Predicting Remission among Perinatal Women with Depression in Rural Pakistan: A Prognostic Model for Task-Shared Interventions in Primary Care Settings
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Predictor Selection
2.3. Model Building Strategy
- Core emotional symptoms: depressed mood, anhedonia, loss of appetite, psychic anxiety, and somatic anxiety.
- Somatic symptoms: loss of weight, psychomotor retardation, hypochondriasis, suicidal ideation, and somatic symptoms.
- Insomnia symptoms: early, middle, and late insomnia.
- Atypical symptoms: hypersomnia, hyperphagia, and weight gain.
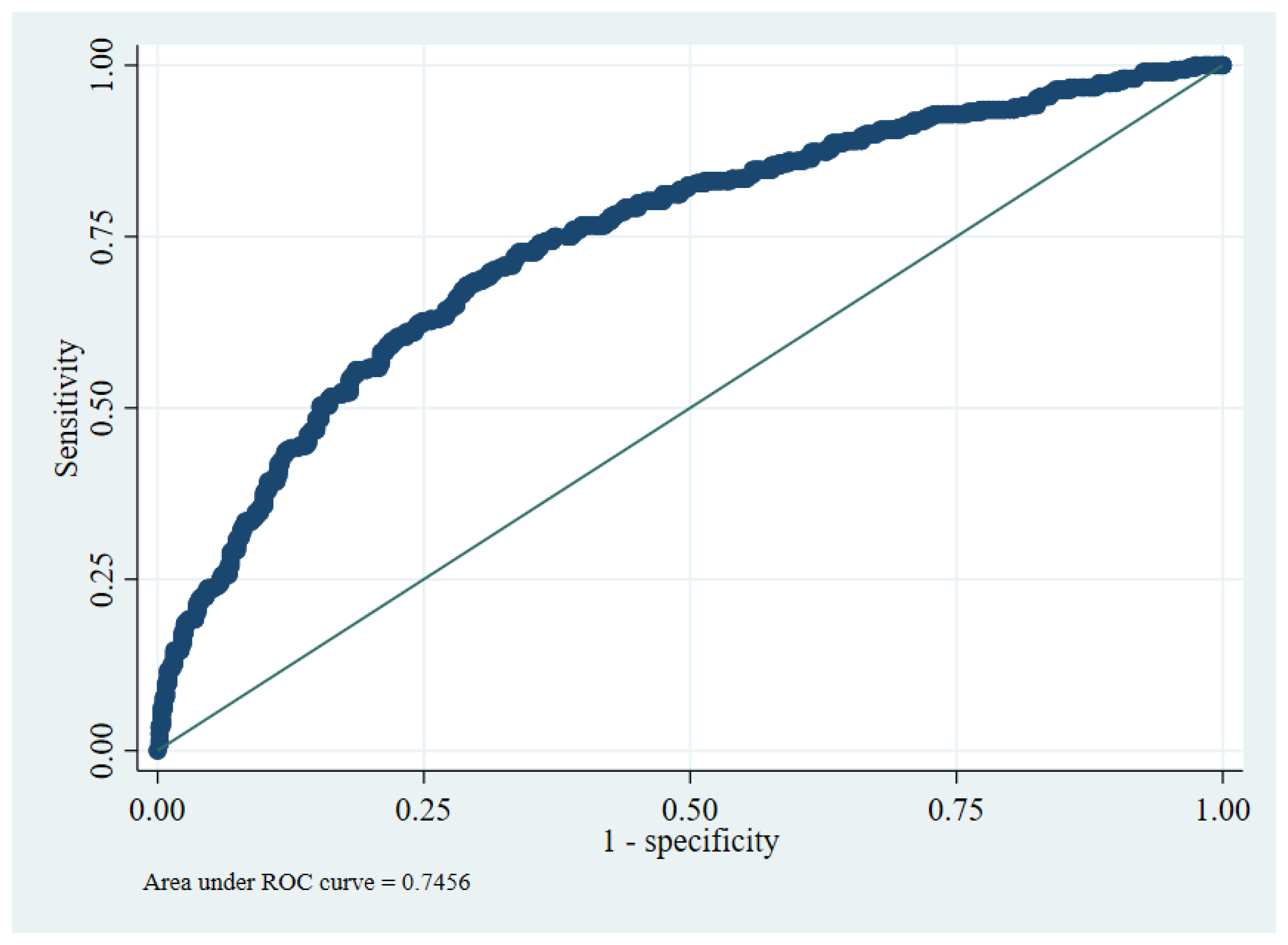
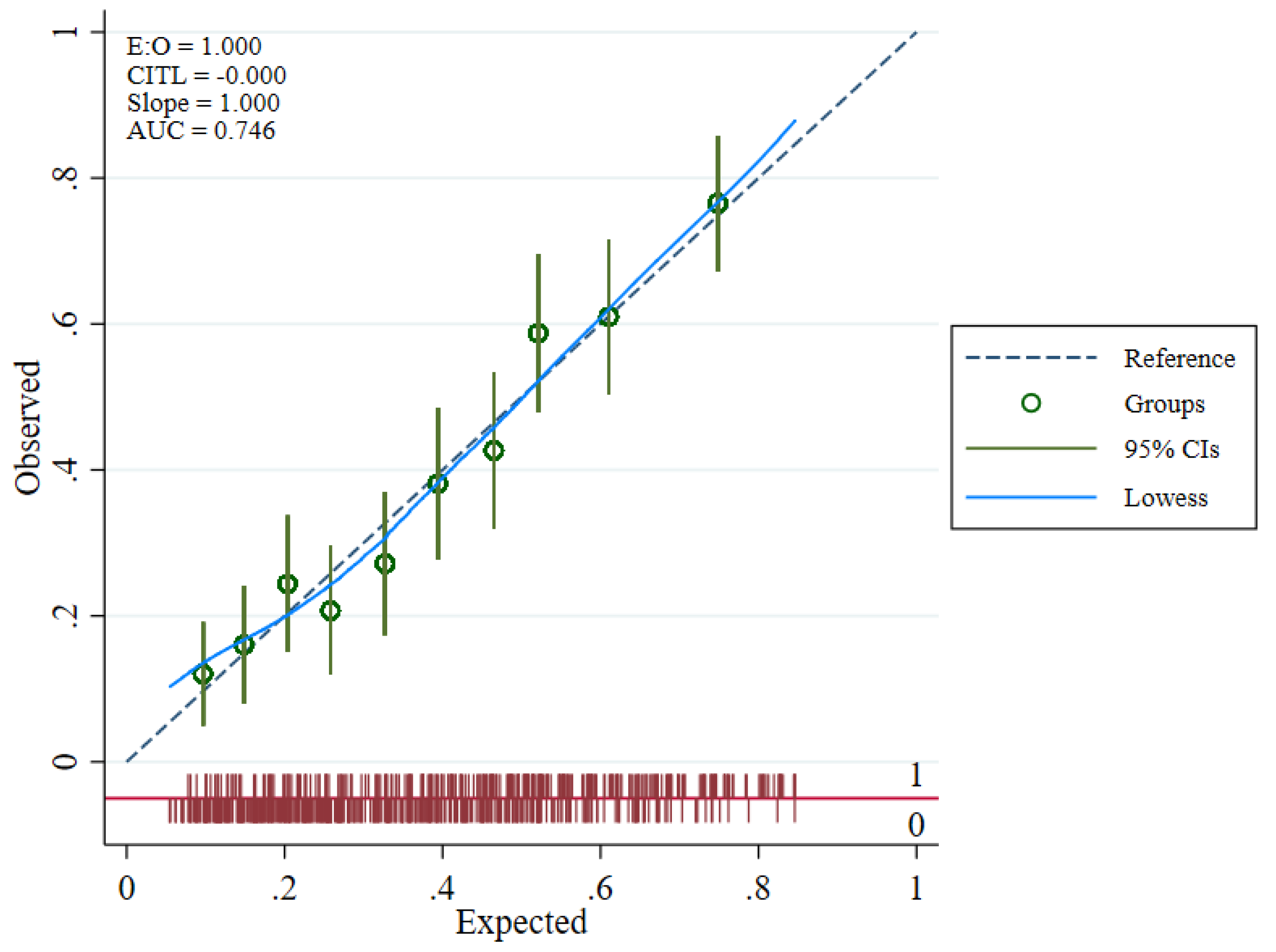
2.4. Internal Validity
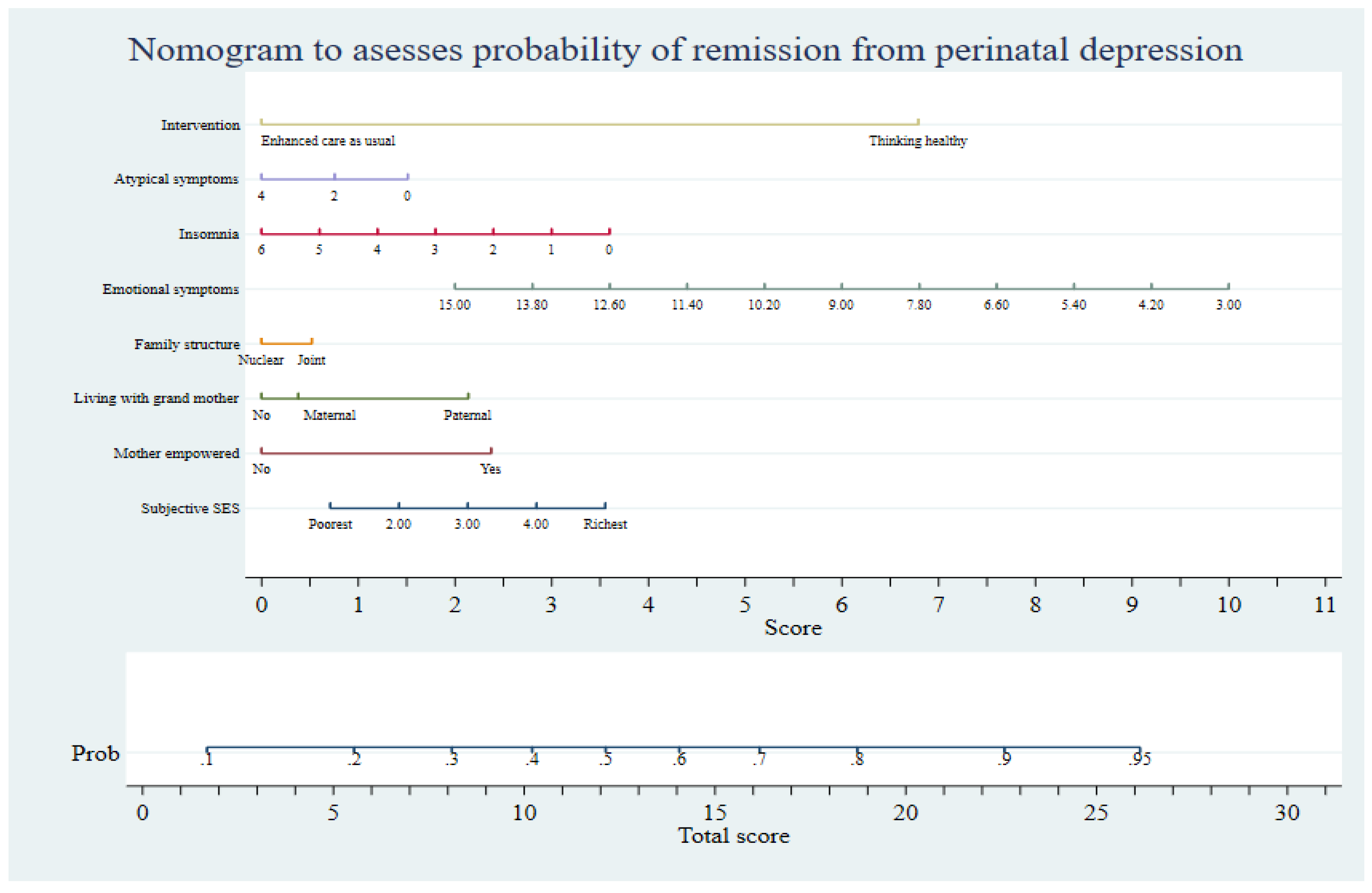
2.5. Utility of Prognostic Tool
2.6. Sample Size Calculation
3. Results
4. Discussion
5. Implications for Future Practice
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Galea, L.A.M.; Frokjaer, V.G. Perinatal Depression: Embracing Variability toward Better Treatment and Outcomes. Neuron 2019, 102, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diagnostic and Statistical Manual of Mental Disorder, 5th ed.; DSM-5; American Psychiatric Association: Washington, DC, USA, 2013.
- Shorey, S.; Chee, C.Y.I.; Ng, E.D.; Chan, Y.H.; Tam, W.W.S.; Chong, Y.S. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 104, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Maselko, J.; Bates, L.; Bhalotra, S.; Gallis, J.A.; O′Donnell, K.; Sikander, S.; Turner, E.L. Socioeconomic status indicators and common mental disorders: Evidence from a study of prenatal depression in Pakistan. SSM Popul. Health 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Atif, M.; Halaki, M.; Raynes-Greenow, C.; Chow, C.M. Perinatal depression in Pakistan: A systematic review and meta-analysis. Birth 2021, 48, 149–163. [Google Scholar] [CrossRef]
- Gelaye, B.; Rondon, M.B.; Araya, R.; Williams, M.A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 2016, 3, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Bureau, J.-F.; Easterbrooks, M.A.; Lyons-Ruth, K. Maternal depressive symptoms in infancy: Unique contribution to children′s depressive symptoms in childhood and adolescence? Dev. Psychopathol. 2009, 21, 519–537. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.H.; Gotlib, I.H. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychol. Rev. 1999, 106, 458–490. [Google Scholar] [CrossRef]
- Letourneau, N.L.; Dennis, C.L.; Cosic, N.; Linder, J. The effect of perinatal depression treatment for mothers on parenting and child development: A systematic review. Depress. Anxiety 2017, 34, 928–966. [Google Scholar] [CrossRef]
- World Health Organization. Improving Early Childhood Development; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/97892400020986 (accessed on 1 June 2022).
- Ransing, R.; Kukreti, P.; Deshpande, S.; Godake, S.; Neelam, N.; Raghuveer, P.; Mahadevaiah, M.; Kataria, D.; Patil, S.; Puri, M.; et al. Perinatal depression-knowledge gap among service providers and service utilizers in India. Asian J. Psychiatr. 2020, 47, 101822. [Google Scholar] [CrossRef]
- Rahman, A.; Waqas, A.; Nisar, A.; Nazir, H.; Sikander, S.; Atif, N. Improving access to psychosocial interventions for perinatal depression in low- and middle-income countries: Lessons from the field. Int. Rev. Psychiatry 2021, 33, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Pearlstein, T. Perinatal depression: Treatment options and dilemmas. J. Psychiatry Neurosci. 2008, 33, 302–318. [Google Scholar] [PubMed]
- Brown, J.V.E.; Wilson, C.A.; Ayre, K.; South, E.; Molyneaux, E.; Trevillion, K.; Howard, L.M.; Khalifeh, H. Antidepressant treatment for postnatal depression. Cochrane Database Syst. Rev. 2020, 2, CD013560. [Google Scholar] [CrossRef]
- Sockol, L.E. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J. Affect. Disord. 2015, 177, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Nyatsanza, M.; Schneider, M.; Davies, T.; Lund, C. Filling the treatment gap: Developing a task sharing counselling intervention for perinatal depression in Khayelitsha, South Africa. BMC Psychiatry 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, N.; Krishna, R.N.; Sikander, S.; Lazarus, A.; Nisar, A.; Ahmad, I.; Raman, R.; Fuhr, D.C.; Patel, V.; Rahman, A. Mother-to-mother therapy in India and Pakistan: Adaptation and feasibility evaluation of the peer-delivered Thinking Healthy Programme. BMC Psychiatry 2017, 17, 79. [Google Scholar] [CrossRef] [Green Version]
- Sikander, S.; Lazarus, A.; Bangash, O.; Fuhr, D.C.; Weobong, B.; Krishna, R.N.; Ahmad, I.; Weiss, H.A.; Price, L.; Rahman, A.; et al. The effectiveness and cost-effectiveness of the peer-delivered Thinking Healthy Programme for perinatal depression in Pakistan and India: The SHARE study protocol for randomised controlled trials. Trials 2015, 16, 534. [Google Scholar] [CrossRef] [Green Version]
- Chondros, P.; Davidson, S.; Wolfe, R.; Gilchrist, G.; Dowrick, C.; Griffiths, F.; Hegarty, K.; Herrman, H.; Gunn, J. Development of a prognostic model for predicting depression severity in adult primary patients with depressive symptoms using the diamond longitudinal study. J. Affect. Disord. 2018, 227, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Chekroud, A.M.; Zotti, R.J.; Shehzad, Z.; Gueorguieva, R.; Johnson, M.K.; Trivedi, M.H.; Cannon, T.D.; Krystal, J.H.; Corlett, P.R. Cross-trial prediction of treatment outcome in depression: A machine learning approach. Lancet Psychiatry 2016, 3, 243–250. [Google Scholar] [CrossRef]
- Eirini Karyotaki, R.A.; Ronald, C.; Kessler, A.W.; Arvin, B.; Atif, R.; Camila, T.M.; Clara, M.; Crick, L.; Emily, C.G.; Etheldreda, N.-M.; et al. Task-Shared Psychological Interventions for Depression in Low- and Middle-Income countries: A Systematic Review and Individual Patient Data Meta-analysis. JAMA Psychiatry 2022, in press. [Google Scholar] [CrossRef]
- Chekroud, A.M.; Gueorguieva, R.; Krumholz, H.M.; Trivedi, M.H.; Krystal, J.H.; McCarthy, G. Reevaluating the Efficacy and Predictability of Antidepressant Treatments: A symptom clustering approach. JAMA Psychiatry 2017, 74, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Keller, M.B.; Leon, A.C.; Mueller, T.I.; Lavori, P.W.; Shea, M.T.; Coryell, W.; Warshaw, M.; Turvey, C.; Maser, J.D.; et al. Multiple recurrences of major depressive disorder. Am. J. Psychiatry 2000, 157, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Thornton, L.M.; Gardner, C.O. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am. J. Psychiatry 2000, 157, 1243–1251. [Google Scholar] [CrossRef]
- Moriarty, A.S.; Meader, N.; Snell, K.I.; Riley, R.D.; Paton, L.W.; Chew-Graham, C.A.; Gilbody, S.; Churchill, R.; Phillips, R.S.; Ali, S.; et al. Prognostic models for predicting relapse or recurrence of major depressive disorder in adults. Cochrane Database Syst. Rev. 2021, 5, CD013491. [Google Scholar] [PubMed]
- Mirza, Z.; Rahman, A. Mental health care in Pakistan boosted by the highest office. Lancet 2019, 394, 2239–2240. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waqas, A.; Rahman, A. Does One Treatment Fit All? Effectiveness of a Multicomponent Cognitive Behavioral Therapy Program in Data-Driven Subtypes of Perinatal Depression. Front. Psychiatry 2021, 12, 736790. [Google Scholar] [CrossRef]
- Rahman, A.; Malik, A.; Sikander, S.; Roberts, C.; Creed, F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: A cluster-randomised controlled trial. Lancet 2008, 372, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.; Zaidi, A.; Waqas, A.; Malik, A.; Hagaman, A.; Maselko, J.; LeMasters, K.; Liaqat, R.; Bilal, S.; Bibi, T.; et al. Psychometric Validation of the Multidimensional Scale of Perceived Social Support During Pregnancy in Rural Pakistan. Front Psychol. 2021, 12, 601563. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Von Korff, M.U.T.; Ormel, J.; Kaplan, I.; Simon, G.E. Self-report disability in an international primary care study of psychological illness. J. Clin. Epidemiol. 1996, 49, 297–303. [Google Scholar] [CrossRef]
- Hall, R.C.W. Global Assessment of Functioning. Psychosomatics 1995, 36, 267–275. [Google Scholar] [CrossRef]
- Moriarty, A.S.; Paton, L.W.; Snell, K.I.E.; Riley, R.D.; Buckman, J.E.J.; Gilbody, S.; Chew-Graham, C.A.; Ali, S.; Pilling, S.; Meader, N.; et al. The development and validation of a prognostic model to PREDICT Relapse of depression in adult patients in primary care: Protocol for the PREDICTR study. Diagn. Progn. Res. 2021, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Spittal, M.J.; Chondros, P.; Palmer, V.J.; Chatterton, M.L.; Densley, K.; Potiriadis, M.; Harris, M.; Bassilios, B.; Burgess, P.; et al. Clinical efficacy of a Decision Support Tool (Link-me) to guide intensity of mental health care in primary practice: A pragmatic stratified randomised controlled trial. Lancet Psychiatry 2021, 8, 202–214. [Google Scholar] [CrossRef]
- Eirini Karyotaki, P.C.; Maria, P.; Ricardo, A.; Ahmed, W.; Atif, R.; Clara, M.; Crick, L.; Emily, C.; Garman, J.; Naslund, A.; et al. An Individualized Treatment Rule for Mean Depressive Symptoms Change after Task-Shared Cognitive Behavioural Therapy in Low- and Middle-Income Countries: An Individual Patient Data Meta-analysis. Preprints 2022, 2022050144. [Google Scholar]
- Mansournia, M.A.; Nazemipour, M.; Naimi, A.I.; Collins, G.S.; Campbell, M.J. Reflection on modern methods: Demystifying robust standard errors for epidemiologists. Int. J. Epidemiol. 2021, 50, 346–351. [Google Scholar] [CrossRef]
- Steyerberg, E. Clinical Prediction Models; Springer International: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Azen, R.; Traxel, N. Using Dominance Analysis to Determine Predictor Importance in Logistic Regression. J. Educ. Behav. Stat. 2009, 34, 319–347. [Google Scholar] [CrossRef]
- Luchman, J.N. Determining relative importance in Stata using dominance analysis: Domin and domme. Stata J. Promot. Commun. Stat. Stata 2021, 21, 510–538. [Google Scholar] [CrossRef]
- Ensor, J. PMSAMPSIZE: Stata Module to Calculate the Minimum Sample Size Required for Developing a Multivariable Prediction Model. 2021. Available online: https://ideas.repec.org/c/boc/bocode/s458569.html (accessed on 1 June 2022).
- Perlis, R.H. A clinical risk stratification tool for predicting treatment resistance in major depressive disorder. Biol. Psychiatry 2013, 74, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Santos Jr., H.; Tan, X.; Salomon, R. Heterogeneity in perinatal depression: How far have we come? A systematic review. Arch. Womens Ment. Health 2017, 20, 11–23. [Google Scholar] [CrossRef]
- Fried, E.I.; Nesse, R.M. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. J. Affect. Disord. 2015, 172, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guintivano, J.; Putnam, K.T.; Sullivan, P.F.; Meltzer-Brody, S. Clinical Phenotypes of Peripartum Depression and Time of Onset the PACT Consortium; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–14. [Google Scholar]
- Uher, R.; Dernovsek, M.Z.; Mors, O.; Hauser, J.; Souery, D.; Zobel, A.; Maier, W.; Henigsberg, N.; Kalember, P.; Rietschel, M.; et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 2011, 132, 112–120. [Google Scholar] [CrossRef]
- Driscoll, O. The importance of transdiagnostic symptom level assessment to understanding prognosis for depressed adults: Analysis of data from six randomized control trials. BMC Med. 2021, 109, 1–21. [Google Scholar]
- Delgadillo, J.; Ali, S.; Fleck, K.; Agnew, C.; Southgate, A.; Parkhouse, L.; Cohen, Z.D.; DeRubeis, R.J.; Barkham, M. Stratified Care vs. Stepped Care for Depression: A Cluster Randomized Clinical Trial. JAMA Psychiatry 2021, 79, 101–108. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Subgroup | Mean (SD) | Frequency | Percentage |
|---|---|---|---|---|
| Outcome | ||||
| Perinatal women with depression post-intervention assessed using the DSM-IV criteria | Enhanced Usual Care | 211 | 52.8% | |
| Thinking Healthy Program | 97 | 23.2% | ||
| Maternal demographic characteristics | ||||
| Mother age at baseline | 26.74 (5.11) | |||
| Maternal education level | 4.06 (4.011) | |||
| Paternal education level | 7.02 (3.965) | |||
| Socioeconomic condition | ||||
| Socioeconomic class | Richest | 12 | 1.3% | |
| Rich | 81 | 9.0% | ||
| Normal | 343 | 38.0% | ||
| Poor | 270 | 29.9% | ||
| Poorest | 197 | 21.8% | ||
| Household debt | No | 371 | 41.1% | |
| Yes | 529 | 58.6% | ||
| Not reported | 3 | 0.3% | ||
| Sufficient money for food | No | 120 | 13.3% | |
| Yes | 783 | 86.7% | ||
| Sufficient money for basic needs | No | 189 | 20.9% | |
| Yes | 714 | 79.1% | ||
| Financial Empowerment | Not empowered | 425 | 47.1% | |
| Empowered | 478 | 52.9% | ||
| Family structure | ||||
| Parity | 0 | 171 | 18.9% | |
| 1 to 3 | 520 | 57.6% | ||
| More than 4 | 212 | 23.5% | ||
| Family structure | Nuclear | 373 | 41.3% | |
| Joint | 530 | 58.7% | ||
| Living with mother or mother-in-law | No | 451 | 49.9% | |
| Maternal | 59 | 6.5% | ||
| Paternal | 393 | 43.5% | ||
| Perceived levels of social support | 45.04 (16.44) | |||
| Clinical profile | ||||
| Hamilton depression scores at baseline | 14.63 (4.09) | |||
| Chronicity (months) | 5.15 (9.08) | |||
| Disability scores (BDQ) | 8.21 (2.69) | |||
| Global assessment of functioning (GAF) | 62.05 (5.22) | |||
| Insomnia symptom dimension of HDRS | 2.33 (1.81) | |||
| Somatic symptom dimension of HDRS | 2.47 (1.47) | |||
| Core emotional symptoms dimension of HDRS | 8.37 (.65) | |||
| Atypical symptoms dimension of HDRS | 0.17 (0.57) | |||
| Major perinatal life events | ||||
| Child death | None | 518 | 57.4% | |
| Yes | 385 | 42.6% | ||
| Still birth | None | 607 | 67.2% | |
| Yes | 296 | 32.8% | ||
| Treatment | ||||
| Enhanced Usual Care | 440 | 48.7% | ||
| Thinking Healthy Program | 463 | 51.3% | ||
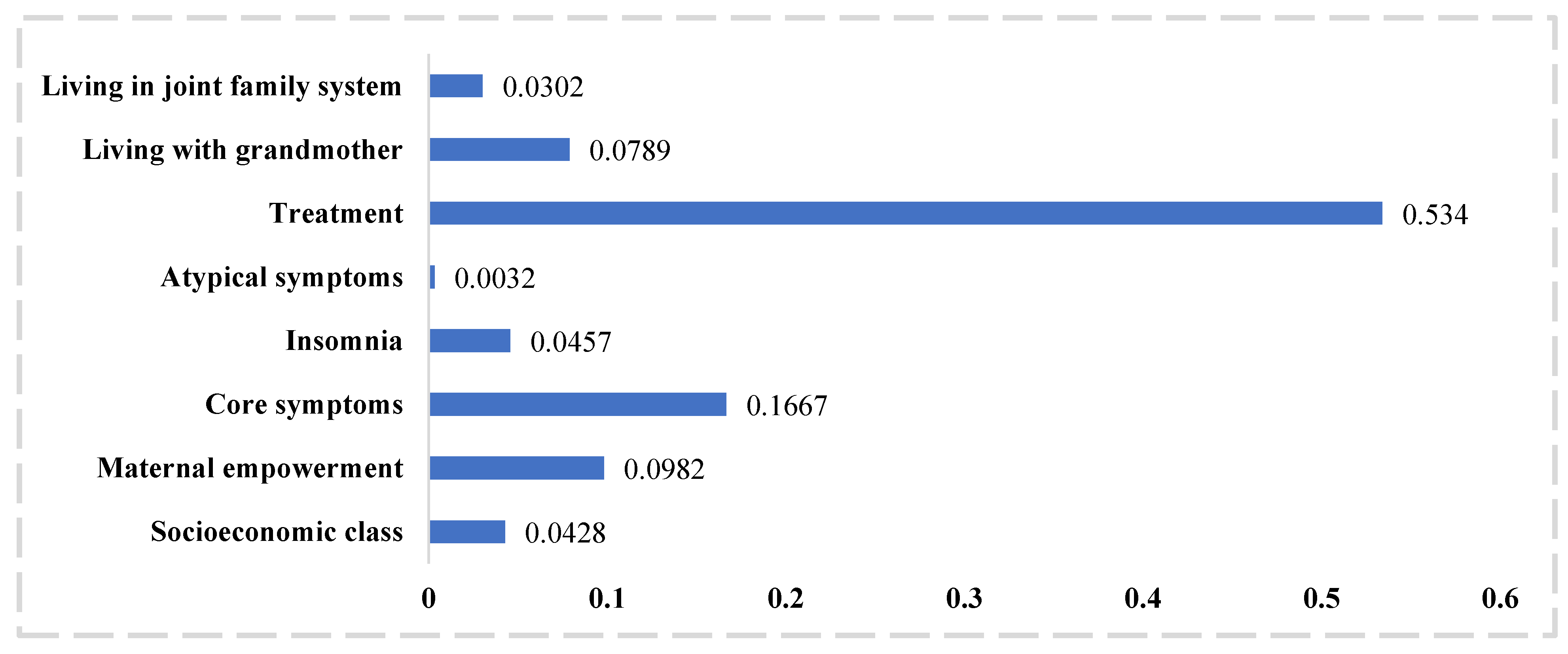
| Variables | Coefficients | Robust S.E. | Coefficients Adjusted for Optimism | z | Predictor Importance | p | 95% CI |
|---|---|---|---|---|---|---|---|
| Socioeconomic class | 0.1495407 | 0.0909545 | 0.139072851 | 1.64 | 6 | 0.1 | (−0.0287269 to 0.3278083) |
| Maternal empowerment | −0.4992032 | 0.1969697 | −0.464258976 | −2.53 | 3 | 0.011 | (−0.8852568 to −0.1131496) |
| Living with mother or mother-in-law | |||||||
| Maternal | −0.0800688 | 0.3280223 | −0.074463984 | −0.24 | 4 | 0.807 | (−0.7229807 to 0.5628432) |
| Paternal | −0.4495611 | 0.2246094 | −0.418091823 | −2 | 0.045 | (−0.8899834 to −0.009388) | |
| Family structure | −0.1090834 | 0.230452 | 0.101447562 | −0.47 | 7 | 0.64 | (−0.5607609 to 0.3425942) |
| Symptom dimensions of depression | |||||||
| Core emotional symptoms | 0.1402178 | 0.0332881 | 0.130402554 | 4.21 | 2 | <0.001 | (0.0749744 to 0.2054612) |
| Insomnia | 0.1261017 | 0.0478054 | 0.117274581 | 2.64 | 5 | 0.008 | (0.0324048 to 0.2197985) |
| Atypical symptoms | 0.0794525 | 0.1272977 | 0.073890825 | 0.62 | 8 | 0.533 | (−0.1700464 to 0.3289514) |
| Treatment | −1.4283 | 0.208072 | −1.328319 | −6.86 | 1 | <0.001 | (−1.836114 to −0.2638574) |
| Constant | −1.372766 | 0.5657802 | −1.31 | −2.43 | 0.015 | (−2.481675 to −0.2638574) | |
| Linear predictor = −0.61 (SD 0.98); Linear predictor adjusted for optimism = −0.599 (0.91) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas, A.; Sikander, S.; Malik, A.; Atif, N.; Karyotaki, E.; Rahman, A. Predicting Remission among Perinatal Women with Depression in Rural Pakistan: A Prognostic Model for Task-Shared Interventions in Primary Care Settings. J. Pers. Med. 2022, 12, 1046. https://doi.org/10.3390/jpm12071046
Waqas A, Sikander S, Malik A, Atif N, Karyotaki E, Rahman A. Predicting Remission among Perinatal Women with Depression in Rural Pakistan: A Prognostic Model for Task-Shared Interventions in Primary Care Settings. Journal of Personalized Medicine. 2022; 12(7):1046. https://doi.org/10.3390/jpm12071046
Chicago/Turabian StyleWaqas, Ahmed, Siham Sikander, Abid Malik, Najia Atif, Eirini Karyotaki, and Atif Rahman. 2022. "Predicting Remission among Perinatal Women with Depression in Rural Pakistan: A Prognostic Model for Task-Shared Interventions in Primary Care Settings" Journal of Personalized Medicine 12, no. 7: 1046. https://doi.org/10.3390/jpm12071046
APA StyleWaqas, A., Sikander, S., Malik, A., Atif, N., Karyotaki, E., & Rahman, A. (2022). Predicting Remission among Perinatal Women with Depression in Rural Pakistan: A Prognostic Model for Task-Shared Interventions in Primary Care Settings. Journal of Personalized Medicine, 12(7), 1046. https://doi.org/10.3390/jpm12071046






