Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder that still lacks an efficient therapy. The barriers between the central nervous system (CNS) and the blood represent a major limiting factor to the development of drugs for CNS diseases, including ALS. Alterations of the blood–brain barrier (BBB) or blood–spinal cord barrier (BSCB) have been reported in this disease but still require further investigations. Interestingly, these alterations might be involved in the complex etiology and pathogenesis of ALS. Moreover, they can have potential consequences on the diffusion of candidate drugs across the brain. The development of techniques to bypass these barriers is continuously evolving and might open the door for personalized medical approaches. Therefore, identifying robust and non-invasive markers of BBB and BSCB alterations can help distinguish different subgroups of patients, such as those in whom barrier disruption can negatively affect the delivery of drugs to their CNS targets. The restoration of CNS barriers using innovative therapies could consequently present the advantage of both alleviating the disease progression and optimizing the safety and efficiency of ALS-specific therapies.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the degeneration of both upper and lower motoneurons. Patients usually die from respiratory failure after 3 to 5 years following the appearance of symptoms [1]. Several mechanisms contribute to the development and progression of ALS, including the aggregation and accumulation of ubiquitinated protein inclusions in motoneurons, alterations of mRNA processing, glutamate-mediated excitotoxicity, oxidative stress, mitochondrial dysfunction, and neuroinflammation [2]. Therapeutic options for ALS patients are very limited and mostly supportive and symptomatic. To date, only two drugs are FDA-approved for ALS: riluzole, which targets mainly the glutamatergic system, and edaravone, which targets oxidative stress. However, these molecules only modestly extend patient survival by a few months. Indeed, numerous drugs that targeted the main pathological mechanisms involved in ALS have been tested in clinical trials but failed to demonstrate a significant benefit in patients [3]. Failures of the numerous tested compounds can be explained in part by the choice of the target in regard to the complex pathophysiology of ALS, the small size of cohorts, and the heterogeneity of ALS patients. Despite continuous advances in drug discovery, the development of therapies targeting disease of the central nervous system (CNS) is complicated and limited by the presence of the blood–brain barrier (BBB) and/or blood–spinal cord barrier (BSCB). BBB/BSCB integrity has been rarely explored in ALS. Although some reports describe a disruption of these barriers, their role in the development and progression of the disease, or their potential consequences on drug delivery into the brain are still debatable. In this context, the objectives of this review are to shed light on BBB and BSCB alterations in ALS and their consequences on CNS-targeting therapeutics, to finally evaluate the interest of restoring the integrity of these barriers versus taking advantage of their alteration for drug administration. First, we will briefly describe the organization of normal BBB/BSCB and then focus on their alterations reported in ALS in regard to their association with the pathophysiology of the disease and their impact on drug pharmacokinetics. Therapeutic options to repair these barriers will be presented, as well as strategies that have been tested in ALS to overcome BBB/BSCB. Finally, we will discuss the interest of restoring its integrity to optimize the safety and distribution of a drug candidate designed to cross the BBB/BSCB versus considering BBB/BSCB disruption as an opportunity to reach the brain.
2. The CNS Barriers: A Protection System
2.1. Organization and Functions of the Normal BBB and BSCB
There are three principal biological interfaces between the blood and the brain [4]. The first barrier is the BBB and the BSCB formed by endothelial cells (ECs). The second barrier is the blood–cerebrospinal fluid barrier (BCSFB) located at the epithelial cells of the choroid plexus. Finally, the arachnoid barrier surrounding the brain under the dura is avascular and presents a small surface area, which limits its role in the exchanges between the blood and the brain [5]. The BBB is the largest interface for these exchanges and therefore represents the most important barrier to prevent the entry of various substances into the brain, including drugs [4]. The composition of BBB, as well as BSCB, is complex and includes numerous entities forming the neurovascular unit (NVU): ECs, mural cells, basement membrane, astrocytes, immune cells and neurons [6] (Figure 1). ECs of the CNS have unique properties as they are joined by tight junctions (TJs), a complex of claudins and occludins linked regulatory proteins, which prevent the paracellular transport of numerous substances into the brain. Adherence junctions (Cadherin, JAMs) are essential to the structural support and the formation of TJs [4]. Mural cells are formed by pericytes and vascular smooth muscular cells that are distributed along the capillaries and partially surround the endothelium [4]. These contractile cells are essential to the BBB functionality as they can regulate the cerebral blood flow [6]. ECs and pericytes secrete and are enclosed by a basement membrane composed of a mixture of laminin, fibronectin and type IV collagen, which is essential to the maintenance of BBB integrity. Astrocytic endfeet form a complex network surrounding the capillaries, which help to the induction and maintenance of the barrier function. The complexity of these barriers ensures a variety of functions combining physical, transport and metabolic barriers [4]. These integrated elements protect the brain from the entry of neurotoxic compounds and are essential to the maintenance of a stable brain homeostasis. However, they also prevent the entry from the blood into the brain of most drugs and represent the major burden in the development of therapeutics for brain disease, including ALS [7].
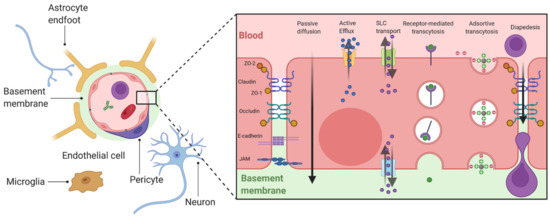
Figure 1.
General organization of the BBB and mechanisms of transport. ECs are polarized cells which present at the apical or basolateral membrane numerous membrane transporters allowing bidirectional exchanges between the brain and the blood. Passive diffusion concerns dissolved gazes and small weight liposoluble molecules (generally <400 Da) [4]. The passage of these molecules can be limited by the fact than they can be substrates of apical efflux transporters, mainly belonging to the ATP Binding Cassette (ABC) family of transporters [8]. Polar nutrients may diffuse across the BBB but mainly enter the brain via carrier transporters such as the solute carrier transporters (SLC) family [5]. Larger molecules such as peptides or proteins can enter into the brain by vesicular transport, including receptor-mediated transport which involves endocytosis by the fixation of a ligand to a receptor (e.g., transferrin and its receptor (TfR)), and adsorptive-mediated transcytosis which concerns cationic molecules [4]. Created with BioRender.com.
The brain represents the main consumer of energy in the body. Additionally, while the BBB/BSCB appear to impassable obstacles, essential nutrients and metabolites can cross these barriers by several mechanisms, including passive diffusion, solute carrier transport, and vesicular transport. Immune cells from the circulation can enter into the brain by diapedesis (under inflammatory conditions) (Figure 1).
2.2. The CNS Barriers: A Burden for Brain-Targeted Therapeutics
Notably, very few drugs with a CNS target can enter the brain via the mechanisms previously described. Moreover, the detection of drug concentration into the CSF does not necessarily indicate a transport across the BBB but only across the BCSFB, much more permeable than the BBB [7]. It is important to keep this in mind as the diffusion of a molecule into the brain from the CSF will be limited near the CSF surface [7]. Multiple approaches have been developed to overcome the BBB for peripherally administered drugs targeting the CNS (Table 1).

Table 1.
Summary of strategies to bypass the BBB/BSCB.
2.2.1. Mode of Administration
One strategy to bypass the BBB is the direct delivery of the drug into the CNS via intrathecal injection or convection-enhanced delivery. However, these methods are challenging and highly invasive. Moreover, they have limited diffusion near the injection site [9]. Bypassing the BBB can also be obtained by drug delivery to the brain through the nasal interface, where the molecule is deposited in the olfactory region and reaches the brain by crossing the olfactory epithelium [10].
2.2.2. Engineering of Drugs
A second option is the re-engineering of the drug with fusion to a Trojan horse molecule, leading to a bifunctional entity. The molecular Trojan horse domain then binds to a BBB receptor such as the insulin receptor or Transferrin Receptor (TfR) to trigger BBB transport via receptor-mediated transcytosis. Drugs can also be combined to nanoparticles which can be organic (such as micelles, liposomes, or nanoemulsions) or inorganic (such as iron oxide or gold nanoparticles) [11,12]. These nanocarriers are able to be functionalized with agents targeting BBB components, with tracers to monitor drug distribution or with chemical compounds to activate the drug release via a stimulus [11].
2.2.3. Permeabilization of the BBB
Other methods aim to transiently increase the permeability of the BBB either by using chemical strategies such as mannitol-mediated osmotic disruption and stimulation of bradykinin B2 receptor or by applying physical methods including radiation or microbubble-assisted focused ultrasound [10,13]. This last technique leads to local and reversible disruption of TJs. Modulation of active efflux transporter (P-gp, breast cancer resistance protein (BCRP), multidrug resistance proteins (MRPs)) by their direct inhibition or transcriptional repression is another possibility, but benefits are limited to the substrates of these transporters.
There is no consensus about an optimal strategy to efficiently deliver substances into the CNS. An important point is that this optimal strategy must take into consideration the state of the BBB in the concerned CNS disease.
3. Strategies to Evaluate the BBB Integrity
Numerous direct and indirect methods have been reported to assess the functionality of the BBB/BSCB. Each method has its specific advantages and limits, and none is used consensually, which limits the comparisons between studies.
Postmortem histological observation of the BBB may provide molecular and ultrastructural information. However, the use of optical imaging (e.g., transmission electronic microscope (TEM), confocal or conventional optical microscopy) before/after tissue immunostaining requires the sacrifice of a large number of animals. In humans, this approach only reflects the end stage of ALS. The approaches based on peripherally administered tracers with various molecular weights such as sodium fluoresceine, fluorescent-labeled dextrans or Evans Blue can directly and quantitatively assess the BBB permeability, but these methods are only available in preclinical models and still require animal sacrifice. Moreover, no tracer presents optimal properties (non-toxic, not bound to other molecules, available in various molecular sizes, viewable and quantifiable) for an accurate determination of the BBB permeability [5].
Dynamic Contrast-enhanced Magnetic Resonance Imaging (DCE-MRI) can be used to quantify the regional BBB permeability (leakage of MR contrast agents) or to observe microhemorrhages in live patients. Positron emission tomography (PET), such as FDG-PET or Verapamil PET, is sometimes used but mainly inform about GLUT1 or P-glycoprotein (P-gp) functionality, respectively. Imaging techniques display the advantages to be performed in live individuals, allowing longitudinal monitoring, but require expensive and high-resolution equipment, especially if used on small animals. Moreover, these techniques are prone to inter-equipment variability (e.g., sensitivity) thus preventing the comparison between research groups.
Indirect markers of BBB/BSCB integrity can be quantified in biological fluids, mainly blood and CSF. These markers include the elevation of blood-derived molecules in the CSF such as total immunoglobulins, proteins, or albumin, but also more specific markers of neurological disease such as glial fibrillary acidic protein (GFAP), neuron-specific enolase (NSE), or S100 beta proteins. Ratio of molecules concentration between CSF and blood can also be used, the albumin quotient (QAlb) being the most routinely employed marker of BBB permeability. However, QAlb does not accurately reflect the BBB permeability, as albumin can be uptaken by the brain macrophages or glial cells and its CSF concentration depends on the fluid production or resorption [14]. It is generally advised to combine the determination of various blood/CSF ratios (e.g., IgG or α2macroglobulin quotients).
4. BBB Alterations in ALS and Their Consequences
Evidence of BBB alterations in ALS have recently been reviewed [15,16]. A summary of these alterations can be visualized in Figure 2. This section will focus on the role of these BBB alterations in the pathophysiology of ALS and their potential consequences on drug distribution in the brain, which are summarized in Table 2.
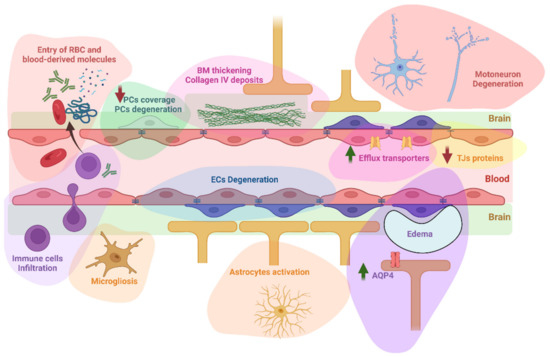
Figure 2.
A summary of BBB/BSCB alterations reported in ALS. These alterations include the infiltration into the brain of circulating erythrocytes and immune cells, but also blood-derived molecules such as immunoglobulins G, complement C3, albumin, thrombin, or fibrin. An activation of astrocytes and microglia has also been reported. Degeneration of endothelial cells and pericytes has been observed, with a decrease of capillary pericytes coverage. The basement membrane was found thickened with observation of collagen IV deposits in humans. Other reported alterations include downregulation of tight junction’s proteins, upregulation of P-glycoprotein, Breast Cancer Resistance Protein, and Aquaporin 4, and formation of extracellular edema. Whether motoneuron degeneration is linked to these alterations still require further investigations as detailed below. AQP4: aquaporin 4; BM: basement membrane ECs: endothelial cells; PCs: pericytes; RBC: red blood cells; TJs: tight junctions. Created with BioRender.com.

Table 2.
Findings of BBB/BSCB alterations in animal models and humans.
4.1. Role of Alteration of the BBB in the Pathogenesis of ALS
4.1.1. Findings from Animal Models
Structural and functional impairment of BBB and BSCB have been demonstrated in a SOD1 mutated (G93A) mouse model of ALS through Evans blue leakage and the ultrastructure observation of the BBB components by TEM showed degenerated endothelial cells and astrocytes, extracellular edema or erythrocytes infiltration, which seem to worsen with disease evolution [17,18]. In various SOD1 mouse models, Zhong et al. also observed signs of BBB alterations (reduction of TJ proteins level, hemosiderin deposits, reduction of capillaries length, and cerebral blood flow) in presymptomatic stages of the disease, before the detection of motoneuron loss and modification of inflammatory markers. These findings suggest that BBB damage might precede neurovascular inflammation and initiate symptoms [19]. A recent study also found signs of BBB alterations before the observation of neuromuscular denervation, within 30 days postnatal [20]. Moreover, TDP-43 overexpression obtained with intracranial injection of adeno-associated virus (AAV) vector containing the gene TARDBP in wild type mice was recently found to induce BBB permeability and inflammation leading to impaired motor learning, motoneuron loss, activation of astrocytes, and microgliosis [21]. The fact that the loss of BBB integrity promotes neuroinflammation, a key feature of ALS pathological mechanism [22], supports its implication in the pathophysiology of the disease. However, on the other side, in a SOD1 mutated (G93A) rat model of ALS, Nicaise et al. observed signs of BBB alteration only at the symptomatic stage while IgG deposits were seen at presymptomatic stages, suggesting that BBB opening could be induced by pro-inflammatory cytokines [23]. The rare imaging studies conducted mostly on SOD1 mutated rat models showed a positive correlation between infiltration of lymphocytes and gadolinium signal [24,25]. However, one study conducted on SOD1 mutated (G93A) mice did not show elevation of Gadolinium leakage despite astrocytes activation and microgliosis, thus suggesting that BBB breakdown might not be a pathological aspect of all ALS cases [26]. Although decreased mRNA or protein levels of TJ proteins (mainly ZO-1, claudin 5, occludin) have been reported at diverse stages of the disease, TJs appeared structurally normal in TEM observation, both in animals and humans, which questions on the impact of their mRNA or protein downregulation on the paracellular pathway [17,23]
4.1.2. Findings from Human Patients
Early evidence of BBB disruptions has been suggested with the observation of IgG and complement C3 deposits as well as lymphocyte and macrophage infiltration in the spinal cord or the cortex of postmortem ALS samples [27,28,29]. Some neuroimaging-based studies performed in ALS patients and regarding BBB permeability reported iron deposits, indicator of microhemorrhages and oxidative stress observed in ALS, but these findings remain debatable [30,31,32]. BBB opening using MR-guided focused ultrasound has been recently performed in four ALS subjects [33]. The gadolinium leakage normalized within 24 h, showing the reversibility of the procedure but also the absence of signs of previous BBB disruption in these patients. Moreover, elevation of QAlb has been reported in only 20–50% of ALS patients, suggesting that BBB disruption would not appear in all individuals [34,35,36]. Recently, Waters et al. showed that BSCB disruption, evidenced by hemoglobin leakage in postmortem human tissues, would be predominant in the thoracic spinal cord while motoneurons loss and TDP-43 deposits were mainly observed in the cervical and lumbar spinal cord. These data suggest that BSCB leakage in ALS is independent from motoneuron pathology [37]. Detection and evaluation of circulatory markers have almost exclusively been performed in human studies, as mouse CSF volume is very limited. Among the most recent ones, Li et al. compared several CSF parameters (total proteins, albumin, IgG, myelin basic protein) in addition to the QAlb and Quotient IgG (QIgG) between 113 ALS patients, 12 FTD-ALS patients, and 40 disease controls [38]. They found that CSF total proteins, CSF IgG, CSF albumin, QAlb and QIgG were significantly elevated in ALS patients. Moreover, CSF total protein, CSF IgG, and QIgG were significant indicators of disease progression. On the other side, Prell et al. evaluated the QAlb in a cohort of 160 ALS patients and 31 ALS mimicking conditions but did not find any significant association with the disease evolution [34].
4.1.3. Further Necessary Investigations
In summary, little is known about the implication of BBB disruption in the pathogenesis of ALS. Histological evaluation in humans is limited to postmortem studies which prevents the estimation of the kinetics of BBB ultrastructure alterations. Therefore, animal models are essential to the longitudinal evaluation of BBB disruption. Despite a large variety of ALS animal models [39], studies evaluating BBB alterations focused almost exclusively on SOD1 mutated rodents which validity is questionable. However, SOD1 mutations represent only 1–2 % of sporadic ALS cases, and the number of genes associated with ALS has risen dramatically [40]. Even if QAlb is not a perfect marker of BBB integrity, the inconsistency of its elevation illustrates, once again, the complex heterogeneity of ALS, and suggests the implication of different pathophysiological mechanisms in different subsets of patients. It also highlights the limits of the different tools used to assess BBB integrity.
To date, studies failed to decipher a clear relationship between BBB disruption and pathological mechanisms. The beneficial effects of the restoration of BBB integrity in SOD1 mutated mice, as discussed below, might support its implication in the pathogenesis of the disease, at least for this specific genetic background. A deep understanding of this phenomenon and its consequences will need: (1) structural and functional investigations in more animal models with various genetic background or even who mimics ALS pathogenesis [39] and (2) robust and accurate markers of BBB integrity routinely available in clinical practice.
4.2. Impact of the BBB Alterations on Drug Pharmacokinetics in ALS
Alterations of BBB ultrastructure and functionality would make this barrier leaky, as evidenced by the accumulation of blood-derived proteins or the infiltration of immune cells [15]. Moreover, permeability assays based on peripherally administered tracers show leakage of molecules with a wide range of molecular weights: from 376 Da sodium fluoresceine [41] to Evans Blue (representative of high molecular weight molecules permeability by binding to 65 kDa-albumin) [23]. This may suggest that a drug initially unable to cross the BBB (from small chemical molecules to high molecular weight antibodies) might still penetrate into the brain of ALS patients and reach its central target, without the need for a bypass strategy. However, it may also increase the cerebral toxicity via the entry of neurotoxic compounds, pathogens or therapies taken by the patient for other comorbidities. As clinical trials conducted on ALS patients continue to fail, this suggests that the tested molecules are either ineffective, or that they do not reach their target despite BBB alterations.
4.2.1. Upregulation of Efflux Transporters
Efflux transporters may explain the impossibility for a drug to reach its target. Indeed, BCRP and P-gp may be upregulated in ALS [15,42,43]. These transporters are widely expressed in various tissues and limit the absorption or accelerate the elimination of numerous conventional drugs, which may suggest their contribution to many treatment failures [44]. As an example, Riluzole is a substrate of these proteins, which may explain its modest efficiency in ALS. Jablonsky et al. observed an increased mRNA and protein expression of these transporters, as well as an elevated transport activity in symptomatic SOD1 (G93A) mutated mice [43]. They also reported an elevation of protein expression in a TDP-43 A315T mouse model and in ALS patients (but comparing only 3 patients to 2 controls) [43]. However, although these results have been successfully replicated for P-gp in other SOD1 ALS models, this was not the case for BRCP [15]. An immunohistochemical evaluation of P-gp and BCRP in 25 ALS patients and 14 controls revealed a strong increase in these proteins in glial cells but not in blood vessels [45]. In 2019, Mohammed et al. found elevated protein expression of P-gp in human-iPS-derived ECs after co-culture with ALS human iPS-derived astrocytes [46]. Notably, P-gp was upregulated for SOD1 and sporadic ALS derived astrocytes but not for C9ORF72, suggesting different alterations according to genetic background. Moreover, they showed that this upregulation of P-gp in ECs seemed mediated by glutamate release from astrocytes.
4.2.2. Drugs Diffusion into the Brain after Crossing the Barrier
When a drug succeeds in crossing the barrier formed by the ECs, it still needs to diffuse across the interstitial fluid to reach its target such as neurons. The distance between ECs and the neurons or glial cells is short, but some of the modifications observed in ALS might limit the diffusion of the molecule to its target. For example, accumulation of collagen IV has been reported in the brain or spinal cord of ALS patients [37,47]. In SOD1 G93A mice, collagen IV staining was progressively reduced in vascular structures but remarkably increased in tissues, as compared to non-transgenic mice [48]. Ultrastructure observations of the BBB in TEM also showed a thickening of the basement membrane in the brainstem, and in the cervical and lumbar spinal cord of SOD1 G93A mice [17]. The accumulation of collagen IV and the subsequent basement membrane thickening or blood-derived molecules deposition might form another barrier limiting the access of the candidate drug to its target cells [49]. This mechanism has been suggested to explain the reduced brain uptake of [3H] diazepam and [3H] propranolol in a mouse model of Alzheimer disease [50].
Moreover, TEM analyses also showed extracellular edema in spinal cord and brainstem in both animals and humans [17,47]. This phenomenon might also affect the penetration and distribution of the drug in the brain. In fact, Binder et al. reported that cytotoxic brain edema produced by water intoxication slowed the diffusion of fluorescein-dextran in the mouse brain and created dead-space microdomains in which free diffusion was prevented [51]. In this study, the deletion of aquaporin 4 (AQP4), a glial water channel enhanced the fluorescein-dextran diffusion in the extracellular space. As AQP4 was found upregulated in ALS [52,53,54], it could also be a limiting factor to the brain diffusion of candidate therapies.
4.2.3. Limitation of Barrier Bypass Strategies by BBB Alterations
Most strategies to bypass the BBB rely on a healthy BBB, but its alteration in ALS could limit the efficiency of these strategies.
TfR is one of the most popular BBB receptors targeted in the molecular Trojan horse technology. However, iron metabolism is altered in ALS, and TfR appears to be dysregulated in different ways depending on the genetic background. Overexpression of wild type SOD1 and SOD1 G93A but not SOD1 H46R mutation induced an increase in the protein expression of TfR in an in vitro experiment [55]. Another study found an elevation in the mRNA expression of TfR in G93A-SOD1 cells, which is consistent with higher iron uptake [56]. On the other hand, mutations in OPT were associated with the degradation of TfR via autophagosomes, although this effect seemed limited to RGC-5 cells [57]. These reports suggest that the efficacy of a therapy using TfR targeted Trojan horse molecular strategy could vary in ALS.
Prevention of drug penetration across the CNS by the accumulation of collagen IV and the thickening of the basement membrane may also limit the efficacy of bypass strategies such as intrathecal administration, nanoparticles, or BBB permeabilization [49]. Focused ultrasounds used for the delivery of therapeutic agents to the brain might need to be adjusted for application in CNS disorders. This hypothesis is illustrated in a preclinical mouse model of Alzheimer’s disease that displays a thickening of the basement membrane and where vessels were less permeable following focused ultrasounds application as compared to non-transgenic mice [58].
4.2.4. Spatial and Temporal Alterations: A Source of Variability
As mentioned above, the kinetics of BBB disruption in ALS are unclear, as some studies reported signs of alteration at presymptomatic stages while others only after the appearance of symptoms. However, almost all studies which evaluated these alterations longitudinally agree that they tend to worsen as the disease progress. Thus, in addition to the inter-individual variability that seems to be reported for BBB disruption in ALS, there might also be an intra-individual variability that must be taken into account when considering the consequence of these alterations on the passage and diffusion of a candidate drug into the CNS.
Moreover, the breakdown of the BBB is not uniform across the spinal cord and the brain. As previously discussed, Waters et al. evaluated the BSCB leakage of hemoglobin across the spinal cord of ALS patients and found that it was more severe at the thoracic level than the cervical or lumbar levels [37]. Moreover, collagen IV for example was only elevated in the white matter of the spinal cord but not in the gray matter. As TDP-43 inclusions and altered motoneurons are predominant in the cervical and lumbar spinal cord, this pattern does not correlate with the BSCB alterations. Therefore, the possible leakage into the brain of a drug candidate via BBB/BSCB disruption might not even benefit the cells that need it the most. The localization of primally affected motoneurons is different between patients with spinal onset (muscle weakness of the limbs) and patients with bulbar onset (dysarthria, dysphagia, speech difficulty) [2]. The localization of the increased BBB permeability reported in ALS might not be associated with that of affected motoneurons in spinal or bulbar forms.
These phenomena illustrate the unpredictable pharmacokinetics of CNS targeted therapies in ALS, both among different patients and throughout the progression of the disease in the same patient, which might be hazardous for drugs with a narrow therapeutic index.
The consequences of BBB alterations on brain distribution of drug candidates are poorly understood. They might vary between subjects, throughout the evolution of the disease, across the different regions of the spinal cord or the brain. Such alterations might even limit the efficacy of a bypass strategy. Thus, monitoring and restoring the BBB integrity may be a valuable therapeutic strategy to optimize the administration of therapeutic molecules (Figure 3).
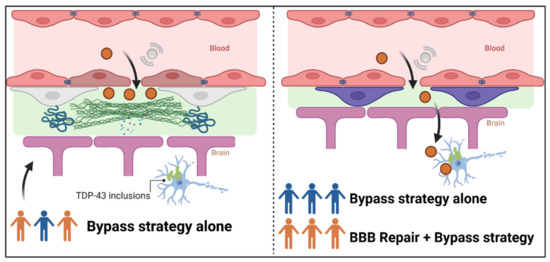
Figure 3.
Interest of restoring BBB alterations to optimize the administration of therapeutic molecules. The left panel represents the distribution into the brain of a molecule with transitory opening of the tight junction proteins via microbubble-associated focused ultrasound. In patients who display BBB alterations (orange patients), the deposit of collagen IV and thickening of basement membrane, for example, might prevent the drug from reaching its target in the degenerating motoneurons presenting TDP-43 inclusions. In the right panel, patients have been stratified according to their BBB integrity: intact (blue patients) or disrupted (orange patients). Combination of the BBB repair and a bypass strategy for patients with a disrupted BBB could lead to the disappearance of deposits and membrane thickening and allow the molecule to reach its target, similarly to blue patients with intact BBB.
5. Therapeutic Strategies by Correcting BBB/BSCB Alterations
Approaches to restore or protect the BBB or BSCB in neurodegenerative or other diseases have already been reviewed elsewhere [14,59]. Briefly, therapeutic methods have focused on the BBB restoration with preservation of ECs and TJs, reducing the formation of edema by targeting AQP4, preventing the degradation of basement membrane by matrix metalloproteinases, elimination of neurotoxic deposits, enhancement of the clearance function and stem cell transplantation therapies to regenerate damaged tissues [14,59]. Here, we will focus on approaches that have been evaluated in ALS models and on recent advances in this field.
5.1. Previous Attempts in ALS
Activated Protein C (APC) and analogs of this molecule have been evaluated in SOD1 mutated (G93A) mice [60,61]. They reduced damage to the BSCB with a blockade of IgG and hemoglobin leakage and reduction of microhemorrhages (evidenced by hemosiderin staining) and free iron. The repair of the barrier function was probably due to the observed restoration of TJ proteins levels. Moreover, APC and its analog increased the lifespan of mice and the duration of the symptomatic phase. Notably, in one of these studies, another therapy evaluated (iron chelation) only alleviated a specific aspect of BBB disruption (iron deposit) with modest beneficial effects. Importantly, therapies given pre-symptomatically [60] extend more the lifespan as compared to post symptomatic treatment [61], meaning that early maintenance or repair of the BBB is more beneficial. These findings also support the role of the BBB disruption in the pathophysiology of the disease. Antagonizing the CXC4 receptor was also found to restore BSCB permeability and improve the survival of SOD1 G93A mice [62].
Stem cells transplantation for BBB restoration has been evaluated in vitro or in ALS preclinical models. Mesenchymal stromal cells and pericytes have been evaluated on presymptomatic SOD1 mutated (G93A) mice [63]. Only pericytes had a slight significant effect on survival. However, no signs of BBB restoration were evaluated here. Other studies aiming to restore the BBB in ALS have been led by Garbuzova-Davis et al. Their goal was to restore the BBB alterations with peripheral administration of cells able to engraft themselves within the capillaries of the spinal cord and to differentiate into functional ECs. In various studies, they evaluated the effects of human bone marrow-derived CD34+ cells (hBM34+) or restricted-lineage endothelial progenitor cells: human bone marrow-derived endothelial progenitor cells (hBMEPCs) [64,65,66,67,68,69]. In summary, they found that both lineages ameliorated ALS outcomes and allowed for BBB repair. These findings were based on the following significant observations: reduction of Evans blue leakage, decreased astrocytosis and microgliosis, amelioration of capillaries ultrastructure, reduction of microhemorrhages, enhancement of basement membrane integrity, restoration of pericyte coverage, endothelial markers and TJ proteins. hBMEPCs displayed better engraftment and differentiation than hBM34+ cells, leading to improved outcomes and BBB integrity restoration. Recently, extracellular vesicles (EV) derived from hBMEPCs were beneficial to mouse brain ECs [70]. This illustrates the potential of EV-derived hBMEPCs as an innovative BBB-restoring therapy in ALS, but preclinical investigation is now necessary.
5.2. Recent Advances with Direct and Indirect BHE Targeting
5.2.1. Stem-Cell Therapies in Human
Stem-cell based therapies have been tested in ALS patients and demonstrated some benefits without substantial improvement in disease progression [71,72,73]. To our knowledge, no evaluation of BBB permeability following stem-cell based therapies was performed on patients. In adrenoleukodystrophy, a X-linked peroxisomal disorder, regions of demyelination are associated with gadolinium enhancement on MRI, sign of BBB disruption [74]. Hematopoietic cell transplant, the only therapy which stops the neurologic progression of the disease, allowed gadolinium resolution (indicator of BBB repair) within 100 days for almost all patients [75]. The underlying proposed mechanism is that donor mononuclear cells or microglia precursors would cross the BBB, differentiate into microglial cells, and attenuate neuroinflammation.
5.2.2. Targeting Oxidative Stress and Inflammation
Caspase-1, a core component of the inflammasome complex is a promising therapeutic target in CNS disorders with neuroinflammation. Inhibition of caspase-1 reduced the transmigration of mononuclear cells across an in vitro BBB model exposed to an inflammasome-dependent pro-inflammatory response and restored the BBB integrity probably via the restoration of cadherin adherens protein [76]. Moreover, inhibition of caspase-1 also demonstrated its efficiency in vivo in various CNS disorders such as Alzheimer’s disease [77], Parkinson’s disease [78] and multiple sclerosis [79] which illustrates its potential as a target for BBB repair [80]. Utilization of inhibitors of caspase-1 may be a promising therapeutic approach via the restoration of BBB integrity, which could allow for a better control of therapeutic administration and an improvement of neuroinflammation-induced symptoms. Interestingly, melatonin, a non-specific inhibitor of caspase-1 activation has previously been administered in SOD1 mutated mice and revealed an improvement in disease progression and an amelioration of motoneuron loss and spinal cord atrophy. However, its effect on BBB permeability were not evaluated [81].
In a mouse model of BBB disruption induced by traumatic brain injury, pharmacological elevation of nicotinamide adenine dinucleotide (NAD) restored the BBB integrity, as suggested by the reduction of IgG infiltration and the number of active microglia [82]. They also observed a restoration of endothelium length and an increase in capillary pericytes and TJ proteins. Moreover, this treatment restored the native BBB permeability to 3kDa dextran, initially enhanced after administration of Liposaccharides (LPS) and protected cultured human microvascular endothelial cells from oxidative stress. Target Nrf2 antioxydative signaling might also be a therapeutic target, as its stimulation by fenretinide protected the BBB against LPS in a mouse model [83]. Neutralization of the pro-inflammatory factor High mobility group box-1, by monoclonal antibody or inhibition of its release also showed a protective effect on BBB integrity [84].
5.2.3. BBB Restoration in Combination of BBB-Opening Strategy
As mentioned above, acoustically mediated BBB opening using microbubble-assisted focused ultrasounds can locally and transiently increase the permeability of BBB for drug delivery. Normally, TJ proteins return to baseline levels within few hours following focused ultrasounds. However, this restoration might be impaired in neurodegenerative diseases with preexisting alterations in TJs, thus leading to a longer exposure of the CNS to neurotoxic blood components. For this matter, Lynch et al. explored the capacity of vasculotide, an angiopoietin 1 mimetic peptide, to restore BBB permeability after focused ultrasounds application [85]. Vasculotide was chronically administrated to a mouse transgenic model of Alzheimer’s disease. BBB restoration was assessed by longitudinal gadolinium enhancement after MRI-guided focused ultrasounds BBB-opening. Vasculotide significantly reduced BBB closure time (without alteration of the opening) and histological analysis (24 h post focused ultrasounds) revealed that the brains of treated animals displayed less infiltration of blood-derived molecules and cells (IgG, fibrinogen, erythrocytes). These results highlight the potential of vasculotide to accelerate BBB restoration after focused ultrasounds exposure with a potential subsequent improvement in safety and efficacy of treatments.
There is a continuous discovery of new approaches for the repair of BBB or BSCB integrity, but rare therapeutics have been successfully translated to humans. The more valuable option would probably be a combination approach to protect all components of the NVU.
6. Therapeutic Strategies to Overcome BBB in ALS
Numerous drugs that focus on main pathological mechanisms involved in ALS have been tested in clinical trials but failed to demonstrate a significant benefit in patients. However, it is difficult to conclude that a given biopharmaceutical is ineffective for a brain disease if the drug never reaches the target site.
Using effective strategies to overcome this barrier and allow a drug to reach its target may give the opportunity to re-evaluate previous disappointing drugs. The benefits of riluzole in ALS might be limited by the upregulation of P-gp observed throughout the disease. In this context, alternative delivery systems have been evaluated in vitro to improve the benefits of this drug, mainly using nanoparticles formulations. Such alternatives include a liposomal formulation capable of co-delivering riluzole with a P-gp inhibitor, verapamil [86], and a lactoferrin-functionalized nanocarriers that allows the receptor-mediated transcytosis of riluzole across the ECs [87]. However, these promising results need to be confirmed in vivo. Other studies aiming to enhance brain distribution of riluzole in preclinical context include intranasal administration of riluzole-loaded nanoemulsion in rats [88], or intraperitoneal injection of riluzole-encapsulated nanoparticles [89,90]. Intracerebroventricular injection of another neuroprotective agent minocycline encapsulated in LPS-modified liposomes showed better efficiency than its conventional formulation in a SOD1 mutated mouse model of ALS [91].
In recent decades, biopharmaceuticals revolutionized the treatment of a broad range of diseases and are increasingly used in many fields of medicine. However, the successful development of biopharmaceuticals is complicated by their limited brain access. Neurotrophic factors, for example, were rapidly tested in clinical trials in ALS but failed, probably because they were never delivered to the CNS [92]. Since then, bypass strategies to deliver therapies to their targets have been evaluated, including intranasal delivery in rats [93], the targeting of the Glial cell line-derived neurotrophic factor (GDNF) expression in the skeletal muscle via AAV vector coding for GDNF [94], or the transplantation of progenitor cells secreting GDNF [95,96,97]. This last strategy is retained in a Phase I clinical trial where the safety of neural progenitor cells secreting GDNF (CNS10-NPC-GDNF) transplantation in the spinal cord of ALS patients will be evaluated (ClinicalTrials.gov Identifier: NCT05306457). The discovery of new potential therapeutic targets leads to an increased number of emerging small molecule approaches which will require a good CNS penetration to be successfully transposed in humans [98]. As drug carriers or therapeutics drugs by themselves, nanoparticles could be a valuable option as evoked for the encapsulation of riluzole in organic nanoparticles. Cerium oxide inorganic nanoparticle administrated in SOD1 mutated mice showed benefit on the symptoms and the lifespan of the transgenic, probably by its antioxidant effects. This compound also displayed a penetration into the CNS as evidenced by cerium concentration into the brain [99].
Bypassing the BBB with direct delivery into the CSF via intrathecal or intracerebroventricular injection only allows the diffusion of the drug into the CNS near the injection site [9]. Indeed, diffusion decreases logarithmically with distance, which is problematic when the target is distant from the injection site. This could contribute to the difficulty of translating promising results found in animals to humans. For instance, intrathecal administration of an antisense oligonucleotide (ASO) targeting SOD1 (Tofersen) recently failed a phase III clinical trial [100], despite promising results in rodents [101] and signs of target engagement with a 30% reduction level of CSF SOD1 for the maximum tested dose (100 mg) in phase II trial [102]. A recent pharmacokinetic model was developed to describe ASO distribution after intrathecal injection based on previous non-human primate data [103]. It predicted that only 4 % of the injection dose would reach the CNS (which remains higher than following peripheral administration), in addition to local differences in the ASO concentrations along the spinal CSF canal, which may limit access to target tissues in humans. This illustrates that ASO therapy in ALS might need a better bypassing strategy than intrathecal administration. In this way, encapsulation of an ASO targeting SOD1 in calcium phosphate lipid nanoparticles displayed uptake in an in vitro model of motoneuron-like cells (NSC-34) and were able to reach the brain and spinal cord after direct injection in a zebrafish model [104]. However, these results still need to be confirmed in mammalian models. Intrathecal administration of AAV allowing gene silencing in the brain or the spinal cord has been conducted in ALS (mostly targeting SOD1) with promising results in animal models and its potential was also demonstrated in two patients [105,106]. AAVs have also been used to deliver therapeutic transgene in specific cells such as insulin-like growth factor 1, GDNF (as mentioned above), hepatocyte growth factor or neuromuscular junction proteins [105]. As mentioned above, safety and feasibility of transitory opening of the BBB using MR-guided focused ultrasounds in ALS has been demonstrated by Abraho et al. [33]. After optimization in future trials, this method could represent a non-invasive opportunity to deliver various therapeutic agents into the CNS, including small molecules and nanoparticles. Moreover, targeting specific regions of the CNS with MR-guided focused ultrasounds could allow specific delivery of the therapeutic agents to the affected motoneurons according to the onset of the disease (spinal or bulbar). Notably, application of this method to the spinal cord will be challenging in primate vertebrae [107].
While strategies to overcome the BBB start to emerge in ALS, bringing new hopes for the disease, translating these bypassing strategies from in vitro or preclinical models to humans still need to prove its efficiency and will probably be hampered by the complex BBB alterations observed in ALS.
7. Conclusions: Taking Advantage of BBB/BSCB Alterations or Restoring the Barriers to Optimize Therapy in ALS?
Despite continuous advances in the understanding of the pathological mechanisms of ALS, conventional pharmacological clinical trials failed to provide any efficient cure. The development of therapies for brain diseases is complicated and limited by the presence of CNS barriers. There is evidence of BBB and BSCB alterations in ALS but their roles in the complex pathogenesis of the disease remain poorly understood and require further investigation. We could suggest that these alterations provide an opportunity for a drug to penetrate into the brain, but the consequences on drug pharmacokinetics and pharmacodynamics are not obvious. First, BBB alterations do not seem to appear in all patients. Second, the upregulation of efflux transporters, formation of extracellular edema and the thickening of the basement membrane might limit the diffusion of the therapy across the interstitial fluid. Moreover, the unclear localization and progression of these alterations is a source of unpredictability and variability concerning the level of the drug that can actually reach its target.
Given these arguments and continuous failures of therapies targeting the CNS over the past decades, we do not recommend assuming that BBB disruption in ALS will allow an optimal access of the candidate molecule to its target. Thus, it appears that efficient therapies for ALS will need the innovative development of an optimal strategy to overcome the transport across the BBB, as direct intrathecal or intracerebroventricular administration alone might be insufficient. However, as mentioned above, some alterations of the BBB could also limit the efficiency of such bypassing strategies. Restoring the BBB/BSCB could be very advantageous in ALS, because it could present the combined advantages of reversing a potential pathological mechanism of the disease, which still needs further investigation, and enhancing the safety and optimization of specific co-medication in ALS.
So, we suggest the following: (1) to find robust and non-invasive biomarkers of BBB/BSCB disruption to stratify patients according to the state of this barrier, (2) to repair these alterations for patients in which the bypass strategy might be less effective, and (3) to use innovative modalities to bypass the BBB, leading to a more personalized medicine approach based on the BBB/BSCB status.
Author Contributions
Conceptualization, H.A. and H.B.; writing—original draft preparation, H.A.; writing—review and editing, all authors; visualization, H.A.; supervision, C.V.-D. and H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Cappella, M.; Pradat, P.-F.; Querin, G.; Biferi, M.G. Beyond the Traditional Clinical Trials for Amyotrophic Lateral Sclerosis and the Future Impact of Gene Therapy. J. Neuromuscul. Dis. 2021, 8, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood-Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef]
- Pardridge, W.M. Delivery of Biologics across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 2017, 31, 503–519. [Google Scholar] [CrossRef]
- Al Ojaimi, Y.; Blin, T.; Lamamy, J.; Gracia, M.; Pitiot, A.; Denevault-Sabourin, C.; Joubert, N.; Pouget, J.-P.; Gouilleux-Gruart, V.; Heuzé-Vourc’h, N.; et al. Therapeutic Antibodies—Natural and Pathological Barriers and Strategies to Overcome Them. Pharmacol. Ther. 2021, 233, 108022. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of Blood-Brain Barrier in Brain Diseases and Related Systemic Nanoscale Brain-Targeting Drug Delivery Strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef]
- Zhu, F.-D.; Hu, Y.-J.; Yu, L.; Zhou, X.-G.; Wu, J.-M.; Tang, Y.; Qin, D.-L.; Fan, Q.-Z.; Wu, A.-G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef]
- Han, L. Modulation of the Blood-Brain Barrier for Drug Delivery to Brain. Pharmaceutics 2021, 13, 2024. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Reviews. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Pan, Y.; Nicolazzo, J.A. Altered Blood-Brain Barrier and Blood-Spinal Cord Barrier Dynamics in Amyotrophic Lateral Sclerosis: Impact on Medication Efficacy and Safety. Br. J. Pharmacol. 2022, 179, 2577–2588. [Google Scholar] [CrossRef]
- Mirian, A.; Moszczynski, A.; Soleimani, S.; Aubert, I.; Zinman, L.; Abrahao, A. Breached Barriers: A Scoping Review of Blood-Central Nervous System Barrier Pathology in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2022, 16, 851563. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Saporta, S.; Kolomey, I.; Nicosia, S.V.; Sanberg, P.R. Ultrastructure of Blood-Brain Barrier and Blood-Spinal Cord Barrier in SOD1 Mice Modeling ALS. Brain Res. 2007, 1157, 126–137. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Saporta, S.; Haller, E.; Kolomey, I.; Bennett, S.P.; Potter, H.; Sanberg, P.R. Evidence of Compromised Blood-Spinal Cord Barrier in Early and Late Symptomatic SOD1 Mice Modeling ALS. PLoS ONE 2007, 2, e1205. [Google Scholar] [CrossRef]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; O’Banion, M.K.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-Causing SOD1 Mutants Generate Vascular Changes Prior to Motor Neuron Degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Aizawa, S.; Oppenheim, R.W.; Milligan, C. Neurovascular Unit Pathology Is Observed Very Early in Disease Progression in the Mutant SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Exp. Neurol. 2022, 353, 114084. [Google Scholar] [CrossRef]
- Zamudio, F.; Loon, A.R.; Smeltzer, S.; Benyamine, K.; Navalpur Shanmugam, N.K.; Stewart, N.J.F.; Lee, D.C.; Nash, K.; Selenica, M.-L.B. TDP-43 Mediated Blood-Brain Barrier Permeability and Leukocyte Infiltration Promote Neurodegeneration in a Low-Grade Systemic Inflammation Mouse Model. J. Neuroinflamm. 2020, 17, 283. [Google Scholar] [CrossRef]
- Béland, L.-C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in Amyotrophic Lateral Sclerosis: Blurred Lines between Excessive Inflammation and Inefficient Immune Responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef]
- Nicaise, C.; Mitrecic, D.; Demetter, P.; De Decker, R.; Authelet, M.; Boom, A.; Pochet, R. Impaired Blood-Brain and Blood-Spinal Cord Barriers in Mutant SOD1-Linked ALS Rat. Brain Res. 2009, 1301, 152–162. [Google Scholar] [CrossRef]
- Andjus, P.R.; Bataveljić, D.; Vanhoutte, G.; Mitrecic, D.; Pizzolante, F.; Djogo, N.; Nicaise, C.; Gankam Kengne, F.; Gangitano, C.; Michetti, F.; et al. In Vivo Morphological Changes in Animal Models of Amyotrophic Lateral Sclerosis and Alzheimer’s-like Disease: MRI Approach. Anat. Rec. 2009, 292, 1882–1892. [Google Scholar] [CrossRef]
- Bataveljić, D.; Stamenković, S.; Bačić, G.; Andjus, P.R. Imaging Cellular Markers of Neuroinflammation in the Brain of the Rat Model of Amyotrophic Lateral Sclerosis. Acta Physiol. Hung. 2011, 98, 27–31. [Google Scholar] [CrossRef]
- Evans, M.C.; Serres, S.; Khrapitchev, A.A.; Stolp, H.B.; Anthony, D.C.; Talbot, K.; Turner, M.R.; Sibson, N.R. T₂-Weighted MRI Detects Presymptomatic Pathology in the SOD1 Mouse Model of ALS. J. Cereb. Blood Flow Metab. 2014, 34, 785–793. [Google Scholar] [CrossRef]
- Donnenfeld, H.; Kascsak, R.J.; Bartfeld, H. Deposits of IgG and C3 in the Spinal Cord and Motor Cortex of ALS Patients. J. Neuroimmunol. 1984, 6, 51–57. [Google Scholar] [CrossRef]
- Engelhardt, J.I.; Appel, S.H. IgG Reactivity in the Spinal Cord and Motor Cortex in Amyotrophic Lateral Sclerosis. Arch. Neurol. 1990, 47, 1210–1216. [Google Scholar] [CrossRef]
- Engelhardt, J.I.; Tajti, J.; Appel, S.H. Lymphocytic Infiltrates in the Spinal Cord in Amyotrophic Lateral Sclerosis. Arch. Neurol. 1993, 50, 30–36. [Google Scholar] [CrossRef]
- Kwan, J.Y.; Jeong, S.Y.; Gelderen, P.V.; Deng, H.-X.; Quezado, M.M.; Danielian, L.E.; Butman, J.A.; Chen, L.; Bayat, E.; Russell, J.; et al. Iron Accumulation in Deep Cortical Layers Accounts for MRI Signal Abnormalities in ALS: Correlating 7 Tesla MRI and Pathology. PLoS ONE 2012, 7, e35241. [Google Scholar] [CrossRef]
- Oba, H.; Araki, T.; Ohtomo, K.; Monzawa, S.; Uchiyama, G.; Koizumi, K.; Nogata, Y.; Kachi, K.; Shiozawa, Z.; Kobayashi, M. Amyotrophic Lateral Sclerosis: T2 Shortening in Motor Cortex at MR Imaging. Radiology 1993, 189, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, E.; Biessels, G.-J.; van Den Heuvel, M.P.; Visser, F.; Luijten, P.R.; van Den Berg, L.H. No Evidence of Microbleeds in ALS Patients at 7 Tesla MRI. Amyotroph. Lateral Scler. 2010, 11, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-Human Trial of Blood–Brain Barrier Opening in Amyotrophic Lateral Sclerosis Using MR-Guided Focused Ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Vlad, B.; Gaur, N.; Stubendorff, B.; Grosskreutz, J. Blood–Brain Barrier Disruption Is Not Associated With Disease Aggressiveness in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2021, 15, 1438. [Google Scholar] [CrossRef]
- Brettschneider, J.; Petzold, A.; Süssmuth, S.D.; Ludolph, A.C.; Tumani, H. Axonal Damage Markers in Cerebrospinal Fluid Are Increased in ALS. Neurology 2006, 66, 852–856. [Google Scholar] [CrossRef]
- Meucci, G.; Rossi, G.; Bettini, R.; Montanaro, D.; Gironelli, L.; Voci, L.; Bianchi, F. Laser Nephelometric Evaluation of Albumin, IgG and Alpha 2-Macroglobulin: Applications to the Study of Alterations of the Blood-Brain Barrier. J. Neurol. Sci. 1993, 118, 73–78. [Google Scholar] [CrossRef]
- Waters, S.; Swanson, M.E.V.; Dieriks, B.V.; Zhang, Y.B.; Grimsey, N.L.; Murray, H.C.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; An, J.; et al. Blood-Spinal Cord Barrier Leakage Is Independent of Motor Neuron Pathology in ALS. Acta Neuropathol. Commun. 2021, 9, 144. [Google Scholar] [CrossRef]
- Li, J.-Y.; Cai, Z.-Y.; Sun, X.-H.; Shen, D.; Yang, X.-Z.; Liu, M.-S.; Cui, L.-Y. Blood–Brain Barrier Dysfunction and Myelin Basic Protein in Survival of Amyotrophic Lateral Sclerosis with or without Frontotemporal Dementia. Neurol. Sci. 2021, 43, 3201–3210. [Google Scholar] [CrossRef]
- Todd, T.W.; Petrucelli, L. Modelling Amyotrophic Lateral Sclerosis in Rodents. Nat. Rev. Neurosci. 2022, 23, 231–251. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging Insights into the Complex Genetics and Pathophysiology of Amyotrophic Lateral Sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, J.D.; Coulson, E.J.; Woodruff, T.M. A Validated Quantitative Method for the Assessment of Neuroprotective Barrier Impairment in Neurodegenerative Disease Models. J. Neurochem. 2021, 158, 807–817. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Markandaiah, S.; Bonanno, S.; Pasinelli, P.; Trotti, D. Blood-Brain Barrier Driven Pharmacoresistance in Amyotrophic Lateral Sclerosis and Challenges for Effective Drug Therapies. AAPS J. 2017, 19, 1600–1614. [Google Scholar] [CrossRef]
- Jablonski, M.R.; Jacob, D.A.; Campos, C.; Miller, D.S.; Maragakis, N.J.; Pasinelli, P.; Trotti, D. Selective Increase of Two ABC Drug Efflux Transporters at the Blood–Spinal Cord Barrier Suggests Induced Pharmacoresistance in ALS. Neurobiol. Dis. 2012, 47, 194–200. [Google Scholar] [CrossRef]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Iyer, A.M.; Mesarosova, L.; Çolakoglu, H.; Anink, J.J.; van Tellingen, O.; Maragakis, N.J.; Shefner, J.; Bunt, T.; Aronica, E. Expression and Cellular Distribution of P-Glycoprotein and Breast Cancer Resistance Protein in Amyotrophic Lateral Sclerosis Patients. J. Neuropathol. Exp. Neurol. 2020, 79, 266–276. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Markandaiah, S.S.; Bonanno, S.; Pasinelli, P.; Trotti, D. Excess Glutamate Secreted from Astrocytes Drives Upregulation of P-Glycoprotein in Endothelial Cells in Amyotrophic Lateral Sclerosis. Exp. Neurol. 2019, 316, 27–38. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Hernandez-Ontiveros, D.G.; Rodrigues, M.C.O.; Haller, E.; Frisina-Deyo, A.; Mirtyl, S.; Sallot, S.; Saporta, S.; Borlongan, C.V.; Sanberg, P.R. Impaired Blood-Brain/Spinal Cord Barrier in ALS Patients. Brain Res. 2012, 1469, 114–128. [Google Scholar] [CrossRef]
- Miyazaki, K.; Ohta, Y.; Nagai, M.; Morimoto, N.; Kurata, T.; Takehisa, Y.; Ikeda, Y.; Matsuura, T.; Abe, K. Disruption of Neurovascular Unit Prior to Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. J. Neurosci. Res. 2011, 89, 718–728. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The Vascular Basement Membrane in the Healthy and Pathological Brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Mehta, D.C.; Short, J.L.; Nicolazzo, J.A. Altered Brain Uptake of Therapeutics in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Pharm. Res. 2013, 30, 2868–2879. [Google Scholar] [CrossRef]
- Binder, D.K.; Papadopoulos, M.C.; Haggie, P.M.; Verkman, A.S. In Vivo Measurement of Brain Extracellular Space Diffusion by Cortical Surface Photobleaching. J. Neurosci. 2004, 24, 8049–8056. [Google Scholar] [CrossRef]
- Bataveljić, D.; Nikolić, L.; Milosević, M.; Todorović, N.; Andjus, P.R. Changes in the Astrocytic Aquaporin-4 and Inwardly Rectifying Potassium Channel Expression in the Brain of the Amyotrophic Lateral Sclerosis SOD1(G93A) Rat Model. Glia 2012, 60, 1991–2003. [Google Scholar] [CrossRef]
- Nicaise, C.; Soyfoo, M.S.; Authelet, M.; De Decker, R.; Bataveljic, D.; Delporte, C.; Pochet, R. Aquaporin-4 Overexpression in Rat ALS Model. Anat. Rec. 2009, 292, 207–213. [Google Scholar] [CrossRef]
- Watanabe-Matsumoto, S.; Moriwaki, Y.; Okuda, T.; Ohara, S.; Yamanaka, K.; Abe, Y.; Yasui, M.; Misawa, H. Dissociation of Blood-Brain Barrier Disruption and Disease Manifestation in an Aquaporin-4-Deficient Mouse Model of Amyotrophic Lateral Sclerosis. Neurosci. Res. 2018, 133, 48–57. [Google Scholar] [CrossRef]
- Danzeisen, R.; Achsel, T.; Bederke, U.; Cozzolino, M.; Crosio, C.; Ferri, A.; Frenzel, M.; Gralla, E.B.; Huber, L.; Ludolph, A.; et al. Superoxide Dismutase 1 Modulates Expression of Transferrin Receptor. J. Biol. Inorg. Chem. 2006, 11, 489–498. [Google Scholar] [CrossRef]
- Hadzhieva, M.; Kirches, E.; Wilisch-Neumann, A.; Pachow, D.; Wallesch, M.; Schoenfeld, P.; Paege, I.; Vielhaber, S.; Petri, S.; Keilhoff, G.; et al. Dysregulation of Iron Protein Expression in the G93A Model of Amyotrophic Lateral Sclerosis. Neuroscience 2013, 230, 94–101. [Google Scholar] [CrossRef]
- Sirohi, K.; Chalasani, M.L.S.; Sudhakar, C.; Kumari, A.; Radha, V.; Swarup, G. M98K-OPTN Induces Transferrin Receptor Degradation and RAB12-Mediated Autophagic Death in Retinal Ganglion Cells. Autophagy 2013, 9, 510–527. [Google Scholar] [CrossRef]
- Burgess, A.; Nhan, T.; Moffatt, C.; Klibanov, A.L.; Hynynen, K. Analysis of Focused Ultrasound-Induced Blood-Brain Barrier Permeability in a Mouse Model of Alzheimer’s Disease Using Two-Photon Microscopy. J. Control. Release 2014, 192, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.-Y.; Li, J.; Wang, K.-F.; Xia, W.-W.; Zhu, Z.-Q.; Wang, C.-R.; Li, X.-F.; Liu, H.-Y. Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Sengillo, J.D.; Sagare, A.P.; Zhao, Z.; Ma, Q.; Zuniga, E.; Wang, Y.; Zhong, Z.; Sullivan, J.S.; Griffin, J.H.; et al. Blood-Spinal Cord Barrier Disruption Contributes to Early Motor-Neuron Degeneration in ALS-Model Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1035–E1042. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Ilieva, H.; Hallagan, L.; Bell, R.; Singh, I.; Paquette, N.; Thiyagarajan, M.; Deane, R.; Fernandez, J.A.; Lane, S.; et al. Activated Protein C Therapy Slows ALS-like Disease in Mice by Transcriptionally Inhibiting SOD1 in Motor Neurons and Microglia Cells. J. Clin. Investig. 2009, 119, 3437–3449. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Ezra, A.; Barbiro, B.; Rabinovich-Toidman, P.; Solomon, B. Chronic Administration of AMD3100 Increases Survival and Alleviates Pathology in SOD1G93A Mice Model of ALS. J. Neuroinflamm. 2016, 13, 123. [Google Scholar] [CrossRef]
- Coatti, G.C.; Frangini, M.; Valadares, M.C.; Gomes, J.P.; Lima, N.O.; Cavaçana, N.; Assoni, A.F.; Pelatti, M.V.; Birbrair, A.; de Lima, A.C.P.; et al. Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient. Stem Cell Rev. Rep. 2017, 13, 686–698. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Kurien, C.; Thomson, A.; Falco, D.; Ahmad, S.; Staffetti, J.; Steiner, G.; Abraham, S.; James, G.; Mahendrasah, A.; et al. Endothelial and Astrocytic Support by Human Bone Marrow Stem Cell Grafts into Symptomatic ALS Mice towards Blood-Spinal Cord Barrier Repair. Sci. Rep. 2017, 7, 884. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Haller, E.; Navarro, S.; Besong, T.E.; Boccio, K.J.; Hailu, S.; Khatib, M.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Transplantation of Human Bone Marrow Stem Cells into Symptomatic ALS Mice Enhances Structural and Functional Blood-Spinal Cord Barrier Repair. Exp. Neurol. 2018, 310, 33–47. [Google Scholar] [CrossRef]
- Eve, D.J.; Steiner, G.; Mahendrasah, A.; Sanberg, P.R.; Kurien, C.; Thomson, A.; Borlongan, C.V.; Garbuzova-Davis, S. Reduction of Microhemorrhages in the Spinal Cord of Symptomatic ALS Mice after Intravenous Human Bone Marrow Stem Cell Transplantation Accompanies Repair of the Blood-Spinal Cord Barrier. Oncotarget 2018, 9, 10621–10634. [Google Scholar] [CrossRef][Green Version]
- Garbuzova-Davis, S.; Kurien, C.; Haller, E.; Eve, D.J.; Navarro, S.; Steiner, G.; Mahendrasah, A.; Hailu, S.; Khatib, M.; Boccio, K.J.; et al. Human Bone Marrow Endothelial Progenitor Cell Transplantation into Symptomatic ALS Mice Delays Disease Progression and Increases Motor Neuron Survival by Repairing Blood-Spinal Cord Barrier. Sci. Rep. 2019, 9, 5280. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Ehrhart, J.; Mustafa, H.; Llauget, A.; Boccio, K.J.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Phenotypic Characteristics of Human Bone Marrow-Derived Endothelial Progenitor Cells in Vitro Support Cell Effectiveness for Repair of the Blood-Spinal Cord Barrier in ALS. Brain Res. 2019, 1724, 146428. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Boccio, K.J.; Llauget, A.; Shell, R.; Hailu, S.; Mustafa, H.; Ehrhart, J.; Sanberg, P.R.; Appel, S.H.; Borlongan, C.V. Beneficial Effects of Transplanted Human Bone Marrow Endothelial Progenitors on Functional and Cellular Components of Blood-Spinal Cord Barrier in ALS Mice. eNeuro 2021, 8, ENEURO.0314-21.2021. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Willing, A.E.; Ehrhart, J.; Wang, L.; Sanberg, P.R.; Borlongan, C.V. Cell-Free Extracellular Vesicles Derived from Human Bone Marrow Endothelial Progenitor Cells as Potential Therapeutics for Microvascular Endothelium Restoration in ALS. Neuromol. Med. 2020, 22, 503–516. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Lindborg, S.R.; Goyal, N.A.; Miller, R.G.; Burford, M.J.; Berry, J.D.; Nicholson, K.A.; Mozaffar, T.; Katz, J.S.; Jenkins, L.J.; et al. A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal Stem Cells Induced to Secrete High Levels of Neurotrophic Factors in Amyotrophic Lateral Sclerosis. Muscle Nerve 2022, 65, 291–302. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Yaghmour, N.E.; Ginzberg, A.; Karussis, D. A Phase II Clinical Trial with Repeated Intrathecal Injections of Autologous Mesenchymal Stem Cells in Patients with Amyotrophic Lateral Sclerosis. Front. Biosci. 2021, 26, 693–706. [Google Scholar] [CrossRef]
- Siwek, T.; Jezierska-Woźniak, K.; Maksymowicz, S.; Barczewska, M.; Sowa, M.; Badowska, W.; Maksymowicz, W. Repeat Administration of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Amyotrophic Lateral Sclerosis. Med. Sci. Monit. 2020, 26, e927484. [Google Scholar] [CrossRef]
- Engelen, M.; Kemp, S.; Poll-The, B.-T. X-Linked Adrenoleukodystrophy: Pathogenesis and Treatment. Curr. Neurol. Neurosci. Rep. 2014, 14, 486. [Google Scholar] [CrossRef]
- Orchard, P.J.; Nascene, D.R.; Miller, W.P.; Gupta, A.; Kenney-Jung, D.; Lund, T.C. Successful Donor Engraftment and Repair of the Blood-Brain Barrier in Cerebral Adrenoleukodystrophy. Blood 2019, 133, 1378–1381. [Google Scholar] [CrossRef]
- Israelov, H.; Ravid, O.; Atrakchi, D.; Rand, D.; Elhaik, S.; Bresler, Y.; Twitto-Greenberg, R.; Omesi, L.; Liraz-Zaltsman, S.; Gosselet, F.; et al. Caspase-1 Has a Critical Role in Blood-Brain Barrier Injury and Its Inhibition Contributes to Multifaceted Repair. J. Neuroinflamm. 2020, 17, 267. [Google Scholar] [CrossRef]
- Flores, J.; Noël, A.; Foveau, B.; Lynham, J.; Lecrux, C.; LeBlanc, A.C. Caspase-1 Inhibition Alleviates Cognitive Impairment and Neuropathology in an Alzheimer’s Disease Mouse Model. Nat. Commun. 2018, 9, 3916. [Google Scholar] [CrossRef]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/Caspase-1/GSDMD Pathway in MPTP Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Mamik, M.K.; Saito, L.B.; Boghozian, R.; Monaco, M.C.; Major, E.O.; Lu, J.-Q.; Branton, W.G.; Power, C. Caspase-1 Inhibition Prevents Glial Inflammasome Activation and Pyroptosis in Models of Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef]
- Rand, D.; Cooper, I. Caspase-1: An Important Player and Possible Target for Repair of the Blood-Brain Barrier Underlying Neurodegeneration. Neural Regen. Res. 2021, 16, 2390–2392. [Google Scholar] [CrossRef]
- Zhang, Y.; Cook, A.; Kim, J.; Baranov, S.V.; Jiang, J.; Smith, K.; Cormier, K.; Bennett, E.; Browser, R.P.; Day, A.L.; et al. Melatonin Inhibits the Caspase-1/Cytochrome c/Caspase-3 Cell Death Pathway, Inhibits MT1 Receptor Loss and Delays Disease Progression in a Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2013, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rosa, E.; Shin, M.-K.; Dhar, M.; Chaubey, K.; Cintrón-Pérez, C.J.; Tang, X.; Liao, X.; Miller, E.; Koh, Y.; Barker, S.; et al. P7C3-A20 Treatment One Year after TBI in Mice Repairs the Blood-Brain Barrier, Arrests Chronic Neurodegeneration, and Restores Cognition. Proc. Natl. Acad. Sci. USA 2020, 117, 27667–27675. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, L.-N.; Han, X.-H. Fenretinide Attenuates Lipopolysaccharide (LPS)-Induced Blood-Brain Barrier (BBB) and Depressive-like Behavior in Mice by Targeting Nrf-2 Signaling. Biomed. Pharmacother. 2020, 125, 109680. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, M.; Wang, D.; Ousaka, D.; Wake, H. High Mobility Group Box-1 and Blood-Brain Barrier Disruption. Cells 2020, 9, 2650. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Heinen, S.; Markham-Coultes, K.; O’Reilly, M.; Van Slyke, P.; Dumont, D.J.; Hynynen, K.; Aubert, I. Vasculotide Restores the Blood-Brain Barrier after Focused Ultrasound-Induced Permeability in a Mouse Model of Alzheimer’s Disease. Int. J. Med. Sci. 2021, 18, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ferrill, L.; Gallant, L.; McGillicuddy, S.; Fernandes, T.; Schields, N.; Bai, S. Verapamil and Riluzole Cocktail Liposomes Overcome Pharmacoresistance by Inhibiting P-Glycoprotein in Brain Endothelial and Astrocyte Cells: A Potent Approach to Treat Amyotrophic Lateral Sclerosis. Eur. J. Pharm. Sci. 2018, 120, 30–39. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Gonçalves, H.; Catita, J.; Silva, A.M.; Rodrigues, F.; Amaral, M.H.; Costa, P.C. Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain. Pharmaceutics 2022, 14, 185. [Google Scholar] [CrossRef]
- Parikh, R.H.; Patel, R.J. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr. Drug Deliv. 2016, 13, 1130–1143. [Google Scholar] [CrossRef]
- Bondì, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-Targeted Solid Lipid Nanoparticles Containing Riluzole: Preparation, Characterization and Biodistribution. Nanomedicine 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Verma, S.K.; Arora, I.; Javed, K.; Akhtar, M.; Samim, M. Enhancement in the Neuroprotective Power of Riluzole Against Cerebral Ischemia Using a Brain Targeted Drug Delivery Vehicle. ACS Appl. Mater. Interfaces 2016, 8, 19716–19723. [Google Scholar] [CrossRef]
- Wiley, N.J.; Madhankumar, A.B.; Mitchell, R.M.; Neely, E.B.; Rizk, E.; Douds, G.L.; Simmons, Z.; Connor, J.R. Lipopolysaccharide Modified Liposomes for Amyotropic Lateral Sclerosis Therapy: Efficacy in SOD1 Mouse Model. Adv. Nanopart. 2012, 1, 44–53. [Google Scholar] [CrossRef]
- The BDNF Study Group (Phase III). A Controlled Trial of Recombinant Methionyl Human BDNF in ALS. Neurology 1999, 52, 1427. [Google Scholar] [CrossRef]
- Alcalá-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H.; McLoon, L.K. Intranasal Delivery of Neurotrophic Factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target 2010, 18, 179–190. [Google Scholar] [CrossRef]
- Mòdol-Caballero, G.; García-Lareu, B.; Herrando-Grabulosa, M.; Verdés, S.; López-Vales, R.; Pagès, G.; Chillón, M.; Navarro, X.; Bosch, A. Specific Expression of Glial-Derived Neurotrophic Factor in Muscles as Gene Therapy Strategy for Amyotrophic Lateral Sclerosis. Neurotherapeutics 2021, 18, 1113–1126. [Google Scholar] [CrossRef]
- Thomsen, G.M.; Avalos, P.; Ma, A.A.; Alkaslasi, M.; Cho, N.; Wyss, L.; Vit, J.-P.; Godoy, M.; Suezaki, P.; Shelest, O.; et al. Transplantation of Neural Progenitor Cells Expressing Glial Cell Line-Derived Neurotrophic Factor into the Motor Cortex as a Strategy to Treat Amyotrophic Lateral Sclerosis. Stem Cells 2018, 36, 1122–1131. [Google Scholar] [CrossRef]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Hayes, A.; Bellantuono, I.; Aebischer, P.; Svendsen, C.N. Direct Muscle Delivery of GDNF with Human Mesenchymal Stem Cells Improves Motor Neuron Survival and Function in a Rat Model of Familial ALS. Mol. Ther. 2008, 16, 2002–2010. [Google Scholar] [CrossRef]
- Klein, S.M.; Behrstock, S.; McHugh, J.; Hoffmann, K.; Wallace, K.; Suzuki, M.; Aebischer, P.; Svendsen, C.N. GDNF Delivery Using Human Neural Progenitor Cells in a Rat Model of ALS. Hum. Gene Ther. 2005, 16, 509–521. [Google Scholar] [CrossRef]
- Brown, D.G.; Shorter, J.; Wobst, H.J. Emerging Small-Molecule Therapeutic Approaches for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Bioorganic Med. Chem. Lett. 2020, 30, 126942. [Google Scholar] [CrossRef]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J.; et al. Cerium Oxide Nanoparticles with Antioxidant Properties Ameliorate Strength and Prolong Life in Mouse Model of Amyotrophic Lateral Sclerosis. Nanomedicine 2016, 12, 2311–2320. [Google Scholar] [CrossRef]
- Mullard, A. ALS Antisense Drug Falters in Phase III. Nat. Rev. Drug Discov. 2021, 20, 883–885. [Google Scholar] [CrossRef]
- McCampbell, A.; Cole, T.; Wegener, A.J.; Tomassy, G.S.; Setnicka, A.; Farley, B.J.; Schoch, K.M.; Hoye, M.L.; Shabsovich, M.; Sun, L.; et al. Antisense Oligonucleotides Extend Survival and Reverse Decrement in Muscle Response in ALS Models. J. Clin. Investig. 2018, 128, 3558–3567. [Google Scholar] [CrossRef]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef]
- Monine, M.; Norris, D.; Wang, Y.; Nestorov, I. A Physiologically-Based Pharmacokinetic Model to Describe Antisense Oligonucleotide Distribution after Intrathecal Administration. J. Pharmacokinet. Pharmacodyn. 2021, 48, 639–654. [Google Scholar] [CrossRef]
- Chen, L.; Watson, C.; Morsch, M.; Cole, N.J.; Chung, R.S.; Saunders, D.N.; Yerbury, J.J.; Vine, K.L. Improving the Delivery of SOD1 Antisense Oligonucleotides to Motor Neurons Using Calcium Phosphate-Lipid Nanoparticles. Front. Neurosci. 2017, 11, 476. [Google Scholar] [CrossRef]
- Amado, D.A.; Davidson, B.L. Gene Therapy for ALS: A Review. Mol. Ther. 2021, 29, 3345–3358. [Google Scholar] [CrossRef]
- Mueller, C.; Berry, J.D.; McKenna-Yasek, D.M.; Gernoux, G.; Owegi, M.A.; Pothier, L.M.; Douthwright, C.L.; Gelevski, D.; Luppino, S.D.; Blackwood, M.; et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N. Engl. J. Med. 2020, 383, 151–158. [Google Scholar] [CrossRef]
- Fletcher, S.-M.P.; O’Reilly, M.A. Analysis of Multifrequency and Phase Keying Strategies for Focusing Ultrasound to the Human Vertebral Canal. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).