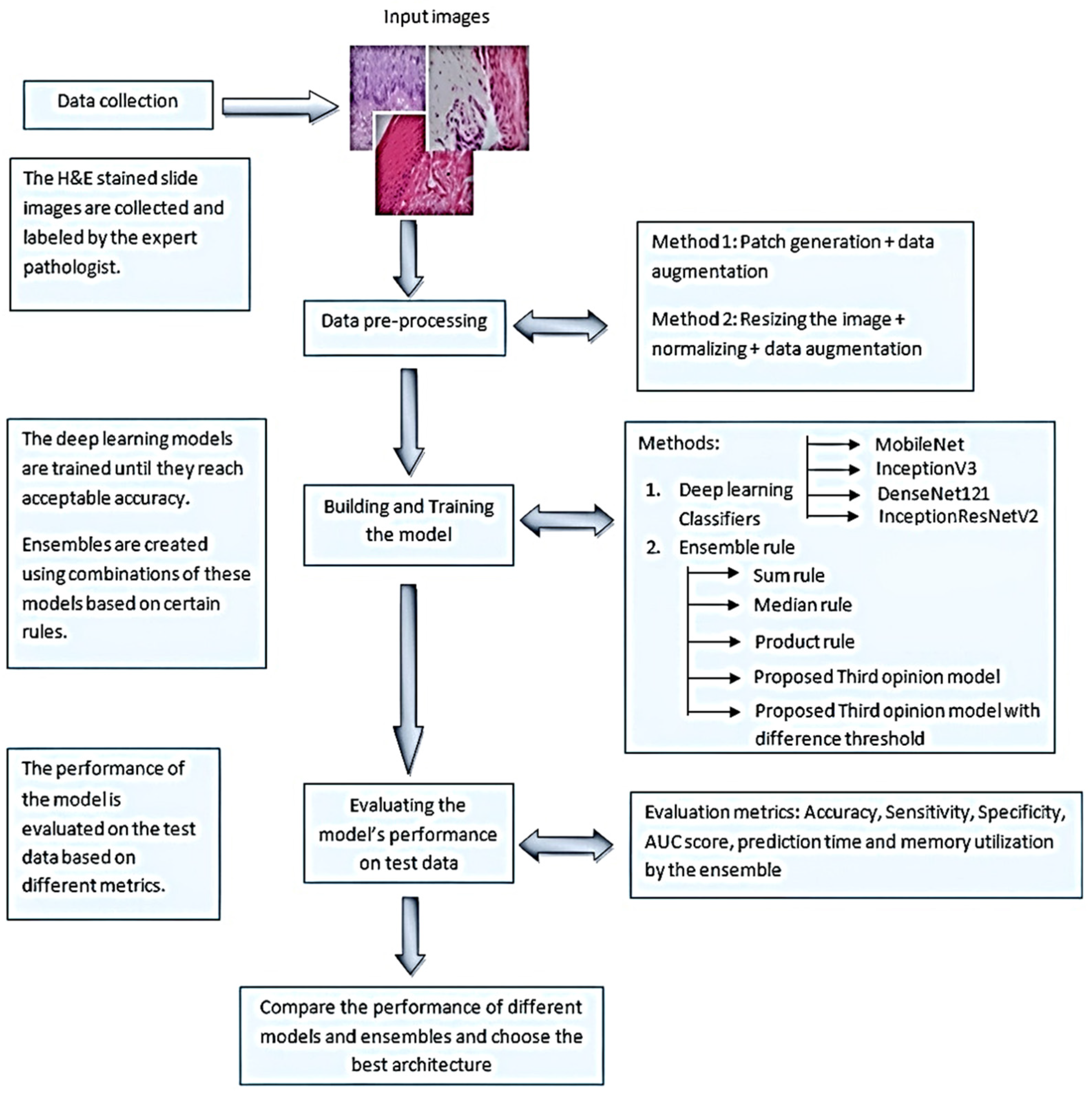
2. Materials and Methods
2.1. Selection of Participants
2.2. Classification of the Dataset
2.3. Image Characteristics
2.4. Deep Learning Classifiers and Computation
2.5. Data Pre-Processing and Training
2.6. Deep Learning Model Classifiers
2.7. The Traditional Ensemble Models
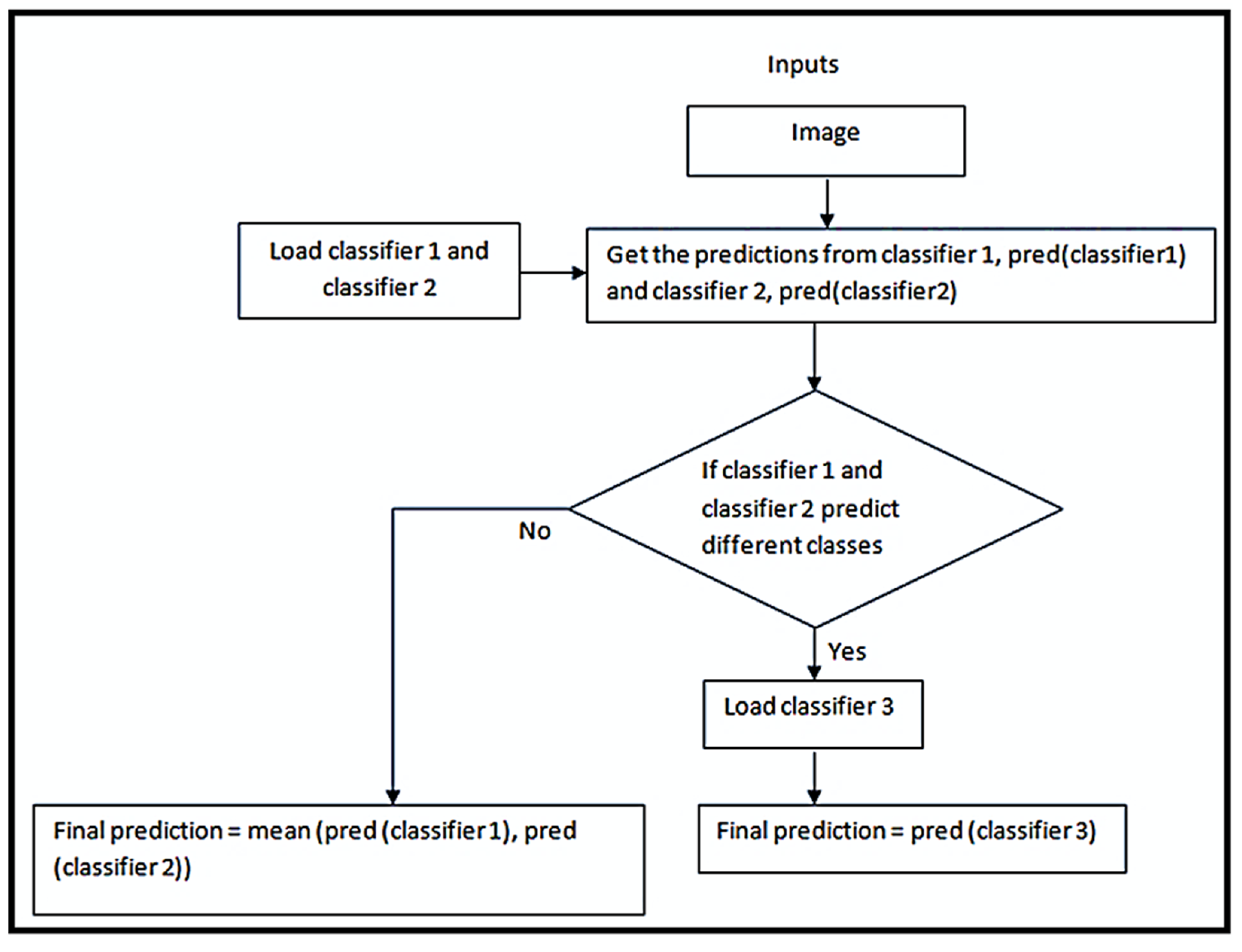
2.8. Novel Ensemble Model
| Algorithm 1 A novel ensemble model |
| Function ensemble_model (X_test): |
| load classifier_1 |
| load classifier_2 |
| for each sample in X_test: |
| p1 = prediction from classfier_1 |
| p2 = prediction from classifier_2 |
| if p1 and p2 predict different classes: |
| load classifier_3 if not already loaded |
| final_prediction = prediction from classifier_3 |
| else |
| final_prediction = mean of p1, p2 |
3. Results
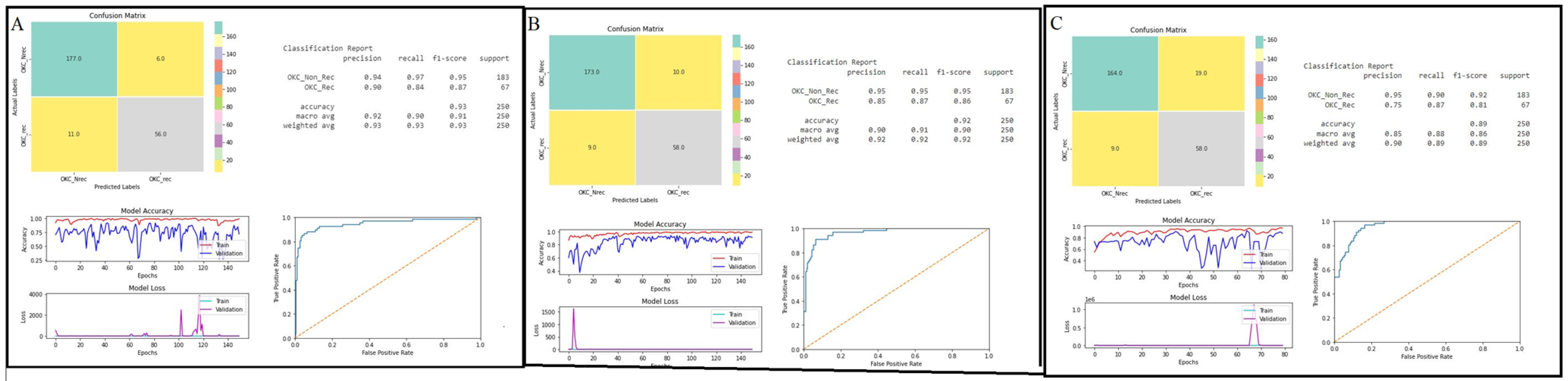
3.1. Evaluating the Model’s Performance
3.2. Ensemble Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuroyanagi, N.; Sakuma, H.; Miyabe, S.; Machida, J.; Kaetsu, A.; Yokoi, M.; Maeda, H.; Warnakulasuriya, S.; Nagao, T.; Shimozato, K. Prognostic factors for keratocystic odontogenic tumor (odontogenic keratocyst): Analysis of clinico-pathologic and immunohistochemical findings in cysts treated by enucleation. J. Oral Pathol. Med. 2009, 38, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Al Qahtani, S.; Dawasaz, A.A.; Saquib, S.A.; Asif, S.M.; Ishfaq, M.; Kota, M.Z.; Ibrahim, M. Management of an extensive odontogenic keratocyst. Medicine 2019, 98, e17987. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.; Rao, R.S.; Lakshminarayana, S.; Prasad, K.; Patil, S. Sub-epithelial hyalinization, incomplete cystic lining, and corrugated surface could be a predictor of recurrence in Odontogenic Keratocysts. J. Oral Biol. Craniofacial Res. 2021, 11, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pindborg, J.J.; Hansen, J. Studies On Odontogenic Cyst Epithelium. 2. Clinical and Roentgenologic Aspects of Odontogenic Keratocysts. Acta Pathol. Microbiol. Scand. 1963, 58, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.F.; Gomes, C.C.; de Mesquita, R.A.; Goulart, E.M.A.; de Castro, W.H.; Gomez, R.S. Clinicopathologic features associated with recurrence of the odontogenic keratocyst: A cohort retrospective analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Passi, D.; Singhal, D. Odontogenic Keratocyst (OKC) or keratocystic odontogenic tumor (KCOT). Journey of OKC from cyst to tumor to cyst again: Comprehensive review with recent updates on WHO classification. Int. J. Curr. Res. 2017, 9, 54080–54086. [Google Scholar]
- Cottom, H.E.; Bshena, F.I.; Speight, P.M.; Craig, G.T.; Jones, A.V. Histopathological features that predict the recurrence of odontogenic keratocysts. J. Oral Pathol. Med. 2011, 41, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Fidele, N.; Yueyu, Z.; Zhao, Y.; Tianfu, W.; Liu, J.; Sun, Y.; Liu, B. Recurrence of odontogenic keratocysts and possible prognostic factors: Review of 455 patients. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e491–e501. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.; Rao, R.S.; Patil, S. Hyalinization as a histomorphological risk predictor in oral pathological lesions. J. Oral Biol. Craniofacial Res. 2021, 11, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.-A.; Sá Mafra, P.-H.; Julia, R.-S.; Travençolo, B.; Silva, P.; Blumenberg, C.; Silva, V.; Paranhos, L. Accuracy of computer-aided image analysis in the diagnosis of odontogenic cysts: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e368–e378. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Lee, S.; Kwon, S.; Nam, W.; Cha, I.-H.; Kim, H.J. Deep learning-based survival prediction of oral cancer patients. Sci. Rep. 2019, 9, 6994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubreville, M.; Knipfer, C.; Oetter, N.; Jaremenko, C.; Rodner, E.; Denzler, J.; Bohr, C.; Neumann, H.; Stelzle, F.; Maier, A. Automatic Classification of Cancerous Tissue in Laserendomicroscopy Images of the Oral Cavity using Deep Learning. Sci. Rep. 2017, 7, 11979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.K.; Bose, S.; Maiti, A.K.; Mitra, B.; Mukherjee, G.; Dutta, P.K. Automatic identification of clinically relevant regions from oral tissue histological images for oral squamous cell carcinoma diagnosis. Tissue Cell 2018, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Koley, S.; Bose, S.; Maiti, A.K.; Mitra, B.; Mukherjee, G.; Dutta, P.K. Computer aided tool for automatic detection and delineation of nucleus from oral histopathology images for OSCC screening. Appl. Soft Comput. 2019, 83, 105642. [Google Scholar] [CrossRef]
- Welikala, R.A.; Remagnino, P.; Lim, J.H.; Chan, C.S.; Rajendran, S.; Kallarakkal, T.G.; Barman, S.A. Automated Detection and Classification of Oral Lesions Using Deep Learning for Early Detection of Oral Cancer. IEEE Access 2020, 8, 132677–132693. [Google Scholar] [CrossRef]
- Rao, R.S.; Shivanna, D.B.; Mahadevpur, K.S.; Shivaramegowda, S.G.; Prakash, S.; Lakshminarayana, S.; Patil, S. Deep Learning-Based Microscopic Diagnosis of Odontogenic Keratocysts and Non-Keratocysts in Haematoxylin and Eosin-Stained Incisional Biopsies. Diagnostics 2021, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Alpaydin, E. Introduction to Machine Learning (Adaptive Computation and Machine Learning Series), 4th ed.; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely Connected Convolutional Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 2261–2269. [Google Scholar]
- Gambarini, G.; Plotino, G.; Grande, N.M.; Testarelli, L.; Prencipe, M.; Messineo, D.; Fratini, L.; D’Ambrosio, F. Differential diagnosis of endodontic-related inferior alveolar nerve paraesthesia with cone beam computed tomography: A case report. Int. Endod. J. 2010, 44, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Längkvist, M.; Karlsson, L.; Loutfi, A. Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning. Pattern Recognit. Lett. 2014, 42, 11–24.a. [Google Scholar] [CrossRef] [Green Version]


| Histopathological Features | Recurrent OKCs | Non-Recurrent OKCs | ||
|---|---|---|---|---|
| Present (%) | Absent (%) | Present (%) | Absent (%) | |
| Subepithelial hyalinization | 75 | 25 | 35 | 65 |
| Lining (complete) | 0 | 100 | 25 | 75 |
| Lining (incomplete) | 100 | 0 | 75 | 25 |
| Keratinization (orthokeratinized) | 50 | 50 | 35 | 65 |
| Keratinization (parakeratinized) | 50 | 50 | 62.5 | 37.5 |
| Keratinization (mixed) | 0 | 100 | 2.5 | 97.5 |
| Keratin layer (thin) | 45 | 55 | 50 | 50 |
| Keratin layer (thick) | 45 | 55 | 50 | 50 |
| Keratin layer (mixed) | 10 | 90 | 0 | 100 |
| Corrugated surface | 70 | 30 | 92.5 | 7.5 |
| Folding of epithelium | 60 | 40 | 60 | 40 |
| Intracellular edema | 35 | 65 | 40 | 60 |
| Reversed polarity | 30 | 70 | 25 | 75 |
| Basilar hyperplasia | 50 | 50 | 35 | 65 |
| Rete pegs | 20 | 80 | 10 | 90 |
| Palisading | 90 | 10 | 95 | 5 |
| EPI/CT separation | 90 | 10 | 85 | 15 |
| Basal off-shoots | 30 | 70 | 17.5 | 82.5 |
| Daughter cysts | 35 | 65 | 20 | 80 |
| Inflammation (nil) | 45 | 55 | 32.5 | 67.5 |
| Inflammation (mild) | 20 | 80 | 42.5 | 57.5 |
| Inflammation (severe) | 35 | 65 | 25 | 75 |
| Histologic Parameters | Recurrence | χ2 | p-Value | ||
|---|---|---|---|---|---|
| Present | Absent | ||||
| Subepithelial hyalinization | Present | 51.7% | 48.3% | 8.543 | 0.004 |
| Absent | 16.1% | 83.9% | |||
| Lining | Complete | 0.0% | 100% | 6.000 | 0.023 |
| Incomplete | 40.0% | 60.0% | |||
| Keratinization | Ortho | 41.7% | 58.3% | 1.607 | 0.448 |
| Para | 28.6% | 71.4% | |||
| Mixed | 0.0% | 100.0% | |||
| Thickness of lining | Thin | 31.0% | 69.0% | 4.138 | 0.126 |
| Thick | 31.0% | 69.0% | |||
| Mixed | 100.0% | 0.0% | |||
| Folding of epithelium | Present | 33.3% | 66.7% | 0.0 | 1.000 |
| Absent | 33.3% | 66.7% | |||
| Corrugated surface | Present | 27.5% | 72.5% | 5.294 | 0.049 |
| Absent | 66.7% | 33.3% | |||
| Intercellular edema | Present | 30.4% | 69.6% | 0.141 | 0.783 |
| Absent | 35.1% | 64.9% | |||
| Reversed polarity | Present | 37.5% | 62.5% | 0.170 | 0.760 |
| Absent | 31.8% | 68.2% | |||
| Basilar hyperplasia | Present | 41.7% | 58.3% | 1.250 | 0.280 |
| Absent | 27.8% | 72.2% | |||
| Rete pegs | Present | 50.0% | 50.0% | 1.154 | 0.422 |
| Absent | 30.8% | 69.2% | |||
| Palisading | Present | 32.1% | 67.9% | 0.536 | 0.595 |
| Absent | 50.0% | 50.0% | |||
| EPI/CT separation | Present | 34.6% | 65.4% | 0.288 | 0.707 |
| Absent | 25.0% | 75.0% | |||
| Basal offshoots | Present | 46.2% | 53.8% | 1.227 | 0.326 |
| Absent | 29.8% | 70.2% | |||
| Daughter cysts | Present | 46.7% | 53.3% | 1.600 | 0.223 |
| Absent | 28.9% | 71.1% | |||
| Inflammation | Absent | 40.9% | 59.1% | 2.967 | 0.227 |
| Mild | 19.0% | 81.0% | |||
| Severe | 41.2% | 58.8% | |||
| Hyperparameter | Classifier 1 | Classifier 2 | Classifier 3 |
|---|---|---|---|
| Number of dense layers | 3 | 1 | 4 |
| Batch size | 72 | 64 | 84 |
| Number of epochs | 82 | 35 | 57 |
| Learning rate | 0.001 | 0.001 | 0.001 |
| Parameter | DenseNet-121 | Inception-Resnet-V2 | Inception-V3 |
|---|---|---|---|
| Performance of the base classifier | |||
| Accuracy (%) | 93 | 88 | 92 |
| AUC | 0.9452 | 0.9602 | 0.9653 |
| Performance of Ensemble Models | |||
| Traditional ensemble model (Sum rule) | Traditional ensemble model (Product rule) | Novel ensemble model | |
| Accuracy (%) | 95 | 88 | 96 |
| Average computational time (in seconds) | 192.9 | 198.5 | 154.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, R.S.; Shivanna, D.B.; Lakshminarayana, S.; Mahadevpur, K.S.; Alhazmi, Y.A.; Bakri, M.M.H.; Alharbi, H.S.; Alzahrani, K.J.; Alsharif, K.F.; Banjer, H.J.; et al. Ensemble Deep-Learning-Based Prognostic and Prediction for Recurrence of Sporadic Odontogenic Keratocysts on Hematoxylin and Eosin Stained Pathological Images of Incisional Biopsies. J. Pers. Med. 2022, 12, 1220. https://doi.org/10.3390/jpm12081220
Rao RS, Shivanna DB, Lakshminarayana S, Mahadevpur KS, Alhazmi YA, Bakri MMH, Alharbi HS, Alzahrani KJ, Alsharif KF, Banjer HJ, et al. Ensemble Deep-Learning-Based Prognostic and Prediction for Recurrence of Sporadic Odontogenic Keratocysts on Hematoxylin and Eosin Stained Pathological Images of Incisional Biopsies. Journal of Personalized Medicine. 2022; 12(8):1220. https://doi.org/10.3390/jpm12081220
Chicago/Turabian StyleRao, Roopa S., Divya Biligere Shivanna, Surendra Lakshminarayana, Kirti Shankar Mahadevpur, Yaser Ali Alhazmi, Mohammed Mousa H. Bakri, Hazar S. Alharbi, Khalid J. Alzahrani, Khalaf F. Alsharif, Hamsa Jameel Banjer, and et al. 2022. "Ensemble Deep-Learning-Based Prognostic and Prediction for Recurrence of Sporadic Odontogenic Keratocysts on Hematoxylin and Eosin Stained Pathological Images of Incisional Biopsies" Journal of Personalized Medicine 12, no. 8: 1220. https://doi.org/10.3390/jpm12081220
APA StyleRao, R. S., Shivanna, D. B., Lakshminarayana, S., Mahadevpur, K. S., Alhazmi, Y. A., Bakri, M. M. H., Alharbi, H. S., Alzahrani, K. J., Alsharif, K. F., Banjer, H. J., Alnfiai, M. M., Reda, R., Patil, S., & Testarelli, L. (2022). Ensemble Deep-Learning-Based Prognostic and Prediction for Recurrence of Sporadic Odontogenic Keratocysts on Hematoxylin and Eosin Stained Pathological Images of Incisional Biopsies. Journal of Personalized Medicine, 12(8), 1220. https://doi.org/10.3390/jpm12081220










