Delirium in ICU Patients after Cardiac Arrest: A Scoping Review
Abstract
:1. Introduction
Aim
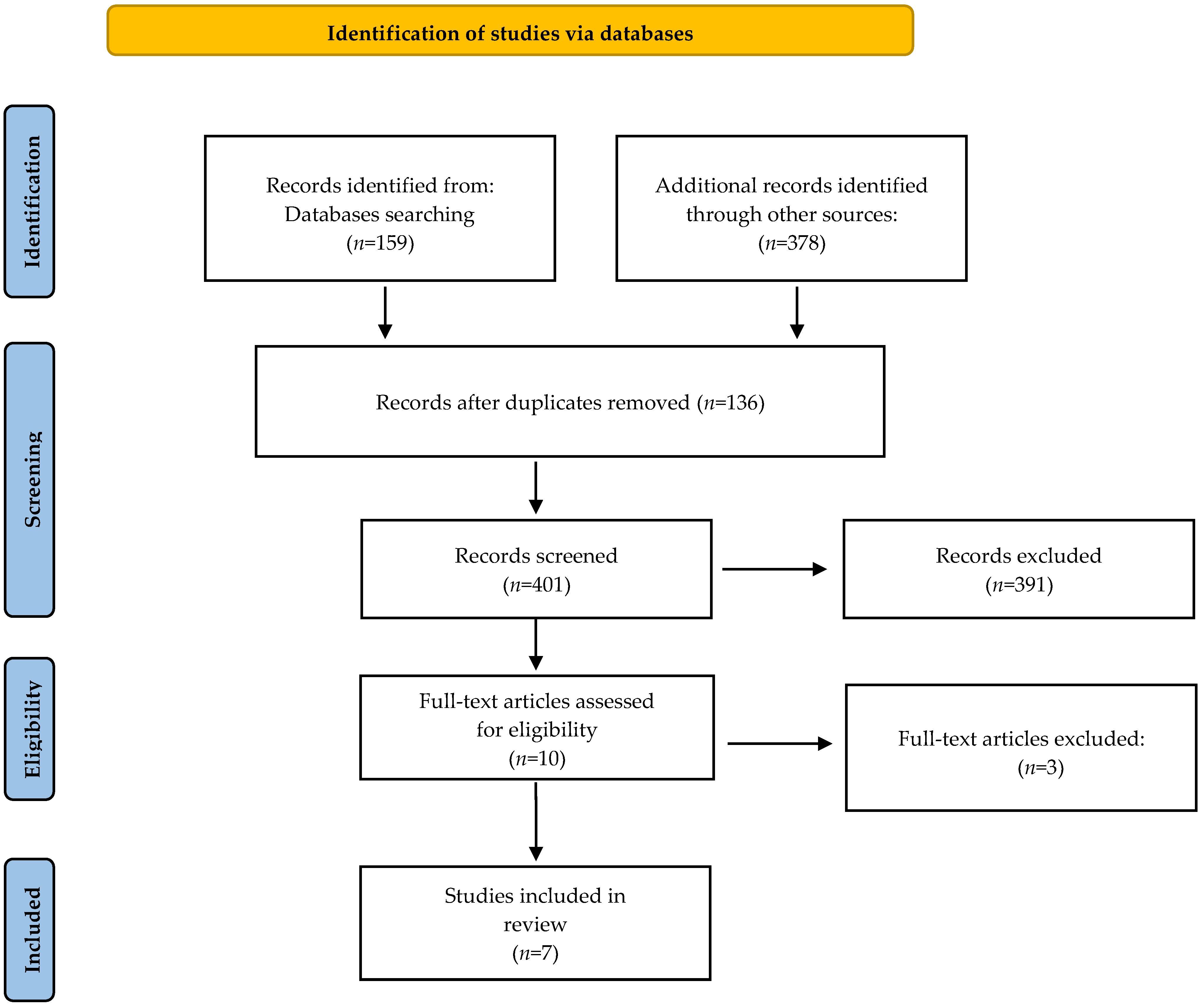
2. Methods
2.1. Study Design
2.2. Review Questions
- What is the incidence of delirium in patients after cardiac arrest?
- Is cardiac arrest associated with higher risk of delirium and what is the impact on delirium duration?
- What impact does delirium after cardiac arrest have on patient outcomes?
- What a type of delirium occurs in patients after cardiac arrest?
2.3. Search Strategy
2.4. Study Selection
2.5. Data Extraction
2.6. Assessment of Study Quality of the Included Studies
 -Yes;
-Yes;  -No; n/a-not applicable.
-No; n/a-not applicable.3. Results
3.1. Incidence of Delirium in Patients after CA
3.2. Cardiac Arrest as a Predictor of Delirium
3.3. Impact of Delirium in Patients after CA on Outcomes
3.4. Subtype of Delirium
4. Discussion
5. Conclusions
6. Implications for Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boncyk, C.S.; Rengel, K.F.; Pandharipande, P.P.; Hughes, C.G. In the ICU—Delirium post cardiac arrest. Curr. Opin. Crit. Care 2019, 25, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Keijzera, H.M.; Klopa, M.; van Puttenc, M.; Hofmeijera, J. Delirium after cardiac arrest: Phenotype, prediction, and outcome. Resuscitation 2020, 151, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of delirium in critically ill patients: Systematic review and meta-analysis. BMJ 2015, 350, h2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, J.S.; Hollenbeck, R.D.; Wang, L.; Holmes, B.; Young, M.N.; Peters, M.; Ely, E.W.; McPherson, J.A.; Vasilevskis, E.E. Hypothermia. Am. J. Crit Care 2016, 25, e81–e89. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Feng, L.; Wang, T.; Li, Y.; Li, Z.; Zhao, B.; Qin, X.; Li, Q.; Wu, S.; Sun, H.; et al. 2020 expert consensus statement on neuro-protection after cardiac arrest in China. Ann. Transl. Med. 2021, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Callaway, C.W. The Brain after Cardiac Arrest. Semin Neurol. 2017, 37, 19–24. [Google Scholar] [CrossRef] [Green Version]
- MacLaren, R.; Gallagher, J.; Shin, J.; Varnado, S.; Nguyen, L. Assessment of Adverse Events and Predictors of Neurological Recovery After Therapeutic Hypothermia. Ann. Pharmacother. 2014, 48, 17–25. [Google Scholar] [CrossRef]
- Stoicea, N.; McVicker, S.; Quinones, A.; Agbenyefia, P.; Bergese, S.D. Delirium-biomarkers and genetic variance. Front. Pharmacol. 2014, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.M.; Colantuoni, E.; Dinglas, V.D.; Hough, C.L.; Wozniak, A.W.; Jackson, J.C.; Morris, P.E.; Mendez-Tellez, P.A.; Ely, E.W.; Hopkins, R.O. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: An ancillary study to a randomised controlled trial. Lancet Respir. Med. 2016, 4, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Foudraine, N.A.; Algargoush, A.; van Osch, F.H.; Bos, A.T. A multimodal sevoflurane-based sedation regimen in combination with targeted temperature management in post-cardiac arrest patients reduces the incidence of delirium: An observational propensity score-matched study. Resuscitation 2021, 159, 158–164. [Google Scholar] [CrossRef]
- Bowman, E.M.L.; Cunningham, E.L.; Page, V.J.; Daniel, F. McAuley. D.F. Phenotypes and subphenotypes of delirium: A review of current categorisations and suggestions for progression. Crit. Care 2021, 25, 334. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 15 May 2022). [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 15 May 2022). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rezar, R.; Wernly, B.; Haslinger, M.; Seelmaier, C.; Schwaiger, P.; Pretsch, I.; Eisl, M.; Jung, C.; Hoppe, U.C.; Lichtenauer, M. Mortality after cardiopulmonary resuscitation on a medical ICU: A sex-specific outcome analysis. Wien Klin. Wochenschr. 2021, 133, 492–499. [Google Scholar] [CrossRef]
- Jäckel, M.; Zotzmann, V.; Wengenmayer, T.; Duerschmied, D.; Biever, P.M.; Spieler, D.; von Zur Mühlen, C.; Stachon, P.; Bode, C.; Staudacher, D.L. Incidence and predictors of delirium on the intensive care unit after acute myocardial infarction, insight from a retrospective registry. Catheter Cardiovasc Interv. 2020, 98, 1072–1081. [Google Scholar] [CrossRef]
- Falsini, G.; Grotti, S.; Porto, I.; Toccafondi, G.; Fraticelli, A.; Angioli, P.; Ducci, K.; Liistro, F.; Pieroni, M.; Taddei, T.; et al. Long-term prognostic value of delirium in elderly patients with acute cardiac diseases admitted to two cardiac intensive care units: A prospective study (DELIRIUM CORDIS). Eur. Heart J. Acute Cardiovasc Care 2018, 7, 661–670. [Google Scholar] [CrossRef]
- Pauley, E.; Lishmanov, A.; Schumann, S.; Gala, G.J.; Van Diepen, S.; Katz, J.N. Delirium is a robust predictor of morbidity and mortality among critically ill patients treated in the cardiac intensive care unit. Am. Heart J. 2015, 170, 79–86. [Google Scholar] [CrossRef]
- Uguz, F.; Kayrak, M.; Çíçek, E.; Kayhan, F.; Ari, H.; Altunbas, G. Delirium following acute myocardial infarction: Incidence, clinical profiles, and predictors. Perspect. Psychiatr. Care 2010, 46, 135–142. [Google Scholar] [CrossRef]
- Laske, R.A.; Stephens, B. Delirium in critical care patients. Nurs. Crit. Care 2016, 11, 18–23. [Google Scholar] [CrossRef]
- Chan, P.S.; McNally, B.; Tang, F.; Kellermann, B.S.A. Recent Trends in Survival from Out-of-Hospital Cardiac Arrest in the United States. Circulation 2014, 130, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit Care 2020, 24, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongardon, N.; Dumas, F.; Ricome, S.; Grimaldi, D.; Hissem, T.; Pène, F.; Cariou, A. Postcardiac arrest syndrome: From immediate resuscitation to long-term outcome. Ann. Intensive Care 2011, 1, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandroni, C.; Cronberg, T.; Sekhon, M. Brain injury after cardiac arrest: Pathophysiology, treatment, and prognosis. Intensive Care Med. 2021, 47, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Aicher, N.; Jäckel, M.; Faye, C.; Wengenmayer, T.; Bode, C.; Staudacher, D.L. Incidence and predictors of delirium after cardiac arrest—A retrospective registry. Resuscitation 2020, 155, S8–S9. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. J. Am. Med. Assoc. 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Ely, W.E. Confusion Assessment Method for the ICU (CAM-ICU): The Complete Training Manual. 2016. Available online: https://uploads-ssl.webflow.com/5b0849daec50243a0a1e5e0c/5bad3d28b04cd592318f45cc_The-Complete-CAM-ICU-training-manual-2016-08-31_Final.pdf (accessed on 18 May 2022).
- Girard, T.D.; Pandharipande, P.P.; Ely, W.E. Delirium in the intensive care unit. Crit Care 2008, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Noordergraaf, G.J.; Hendriksen, E. Not a whiff: Sevoflurane for post-ROSC sedation on the ICU. Try it, you might like it. Resuscitation 2021, 159, 170–171. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Participants (P) | Adult ICU patients | Children (>18 years) Non-ICU patients |
| Concept (C) | Delirium | No-delirium |
| Context (C) | Cardiac arrest | Other diseases |
| Types of evidence source | Observational, prospective, retrospective studies | Single-case report, cases report, letters to the editor |
| Years considered/time period | All evidence published in the past 10 years, period 2010–2020 | Publications prior to 2010 |
| Language | English | Other languages |
| Databases | MEDLINE (PubMed), Web of Science, EBSCO, Cochrane Library | Other databases |
| Keywords | Delirium, resuscitation, cardiac arrest | n/a |
| Additional search terms, with which the central search terms were combined | “ICU”, “intensive care”, “delirium”, “cardiac arrest”, “resuscitation”, “delirium after cardiac arrest”, “delirium after CPR”, “post-cardiac arrest” “incidence of delirium” | n/a |
| Author, year. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rezar, R. et al. 2020 [16] |  |  |  |  |  |  |  |  |  | n/a |  |
| Keijze, H.M. et al. 2020 [2] |  |  |  |  |  |  |  |  |  |  |  |
| Jäckel, M. et al. 2020 [17] |  |  |  |  |  |  |  |  |  |  |  |
| Falsini, G. et al. 2018 [18] |  |  |  |  |  |  |  |  |  | n/a |  |
| Pollock, J.S. et al. 2016 [4] |  |  |  |  |  |  |  |  |  |  |  |
| Pauley, E. et al. 2015 [19] |  |  |  |  |  |  |  |  |  | n/a |  |
| Uguz, F. et al. 2010 [20] |  |  |  |  |  |  |  |  |  | n/a |  |
| Author, Year | Study Design | Participants | Delirium Assessment Tool | No. of Delirium Incidents after CA | Findings |
|---|---|---|---|---|---|
| Rezar, R. et al. 2020 [16] | A prospective analysis | Adult patients hospitalized at a medical ICU after CPR | No data | 24/106 (23%) | -Delirium occurred in 22.6% of patients after CA -There was no statistically significant difference in the incidence of delirium after CA in males and females |
| Keijze, H.M. et al. 2020 [2] | An ad hoc analysis of a multicenter prospective cohort study | Patients with recovery of consciousness, who survived until hospital discharge | Psychiatric consultation (DSM-V criteria) | 47/141 (33%) | -Delirium is common after CA -Delirium leads to longer hospitalization and poorer outcome |
| Jäckel, M. et al. 2020 [17] | A retrospective study | Patients (ICU) hospitalized for MI treated with coronary angiography | RASS and NuDesc | 15/68 (22%) | -CA was an independent predictor of delirium |
| Falsini, G. et al. 2018 [18] | A prospective, observational cohort study | CICU patients | RASS and CAM | 9/111 (8%) | -CA was not a predictor of delirium |
| Pollock, J.S. et al. 2016 [4] | A retrospective observational study | Patients (CICU) treated with therapeutic hypothermia after cardiac arrest | RASS and CAM-ICU | 107/107 (100%) | -High prevalence of delirium during the ICU stay in patients treated with TH after cardiac arrest -Most of the episodes of delirium were hypoactive -Older ages, longer times from initiation of CPR to ROSC were associated with increased duration of delirium. |
| Pauley, E. et al. 2015 [19] | A retrospective study | Patients admitted to CICU with a primary cardiovascular diagnosis | RASS and CAM-ICU | 21/120 (18%) | -Patients admitted after cardiac arrest were more likely to be CAM-ICU positive |
| Uguz, F. et al.2010 [20] | A retrospective study | Patients with acute MI admitted to the CICU | Psychiatric assess (DSM-IV-TR criteria | 3/12 (25%) | -CA during MI was an independent predictor of development of delirium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mędrzycka-Dąbrowska, W.; Lange, S.; Religa, D.; Dąbrowski, S.; Friganović, A.; Oomen, B.; Krupa, S. Delirium in ICU Patients after Cardiac Arrest: A Scoping Review. J. Pers. Med. 2022, 12, 1047. https://doi.org/10.3390/jpm12071047
Mędrzycka-Dąbrowska W, Lange S, Religa D, Dąbrowski S, Friganović A, Oomen B, Krupa S. Delirium in ICU Patients after Cardiac Arrest: A Scoping Review. Journal of Personalized Medicine. 2022; 12(7):1047. https://doi.org/10.3390/jpm12071047
Chicago/Turabian StyleMędrzycka-Dąbrowska, Wioletta, Sandra Lange, Dorota Religa, Sebastian Dąbrowski, Adriano Friganović, Ber Oomen, and Sabina Krupa. 2022. "Delirium in ICU Patients after Cardiac Arrest: A Scoping Review" Journal of Personalized Medicine 12, no. 7: 1047. https://doi.org/10.3390/jpm12071047
APA StyleMędrzycka-Dąbrowska, W., Lange, S., Religa, D., Dąbrowski, S., Friganović, A., Oomen, B., & Krupa, S. (2022). Delirium in ICU Patients after Cardiac Arrest: A Scoping Review. Journal of Personalized Medicine, 12(7), 1047. https://doi.org/10.3390/jpm12071047









