Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems
Abstract
:1. Introduction
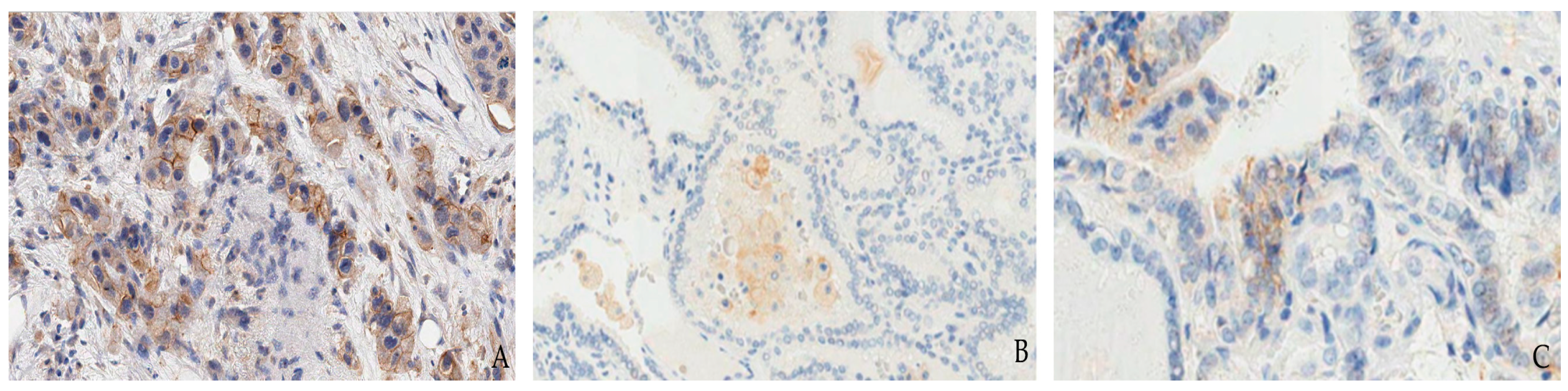
1.1. Lung Cancer
- (1)
- TPS/TC (tumor cell score): percentage of tumor cells with membranous positivity, regardless of staining pattern and intensity, evaluated on at least 100 viable tumor cells.
- (2)
- IC, considering a tumor area of at least 50 tumor cells, regardless of staining pattern and intensity. Like TC, the IC value is also divided into cut-off groups: IC0: <1%; IC1: 1–4%; IC2: 5–9%; IC3: ≥10%.
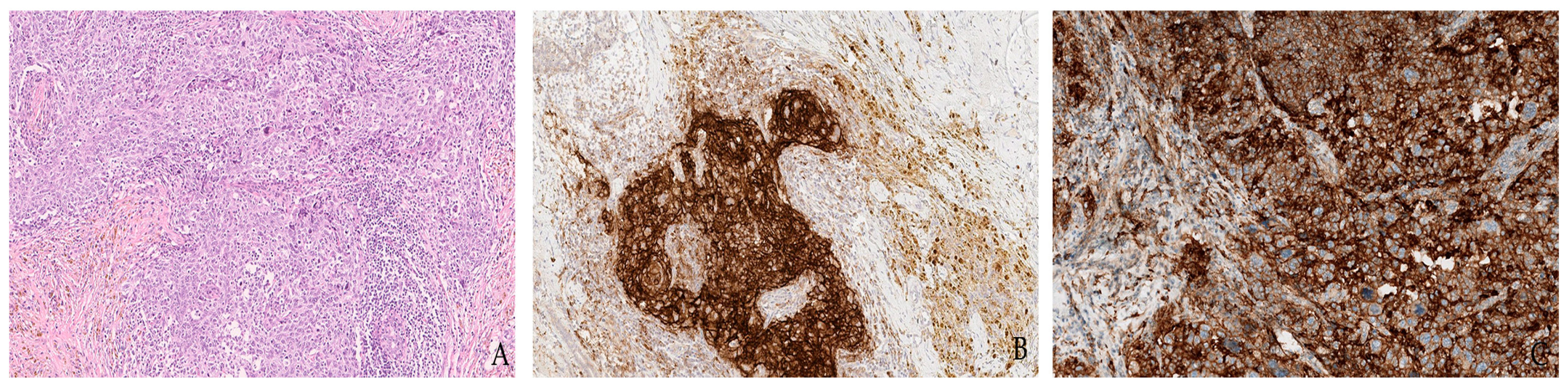
1.2. Gastrointestinal Cancers
1.3. Breast Cancer
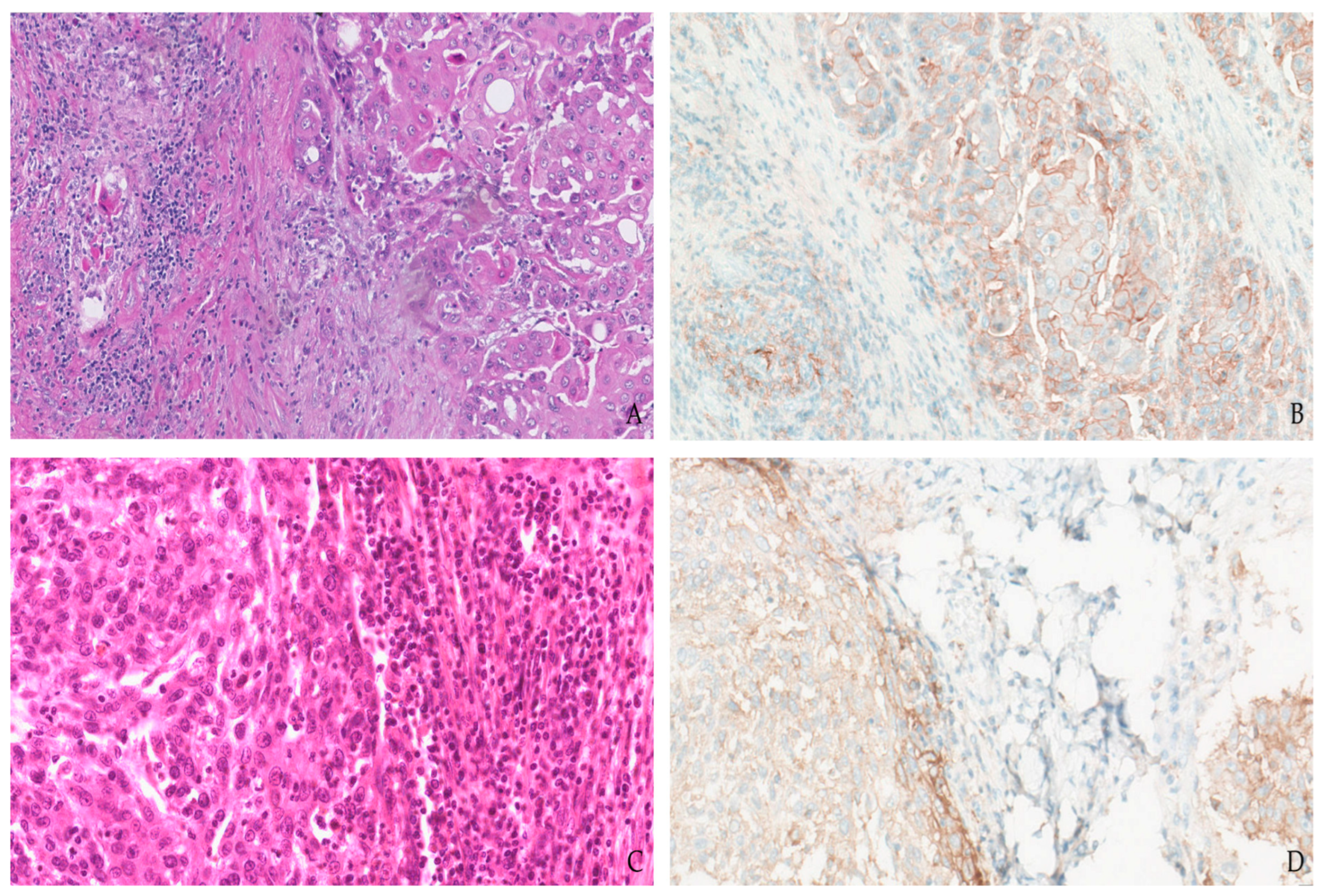
1.4. Urothelial Cancer
1.5. Kidney Cancer
1.6. Melanoma
1.7. Head and Neck Cancer
1.8. Cytopathology
1.9. Hematological Disorders
2. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munari, E.; Mariotti, F.R.; Quatrini, L.; Bertoglio, P.; Tumino, N.; Vacca, P.; Eccher, A.; Ciompi, F.; Brunelli, M.; Martignoni, G.; et al. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int. J. Mol. Sci. 2021, 22, 5123. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.; Labarriere, N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology 2017, 7, e1364828. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-Y.; Yan, Y.-Y.; Li, J.-J.; Adhikari, R.; Fu, L.-W. PD-1/PD-L1 Based Combinational Cancer Therapy: Icing on the Cake. Front. Pharmacol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Seliger, B. Basis of PD1/PD-L1 Therapies. J. Clin. Med. 2019, 8, 2168. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Irving, B.A.; Hodi, F.S. Molecular pathways: Next-generation immunotherapy—Inhibiting programmed death-ligand 1 and programmed death-1. Clin. Cancer Res. 2012, 18, 6580–6587. [Google Scholar] [CrossRef] [Green Version]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Sukari, A.; Nagasaka, M.; Al-Hadidi, A.; Lum, L.G. Cancer Immunology and Immunotherapy. Anticancer Res. 2016, 36, 5593–5606. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.-S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Vigliar, E.; Malapelle, U.; Bono, F.; Fusco, N.; Cortinovis, D.; Valtorta, E.; Spyridon, A.; Bimbatti, M.; Zocchi, M.; Piva, C.; et al. The Reproducibility of the Immunohistochemical PD-L1 Testing in Non-Small-Cell Lung Cancer: A Multicentric Italian Experience. Biomed Res. Int. 2019, 2019, 6832909. [Google Scholar] [CrossRef]
- Fusco, N.; Vaira, V.; Righi, I.; Sajjadi, E.; Venetis, K.; Lopez, G.; Cattaneo, M.; Castellani, M.; Rosso, L.; Nosotti, M.; et al. Characterization of the immune microenvironment in malignant pleural mesothelioma reveals prognostic subgroups of patients. Lung Cancer 2020, 150, 53–61. [Google Scholar] [CrossRef]
- Pagni, F.; Guerini-Rocco, E.; Schultheis, A.M.; Grazia, G.; Rijavec, E.; Ghidini, M.; Lopez, G.; Venetis, K.; Croci, G.A.; Malapelle, U.; et al. Targeting Immune-Related Biological Processes in Solid Tumors: We do Need Biomarkers. Int. J. Mol. Sci. 2019, 20, 5452. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, C.; Guerini-Rocco, E.; Vacirca, D.; Passaro, A.; de Marinis, F.; Barberis, M. The immune profile of EGFR-mutated non-small-cell lung cancer at disease onset and progression after tyrosine kinase inhibitors therapy. Immunotherapy 2018, 10, 1041–1045. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Mete, O.; Brunelli, M.; Marletta, S.; Colato, C.; Trimboli, P.; Crescenzi, A.; Bongiovanni, M.; Barbareschi, M.; et al. Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma. Endocr. Pathol. 2020, 31, 291–300. [Google Scholar] [CrossRef]
- Munari, E.; Rossi, G.; Zamboni, G.; Lunardi, G.; Marconi, M.; Sommaggio, M.; Netto, G.J.; Hoque, M.O.; Brunelli, M.; Martignoni, G.; et al. PD-L1 Assays 22C3 and SP263 Are Not Interchangeable in Non-Small Cell Lung Cancer When Considering Clinically Relevant Cutoffs: An Interclone Evaluation by Differently Trained Pathologists. Am. J. Surg. Pathol. 2018, 42, 1384–1389. [Google Scholar] [CrossRef]
- Straub, M.; Drecoll, E.; Pfarr, N.; Weichert, W.; Langer, R.; Hapfelmeier, A.; Götz, C.; Wolff, K.-D.; Kolk, A.; Specht, K. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget 2016, 7, 12024–12034. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.M.; Tsao, M.-S.; Nicholson, A.G.; Yatabe, Y.; Wistuba, I.I.; Hirsch, F.R. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennapusa, B.; Baker, B.; Kowanetz, M.; Boone, J.; Menzl, I.; Bruey, J.-M.; Fine, G.; Mariathasan, S.; McCaffery, I.; Mocci, S.; et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Jöhrens, K.; Rüschoff, J. The Challenge to the Pathologist of PD-L1 Expression in Tumor Cells of Non-Small-Cell Lung Cancer—An Overview. Curr. Oncol. 2021, 28, 5227–5239. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.J.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- FDA Expands Pembrolizumab Indication for First-Line Treatment of NSCLC (TPS ≥ 1%). Available online: https://www.fda.gov/drugs/fda-expands-pembrolizumab-indication-first-line-treatment-nsclc-tps-1 (accessed on 13 June 2022).
- FDA Approves Atezolizumab for First-Line Treatment of Metastatic NSCLC with High PD-L1 Expression. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclc-high-pd-l1-expression (accessed on 13 June 2022).
- FDA Approves Durvalumab for Extensive-Stage Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-extensive-stage-small-cell-lung-cancer (accessed on 13 June 2022).
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Högner, A.; Moehler, M. Immunotherapy in Gastric Cancer. Curr. Oncol. 2022, 29, 1559–1574. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Zhou, Y.; Yang, C.; Wei, S.; Bian, X.; Christopher, O.; Xie, L. The Prognostic and Clinicopathological Roles of PD-L1 Expression in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.I.; Lim, K.-H. Evolving Paradigms in the Systemic Treatment of Advanced Gallbladder Cancer: Updates in Year 2022. Cancers 2022, 14, 1249. [Google Scholar] [CrossRef]
- Thuss-Patience, P.; Stein, A. Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr. Oncol. 2022, 29, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.-H.; Doi, T.; Moriwaki, T.; Kim, S.-B.; Lee, S.-H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, K.-M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef]
- Fassan, M.; Brignola, S.; Pennelli, G.; Alberti, G.; Angerilli, V.; Bressan, A.; Pellino, A.; Lanza, C.; Salmaso, R.; Lonardi, S.; et al. PD-L1 expression in gastroesophageal dysplastic lesions. Virchows Arch. 2020, 477, 151–156. [Google Scholar] [CrossRef]
- Schrock, A.B.; Ouyang, C.; Sandhu, J.; Sokol, E.; Jin, D.; Ross, J.S.; Miller, V.A.; Lim, D.; Amanam, I.; Chao, J.; et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019, 30, 1096–1103. [Google Scholar] [CrossRef]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J., II; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: Histology, molecular pathology and clinical implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef]
- Grant, R.C.; Denroche, R.; Jang, G.H.; Nowak, K.M.; Zhang, A.; Borgida, A.; Holter, S.; Topham, J.T.; Wilson, J.; Dodd, A.; et al. Clinical and genomic characterisation of mismatch repair deficient pancreatic adenocarcinoma. Gut 2021, 70, 1894–1903. [Google Scholar] [CrossRef]
- Hu, Z.I.; Shia, J.; Stadler, Z.K.; Varghese, A.M.; Capanu, M.; Salo-Mullen, E.; Lowery, M.A.; Diaz, L.A.J.; Mandelker, D.; Yu, K.H.; et al. Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin. Cancer Res. 2018, 24, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, R.T.; Mattiolo, P.; Mafficini, A.; Hong, S.-M.; Piredda, M.L.; Taormina, S.V.; Malleo, G.; Marchegiani, G.; Pea, A.; Salvia, R.; et al. Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Pancreatic Cancer: Systematic Review and Still-Open Questions. Cancers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Luchini, C.; Cros, J.; Pea, A.; Pilati, C.; Veronese, N.; Rusev, B.; Capelli, P.; Mafficini, A.; Nottegar, A.; Brosens, L.A.A.; et al. PD-1, PD-L1, and CD163 in pancreatic undifferentiated carcinoma with osteoclast-like giant cells: Expression patterns and clinical implications. Hum. Pathol. 2018, 81, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastaki, S.; Irandoust, M.; Ahmadi, A.; Hojjat-Farsangi, M.; Ambrose, P.; Hallaj, S.; Edalati, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M.; et al. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. 2020, 247, 117437. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Criscitiello, C.; Guerini-Rocco, E.; Viale, G.; Fumagalli, C.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Malapelle, U.; Fusco, N. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anticancer. Agents Med. Chem. 2022, 22, 787–800. [Google Scholar] [CrossRef]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Ivanova, M.; Andaloro, S.; Guerini-Rocco, E.; Montagna, E.; Caldarella, P.; Veronesi, P.; Colleoni, M.; et al. Low-risk triple-negative breast cancers: Clinico-pathological and molecular features. Crit. Rev. Oncol. Hematol. 2022, 172, 103643. [Google Scholar] [CrossRef]
- Tokumaru, Y.; Joyce, D.; Takabe, K. Current status and limitations of immunotherapy for breast cancer. Surgery 2020, 167, 628–630. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Sousa, R.; Tolaney, S.M. Clinical Development of PD-1/PD-L1 Inhibitors in Breast Cancer: Still a Long Way to Go. Curr. Treat. Options Oncol. 2020, 21, 59. [Google Scholar] [CrossRef]
- Rugo, H.S.; Loi, S.; Adams, S.; Schmid, P.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.C.; Winer, E.P.; Kockx, M.; et al. LBA20—Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): Post-hoc analysis of IMpassion130. In Proceedings of the ESMO 2019 Congress, Barcelona, Spain, 3–6 July 2019; pp. v858–v859. [Google Scholar]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.; Scorer, P.; Barker, C.; Al-Masri, H. Comparison of patient populations identified by different PD-L1 assays in in triple-negative breast cancer (TNBC). Ann. Oncol. 2019, 30, iii4. [Google Scholar] [CrossRef]
- De Melo Gagliato, D.; Buzaid, A.C.; Perez-Garcia, J.; Cortes, J. Immunotherapy in Breast Cancer: Current Practice and Clinical Challenges. BioDrugs 2020, 34, 611–623. [Google Scholar] [CrossRef]
- FDA Approves Atezolizumab for PD-L1 Positive Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (accessed on 12 June 2022).
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Available online: https://www.nccn.org/guidelines/recently-published-guidelines (accessed on 12 June 2022).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- VENTANA PD-L1 (SP142) Assay Interpretation Guide for Triple-Negative Breast Carcinoma (TNBC). Ventana. Roche. Available online: https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp142-assay2.html (accessed on 12 June 2022).
- EAU. EAU Guidelines. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 1–4 July 2022; ISBN 978-94-92671-16-5. [Google Scholar]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet. Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- FDA Updates Prescribing Information for Keytruda and Tecentriq. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-updates-prescribing-information-keytruda-and-tecentriq (accessed on 12 June 2022).
- Cimadamore, A.; Scarpelli, M.; Massari, F.; Eckstein, M.; Gevaert, T.; Cheng, L.; Lopez-Beltran, A.; Montironi, R. Immunotherapy for urothelial cancer: From the diagnostic pathologist’s point of view. Expert Opin. Biol. Ther. 2020, 20, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef]
- Iacovelli, R.; Nolè, F.; Verri, E.; Renne, G.; Paglino, C.; Santoni, M.; Cossu Rocca, M.; Giglione, P.; Aurilio, G.; Cullurà, D.; et al. Prognostic Role of PD-L1 Expression in Renal Cell Carcinoma. A Systematic Review and Meta-Analysis. Target. Oncol. 2016, 11, 143–148. [Google Scholar] [CrossRef]
- Brunelli, M.; Martignoni, G.; Malpeli, G.; Volpe, A.; Cima, L.; Raspollini, M.R.; Barbareschi, M.; Tafuri, A.; Masi, G.; Barzon, L.; et al. Validation of a Novel Three-Dimensional (3D Fusion) Gross Sampling Protocol for Clear Cell Renal Cell Carcinoma to Overcome Intratumoral Heterogeneity: The Meet-Uro 18 Study. J. Pers. Med. 2022, 12, 727. [Google Scholar] [CrossRef]
- FDA Approves Pembrolizumab Plus Axitinib for Advanced Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma (accessed on 12 June 2022).
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Darmon-Novello, M.; Adam, J.; Lamant, L.; Battistella, M.; Ortonne, N.; Balme, B.; de la Fouchardière, A.; Chaltiel, L.; Gerard, E.; Franchet, C.; et al. Harmonization of programmed death-ligand 1 immunohistochemistry and mRNA expression scoring in metastatic melanoma: A multicentre analysis. Histopathology 2022, 80, 1091–1101. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet. Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Daud, A.I.; Wolchok, J.D.; Robert, C.; Hwu, W.-J.; Weber, J.S.; Ribas, A.; Hodi, F.S.; Joshua, A.M.; Kefford, R.; Hersey, P.; et al. Programmed Death-Ligand 1 Expression and Response to the Anti-Programmed Death 1 Antibody Pembrolizumab in Melanoma. J. Clin. Oncol. 2016, 34, 4102–4109. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Meshcheryakov, A.; Atkinson, V.; Blank, C.U.; Mandala, M.; Long, G.V.; Barrow, C.; Di Giacomo, A.M.; Fisher, R.; Sandhu, S.; et al. Crossover and rechallenge with pembrolizumab in recurrent patients from the EORTC 1325-MG/Keynote-054 phase III trial, pembrolizumab versus placebo after complete resection of high-risk stage III melanoma. Eur. J. Cancer 2021, 158, 156–168. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet. Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G.J.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs. investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G.; et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef]
- Crosta, S.; Boldorini, R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef]
- Girolami, I.; Pantanowitz, L.; Barberis, M.; Paolino, G.; Brunelli, M.; Vigliar, E.; Munari, E.; Satturwar, S.; Troncone, G.; Eccher, A. Challenges facing pathologists evaluating PD-L1 in head & neck squamous cell carcinoma. J. Oral Pathol. Med. 2021, 50, 864–873. [Google Scholar] [CrossRef]
- Paolino, G.; Pantanowitz, L.; Barresi, V.; Pagni, F.; Munari, E.; Moretta, L.; Brunelli, M.; Bariani, E.; Vigliar, E.; Pisapia, P.; et al. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol. Res. Pract. 2021, 226, 153605. [Google Scholar] [CrossRef]
- Ock, C.-Y.; Kim, S.; Keam, B.; Kim, S.; Ahn, Y.-O.; Chung, E.-J.; Kim, J.-H.; Kim, T.M.; Kwon, S.K.; Jeon, Y.K.; et al. Changes in programmed death-ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget 2017, 8, 97920–97927. [Google Scholar] [CrossRef] [Green Version]
- Pflumio, C.; Thomas, J.; Salleron, J.; Faivre, J.-C.; Borel, C.; Dolivet, G.; Sastre-Garau, X.; Geoffrois, L. Expression of immune response biomarkers (PD-L1, p16, CD3+ and CD8+ TILs) in recurrent head and neck squamous cell carcinoma within previously irradiated areas. Oncol. Rep. 2021, 45, 1273–1283. [Google Scholar] [CrossRef]
- Cheung, C.C.; Barnes, P.; Bigras, G.; Boerner, S.; Butany, J.; Calabrese, F.; Couture, C.; Deschenes, J.; El-Zimaity, H.; Fischer, G.; et al. Fit-For-Purpose PD-L1 Biomarker Testing For Patient Selection in Immuno-Oncology: Guidelines For Clinical Laboratories From the Canadian Association of Pathologists-Association Canadienne Des Pathologistes (CAP-ACP). Appl. Immunohistochem. Mol. Morphol. 2019, 27, 699–714. [Google Scholar] [CrossRef]
- Lantuejoul, S.; Damotte, D.; Hofman, V.; Adam, J. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J. Thorac. Dis. 2019, 11, S89–S101. [Google Scholar] [CrossRef]
- Iaccarino, A.; Salatiello, M.; Migliatico, I.; De Luca, C.; Gragnano, G.; Russo, M.; Bellevicine, C.; Malapelle, U.; Troncone, G.; Vigliar, E. PD-L1 and beyond: Immuno-oncology in cytopathology. Cytopathology 2021, 32, 596–603. [Google Scholar] [CrossRef]
- Gosney, J.R.; Boothman, A.-M.; Ratcliffe, M.; Kerr, K.M. Cytology for PD-L1 testing: A systematic review. Lung Cancer 2020, 141, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, M.S.; Kerr, K.M.; Kockx, M.; Beasley, M.-B.; Borczuk, A.C.; Botling, J.; Bubendorf, L.; Chirieac, L.; Chen, G.; Chou, T.-Y.; et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018, 13, 1302–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paintal, A.S.; Brockstein, B.E. PD-L1 CPS Scoring Accuracy in Small Biopsies and Aspirate Cell Blocks from Patients with Head and Neck Squamous Cell Carcinoma. Head Neck Pathol. 2020, 14, 657–665. [Google Scholar] [CrossRef]
- Liu, Z.; Williams, M.; Stewart, J.; Glisson, B.S.; Fuller, C.; Roy-Chowdhuri, S. Evaluation of programmed death ligand 1 expression in cytology to determine eligibility for immune checkpoint inhibitor therapy in patients with head and neck squamous cell carcinoma. Cancer Cytopathol. 2022, 130, 110–119. [Google Scholar] [CrossRef]
- Xie, W.; Medeiros, L.J.; Li, S.; Yin, C.C.; Khoury, J.D.; Xu, J. PD-1/PD-L1 Pathway and Its Blockade in Patients with Classic Hodgkin Lymphoma and Non-Hodgkin Large-Cell Lymphomas. Curr. Hematol. Malig. Rep. 2020, 15, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Mihalyova, J.; Kascak, M.; Duras, J.; Hajek, R. PD-1/PD-L1 inhibitors in haematological malignancies: Update 2017. Immunology 2017, 152, 357–371. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef] [Green Version]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.-M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J. Clin. Oncol. 2016, 34, 3733–3739. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, S.; Kim, P.-J.; Go, H.; Nam, S.J.; Paik, J.H.; Kim, Y.A.; Kim, T.M.; Heo, D.S.; Kim, C.W.; et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 2016, 68, 1079–1089. [Google Scholar] [CrossRef]
- Shi, M.; Roemer, M.G.M.; Chapuy, B.; Liao, X.; Sun, H.; Pinkus, G.S.; Shipp, M.A.; Freeman, G.J.; Rodig, S.J. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am. J. Surg. Pathol. 2014, 38, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Ribrag, V.; Moskowitz, C.H.; Michot, J.-M.; Kuruvilla, J.; Balakumaran, A.; Zhang, Y.; Chlosta, S.; Shipp, M.A.; Armand, P. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017, 130, 267–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokoph, N.; Larose, H.; Lim, M.S.; Burke, G.A.A.; Turner, S.D. Treatment Options for Paediatric Anaplastic Large Cell Lymphoma (ALCL): Current Standard and beyond. Cancers 2018, 10, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panjwani, P.K.; Charu, V.; DeLisser, M.; Molina-Kirsch, H.; Natkunam, Y.; Zhao, S. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018, 71, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Tsesmetzis, N.; Chioureas, D.; Kis, L.; Leventaki, V.; Drakos, E.; Panaretakis, T.; Grander, D.; Medeiros, L.J.; Young, K.H.; et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia 2017, 31, 1633–1637. [Google Scholar] [CrossRef]
- Chan, T.S.Y.; Khong, P.-L.; Kwong, Y.-L. Pembrolizumab for relapsed anaplastic large cell lymphoma after allogeneic haematopoietic stem cell transplantation: Efficacy and safety. Ann. Hematol. 2016, 95, 1913–1915. [Google Scholar] [CrossRef]
- Rigaud, C.; Abbou, S.; Minard-Colin, V.; Geoerger, B.; Scoazec, J.Y.; Vassal, G.; Jaff, N.; Heuberger, L.; Valteau-Couanet, D.; Brugieres, L. Efficacy of nivolumab in a patient with systemic refractory ALK+ anaplastic large cell lymphoma. Pediatr. Blood Cancer 2018, 65, e26902. [Google Scholar] [CrossRef]
- Wilcox, R.A.; Feldman, A.L.; Wada, D.A.; Yang, Z.-Z.; Comfere, N.I.; Dong, H.; Kwon, E.D.; Novak, A.J.; Markovic, S.N.; Pittelkow, M.R.; et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 2009, 114, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Pagni, F.; Malapelle, U.; Doglioni, C.; Fontanini, G.; Fraggetta, F.; Graziano, P.; Marchetti, A.; Guerini Rocco, E.; Pisapia, P.; Vigliar, E.V.; et al. Digital Pathology and PD-L1 Testing in Non Small Cell Lung Cancer: A Workshop Record. Cancers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Eccher, A.; Girolami, I.; Troncone, G.; Pantanowitz, L. Digital Slide Assessment for Programmed Death-Ligand 1 Combined Positive Score in Head and Neck Squamous Carcinoma: Focus on Validation and Vision. Front. Artif. Intell. 2021, 4, 684034. [Google Scholar] [CrossRef]
- Eccher, A.; Fontanini, G.; Fusco, N.; Girolami, I.; Graziano, P.; Rocco, E.G.; Martini, M.; Morbini, P.; Pantanowitz, L.; Parwani, A.; et al. Digital Slides as an Effective Tool for Programmed Death Ligand 1 Combined Positive Score Assessment and Training: Lessons Learned from the “Programmed Death Ligand 1 Key Learning Program in Head-and-Neck Squamous Cell Carcinoma”. J. Pathol. Inform. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Srinivas, C.; Bai, I.; Frederick, J.; Liu, W.; Sarkar, A.; Wang, X.; Nie, Y.; Portier, B.; Kapadia, M.; et al. Whole tumor section quantitative image analysis maximizes between-pathologists’ reproducibility for clinical immunohistochemistry-based biomarkers. Lab. Investig. 2017, 97, 1508–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inge, L.J.; Dennis, E. Development and applications of computer image analysis algorithms for scoring of PD-L1 immunohistochemistry. Immuno-Oncol. Technol. 2020, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, L.; Lv, L.; Fu, C.-C.; Jin, Y.; Zheng, Q.; Wang, B.; Ye, Q.; Fang, Q.; Li, Y. A new AI-assisted scoring system for PD-L1 expression in NSCLC. Comput. Methods Programs Biomed. 2022, 221, 106829. [Google Scholar] [CrossRef]

| Tumor | Indications | Scoring System (Clones) and Therapeutic Guidelines |
|---|---|---|
| Lung cancer | 1L/2L in stage IV NSCL or diffuse SCLC | TPS ≥ 1% (22C3, SP142, SP263) and IC ≥ 10% (SP142) * |
| GE cancer | 1L or following lines in stage IV | CPS ≥ 1 (22C3, 28-8) |
| Colon and pancreas cancer | 1L or following lines in stage IV MSI-H | IC ≥ 1% (28-8) (registration trial Check-Mate 142) |
| Breast cancer | 1L or following lines in stage IV TNBC | IC ≥ 1% (SP142) |
| Urothelial carcinoma | 1L platinum-unfit, 2L platinum-fit both in stage IV | CPS > 10 (22C3) and IC ≥ 5% (SP142) |
| Kidney cancer | 1L in stage IV RCC | Therapy given regardless of PD-L1 status (22C3, SP142, SP263) |
| Melanoma | 1L in stage IV melanoma | TPS ≥ 1% (22C3, 28-8, SP263) and MEL score > 2 (22C3) |
| HNSCC | 1L in recurrent or stage IV HNSCC +/− platinum | CPS ≥ 1 (22C3, SP263) or regardless of PD-L1 status (+ platinum) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marletta, S.; Fusco, N.; Munari, E.; Luchini, C.; Cimadamore, A.; Brunelli, M.; Querzoli, G.; Martini, M.; Vigliar, E.; Colombari, R.; et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J. Pers. Med. 2022, 12, 1073. https://doi.org/10.3390/jpm12071073
Marletta S, Fusco N, Munari E, Luchini C, Cimadamore A, Brunelli M, Querzoli G, Martini M, Vigliar E, Colombari R, et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. Journal of Personalized Medicine. 2022; 12(7):1073. https://doi.org/10.3390/jpm12071073
Chicago/Turabian StyleMarletta, Stefano, Nicola Fusco, Enrico Munari, Claudio Luchini, Alessia Cimadamore, Matteo Brunelli, Giulia Querzoli, Maurizio Martini, Elena Vigliar, Romano Colombari, and et al. 2022. "Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems" Journal of Personalized Medicine 12, no. 7: 1073. https://doi.org/10.3390/jpm12071073
APA StyleMarletta, S., Fusco, N., Munari, E., Luchini, C., Cimadamore, A., Brunelli, M., Querzoli, G., Martini, M., Vigliar, E., Colombari, R., Girolami, I., Pagni, F., & Eccher, A. (2022). Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. Journal of Personalized Medicine, 12(7), 1073. https://doi.org/10.3390/jpm12071073











