Imaging Assessment of Interval Metastasis from Melanoma
Abstract
:1. Introduction
2. Methods
2.1. Search Criteria
2.2. Results
2.3. Assessment and Imaging in a Clinical Setting
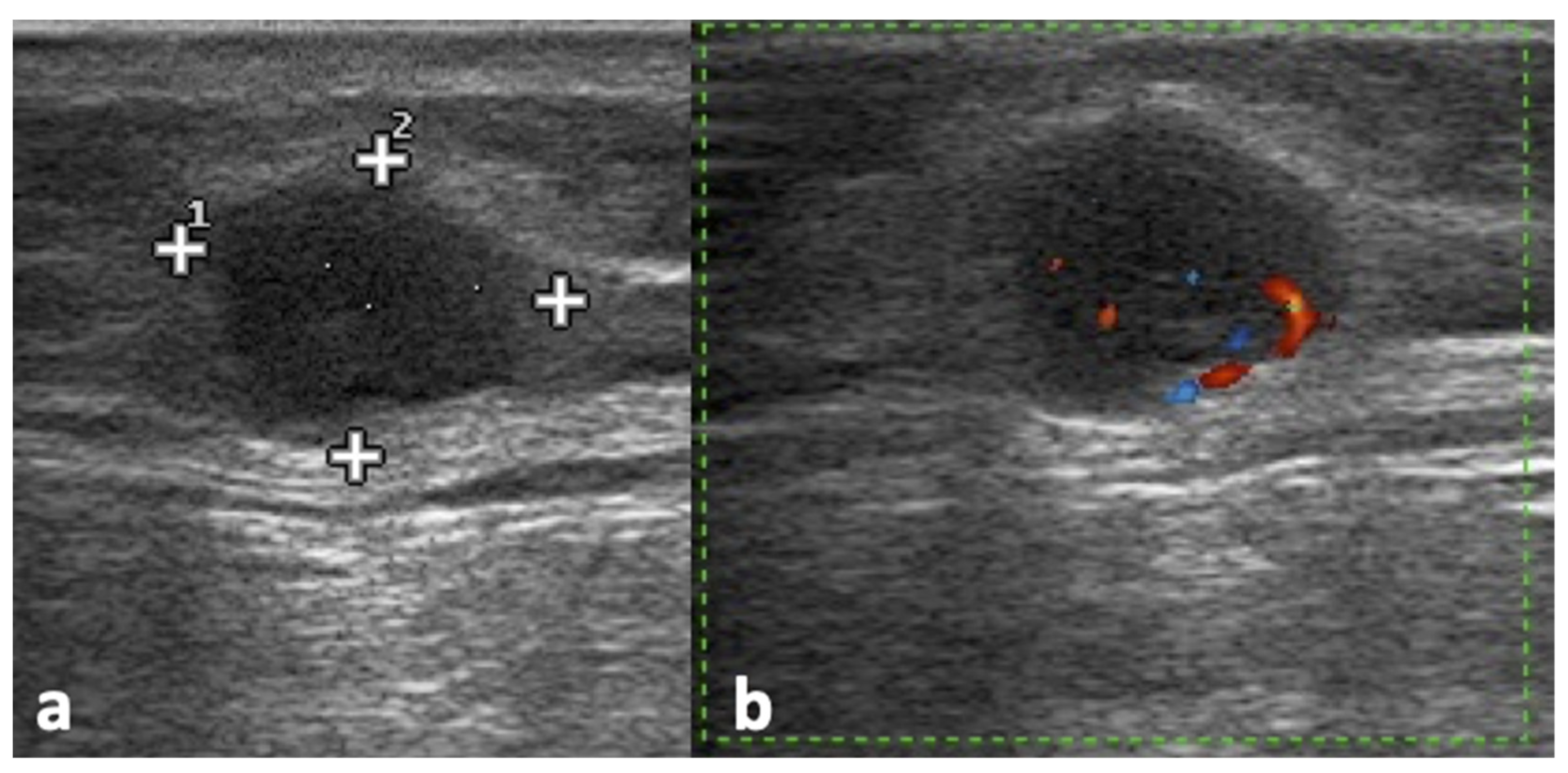
2.4. Ultrasound Assessment
2.5. Assessment with Other Imaging Techniques (CT, MRI, PET)
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sanki, A.; Uren, R.F.; Moncrieff, M.; Tran, K.L.; Scolyer, R.A.; Lin, H.Y.; Thompson, J.F. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J. Clin. Oncol. 2009, 27, 5614–5619. [Google Scholar] [CrossRef] [PubMed]
- Mcmasters, K.M.; Chao, C.; Wong, S.L.; Wrightson, W.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Cerrito, P.B. Interval Sentinel Lymph Nodes in Melanoma. Arch. Surg. 2020, 137, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, J.A.; Thompson, J.F.; Uren, R.F.; Howman-Giles, R.; Harman, C.R. Epitrochlear lymph nodes as a site of melanoma metastasis. Ann. Surg. Oncol. 1998, 5, 248–252. [Google Scholar] [CrossRef]
- Ishihara, T.; Kageshita, T.; Matsushita, S.; Ono, T. Investigation of sentinel lymph nodes of the axillary and cubital regions in upper-extremity malignant skin tumors: A series of 15 patients. Int. J. Clin. Oncol. 2003, 8, 297–300. [Google Scholar] [CrossRef]
- Uren, R.F.; Howman-Giles, R.; Thompson, J.F.; McCarthy, W.H.; Quinn, M.J.; Roberts, J.M.; Shaw, H.M. Interval nodes: The forgotten sentinel nodes in patients with melanoma. Arch. Surg. 2000, 135, 1168–1172. [Google Scholar] [CrossRef] [Green Version]
- Balch, C.M.; Buzaid, A.C.; Soong, S.J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.J.; Kirkwood, J.M.; et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J. Clin. Oncol. 2001, 19, 3635–3648. [Google Scholar] [CrossRef]
- Roozendaal, G.K.; De Vries, J.D.H.; Van Poll, D.; Jansen, L.; Schraffordt Koops, H.; Nieweg, O.E.; Kroon, B.B.R. Sentinel nodes outside lymph node basins in patients with melanoma. Br. J. Surg. 2001, 88, 305–308. [Google Scholar] [CrossRef]
- Thelmo, M.C.; Morita, E.T.; Treseler, P.A.; Nguyen, L.H.; Allen, R.E.; Sagebiel, R.W.; Kashani-sabet, M.; Leong, S.P.L. Micrometastasis to In-Transit Lymph Nodes From Extremity and Truncal Malignant Melanoma. Ann. Surg. Oncol. 2001, 8, 444–448. [Google Scholar] [CrossRef]
- Exhibit, S.; Nunziata, A.; Catalano, O.; Sandomenico, F.; Saturnino, P.P. The tail sign and the string sign: A new sonography (US) finding in patients with superficial melanoma metastasis. In Proceedings of the European Congress of Radiology-ECR 2012, Vienna, Austria, 1–5 March 2012; pp. 1–26. [Google Scholar]
- Uren, R.F.; Howman-Giles, R.B.; Thompson, J.F.; Shaw, H.M.; McCarthy, W.H. Lymphatic drainage from peri-umbilical skin to internal mammary nodes. Clin. Nucl. Med. 1995, 20, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Patnana, M.; Bronstein, Y.; Szklaruk, J.; Bedi, D.G.; Hwu, W.; Gershenwald, J.E.; Prieto, V.G.; Ng, C.S. Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin. Radiol. 2011, 66, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Catalano, O.; Setola, S.V.; Vallone, P.; Raso, M.M.; D’Errico, A.G. Sonography for locoregional staging and follow-up of cutaneous melanoma: How we do it. J. Ultrasound Med. 2010, 29, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, A.; Chung, D.; London, K.; Uren, R. Ultrasound Examination of the Lymphatic Drainage Area and Regional Lymph Nodes in Melanoma Patients with In-Transit Metastases. Ann. Surg. Oncol. 2021, 28, 1625–1631. [Google Scholar] [CrossRef]
- Lomoro, P.; Simonetti, I.; Nanni, A.L.; Corsani, G.; Togni, G.; Fichera, V.; Verde, F.; Formica, M.; Trovato, P.; Vallone, G.; et al. Imaging of head and neck lipoblastoma: Case report and systematic review. J. Ultrasound 2021, 24, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Testori, A.; Ribero, S.; Bataille, V. Diagnosis and treatment of in-transit melanoma metastases. Eur. J. Surg. Oncol. 2017, 43, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Voit, C.; Mayer, T.; Kr, M.; Schoengen, A.; Sterry, W.; Weber, L.; Proebstle, T.M. Efficacy of ultrasound B-scan compared with physical examination in follow-up of melanoma patients. Cancer 2001, 91, 2409–2416. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Kiyohara, Y. B-Mode Ultrasound Imaging, Doppler Imaging, and Real-Time Elastography in Cutaneous Malignant Melanoma and Lymph Node Metastases. Healthcare 2013, 1, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Ogata, D.; Uematsu, T.; Yoshikawa, S. Accuracy of real-time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): A pilot study. Int. J. Clin. Oncol. 2013, 19, 716–721. [Google Scholar] [CrossRef]
- Catalano, O.; Nunziata, A.; Siani, A. Fundamentals in Oncologic Ultrasound: Sonographic Imaging and Intervention in the Cancer Patient; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 9788847013544. [Google Scholar]
- Shirakawa, T.; Miyamoto, Y.; Yamagishi, J.; Fukuda, K.; Tada, S. Color/power Doppler sonographic differential diagnosis of superficial lymphadenopathy: Metastasis, malignant lymphoma, and benign process. J. Ultrasound Med. 2001, 20, 525–532. [Google Scholar] [CrossRef]
- Catalano, O.; Nunziata, A.; Saturnino, P.P.; Siani, A. Epitrochlear lymph nodes: Anatomy, clinical aspects, and sonography features. Pictorial essay. J. Ultrasound 2010, 13, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, C.R.; Seno, A.; Vecchiato, A.; Foletto, M.; Tregnaghi, A.; De Candia, A.; Rubaltelli, L.; Montesco, C.; Lise, M. The impact of ultrasound scanning in the staging and follow-up of patients with clinical stage I cutaneous melanoma. Eur. J. Cancer Part A 1997, 33, 200–203. [Google Scholar] [CrossRef]
- Brountzos, E.N.; Panagiotou, I.E.; Bafaloukos, D.I.; Kelekis, D.A. Ultrasonographic detection of regional lymph node metastases in patients with intermediate or thick malignant melanoma. Oncol. Rep. 2003, 10, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Schuh, T.; Eberle, J.Y.; Baumert, J.; Konz, B.; Volkenandt, M.; Ruzicka, T.; Schmid-Wendtner, M.H. The use of high-resolution ultrasonography for preoperative detection of metastases in sentinel lymph nodes of patients with cutaneous melanoma. Dermatol. Surg. 2009, 35, 1757–1765. [Google Scholar] [CrossRef]
- Testori, A.; Lazzaro, G.; Baldini, F.; Tosti, G.; Mosconi, M.; Lovati, E.; Bossi, C.; Sanvito, S.; Stanganelli, I.; Mazzarol, G.; et al. The role of ultrasound of sentinel nodes in the pre- and post-operative evaluation of stage I melanoma patients. Melanoma Res. 2005, 15, 191–198. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Dill-Müller, D.; Baumert, J.; Wagner, A.; Eberle, J.; Tilgen, W.; Plewig, G. Lymph node metastases in patients with cutaneous melanoma: Improvements in diagnosis by signal-enhanced color Doppler sonography. Melanoma Res. 2004, 14, 269–276. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Burgdorf, W. Ultrasound scanning in dermatology. Arch. Dermatol. 2005, 141, 217–224. [Google Scholar] [CrossRef]
- Tregnaghi, A.; De Candia, A.; Calderone, M.; Cellini, L.; Rossi, C.R.; Talenti, E.; Blandamura, S.; Borsato, S.; Muzzio, P.C.; Rubaltelli, L. Ultrasonographic evaluation of superficial lymph node metastases in melanoma. Eur. J. Radiol. 1997, 24, 216–221. [Google Scholar] [CrossRef]
- Bedi, D.G.; Krishnamurthy, R.; Krishnamurthy, S.; Edeiken, B.S.; Le-Petross, H.; Fornage, B.D.; Bassett, R.L.; Hunt, K.K. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: In vitro sonographic study. Am. J. Roentgenol. 2008, 191, 646–652. [Google Scholar] [CrossRef]
- Schäfer-Hesterberg, G.; Schoengen, A.; Sterry, W.; Voit, C. Use of ultrasound to early identify, diagnose and localize metastases in melanoma patients. Expert Rev. Anticancer Ther. 2007, 7, 1707–1716. [Google Scholar] [CrossRef]
- Catalano, O.; Siani, A. Cutaneous Melanoma: Role of Ultrasound in the Assessment of Locoregional Spread. Curr. Probl. Diagn. Radiol. 2010, 39, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Pow, S.Y.; Teo, M. Unusual presentation of primary cutaneous melanoma of the forearm with epitrochlear and axillary lymphadenopathy. BMJ Case Rep. 2018, 2018, bcr2017222518. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Schlagenhauff, B.; Stroebel, W.; Breuninger, H.; Rassner, G.; Garbe, C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow-up of patients with cutaneous melanoma: Results of a prospective study of 1288 patients. Cancer 2000, 88, 2534–2539. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Soyer Güldoğan, E.; Ergun, O.; Taşkın Türkmenoğlu, T.; Yılmaz, K.B.; Akdağ, T.; Özbal Güneş, S.; Durmaz, H.A.; Hekimoğlu, B. The impact of TI-RADS in detecting thyroid malignancies: A prospective study. Radiol. Med. 2021, 126, 1335–1344. [Google Scholar] [CrossRef]
- Celletti, I.; Fresilli, D.; De Vito, C.; Bononi, M.; Cardaccio, S.; Cozzolino, A.; Durante, C.; Grani, G.; Grimaldi, G.; Isidori, A.M.; et al. TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance? Radiol. Med. 2021, 126, 1189–1200. [Google Scholar] [CrossRef]
- Lomoro, P.; Simonetti, I.; Vinci, G.; Fichera, V.; Prevedoni Gorone, M.S. Pancake kidney, a rare and often misdiagnosed malformation: A case report and radiological differential diagnosis. J. Ultrasound 2019, 22, 207–213. [Google Scholar] [CrossRef]
- Sandomenico, F.; Corvino, A.; Setola, S.V.; Simonetti, I.; Porcaro, M.; Trovato, P.; Catalano, O.; Petrillo, A. Post-amputation neuroma of radial nerve in a patient with ephitelioid sarcoma: Case report and literature review. Acta Biomed. 2020, 91, 122–127. [Google Scholar] [CrossRef]
- De Muzio, F.; Cutolo, C.; Aversana, F.D.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agents Cancer 2017, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Fusco, R.; de Lutio di Castelguidone, E.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid-enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agents Cancer 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Hedgire, S.; Harisinghani, M. Radiologic Assessment of Lymph Nodes in Oncologic Patients. Curr. Radiol. Rep. 2014, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, S.E.; Carrasquillo, J.A.; Hoffman, J.M.; Galen, B.; Choyke, P.; White, D.E.; Rosenberg, S.A.; Sherry, R.M. A prospective analysis of positron emission tomography and conventional imaging for detection of stage IV metastatic melanoma in patients undergoing metastasectomy. Ann. Surg. Oncol. 2004, 11, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Petrillo, M.; Fusco, R.; Setola, S.V.; De Lutio Di Castelguidone, E.; Catalano, O.; Piccirillo, M.; Albino, V.; Izzo, F.; Petrillo, A. Surveillance of HCC patients after liver RFA: Role of MRI with hepatospecific contrast versus three-phase CT scan—Experience of high volume oncologic institute. Gastroenterol. Res. Pract. 2013, 2013, 469097. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative Radiotherapy Indications during the COVID-19 Pandemic and in Future Complex Logistic Settings: The NORMALITY Model; Springer: Milan, Italy, 2021; Volume 126, ISBN 0123456789. [Google Scholar]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. La Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Saokar, A.; Islam, T.; Jantsch, M.; Saksena, M.A.; Hahn, P.F.; Harisinghani, M.G. Detection of lymph nodes in pelvic malignancies with computed tomography and magnetic resonance imaging. Clin. Imaging 2010, 34, 361–366. [Google Scholar] [CrossRef]
- Simonetti, I.; Sandomenico, F.; Rocco, M.P.; Fusco, R.; Setola, S.V.; Granata, V.; Iasevoli, D.M.; Ascierto, P.A.; Grassi, R.; Petrillo, A. Metastatic endo and perineural involvement of the ulnar nerve from malignant melanoma: Ultrasound (US) and magnetic resonance imaging (MRI) findings. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3478–3482. [Google Scholar] [CrossRef]
- Fusco, R.; Petrillo, M.; Granata, V.; Filice, S.; Sansone, M.; Catalano, O.; Petrillo, A. Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: A systematic review. Radiol. Oncol. 2017, 51, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.; et al. Standardized Index of Shape (DCE-MRI) and Standardized Uptake Value (PET/CT): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017, 8, 8143–8153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avallone, A.; Pecori, B.; Bianco, F.; Aloj, L.; Tatangelo, F.; Romano, C.; Granata, V.; Marone, P.; Leone, A.; Botti, G.; et al. Critical role of bevacizumab scheduling in combination with presurgical chemo-radiotherapy in MRI-defined high-risk locally advanced rectal cancer: Results of the branch trial. Oncotarget 2015, 6, 30394–30407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossola, C.; Curti, M.; Calvi, M.; Tack, S.; Mazzoni, S.; Genesio, L.; Venturini, M.; Genovese, E.A. Role of ultrasound and magnetic resonance imaging in the prognosis and classification of muscle injuries in professional football players: Correlation between imaging and return to sport time. Radiol. Med. 2021, 126, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: A meta-analysis. J. Natl. Cancer Inst. 2011, 103, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Billé, A.; Pelosi, E.; Skanjeti, A.; Arena, V.; Errico, L.; Borasio, P.; Mancini, M.; Ardissone, F. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: Accuracy of integrated positron emission tomography and computed tomography. Eur. J. Cardiothorac. Surg. 2009, 36, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Bimonte, S.; Leongito, M.; Barbieri, A.; Del Vecchio, V.; Barbieri, M.; Albino, V.; Piccirillo, M.; Amore, A.; Di Giacomo, R.; Nasto, A.; et al. Inhibitory effect of (-)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect. Agents Cancer 2015, 10, 22. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Scrofani, A.R.; Marra, P.; Coniglio, G.; Failla, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef]
- Chen, H.; Thompson, L.D.R.; Aguilera, N.S.I.; Abbondanzo, S.L. Kimura Disease: A Clinicopathologic Study of 21 Cases. Am. J. Surg. Pathol. 2004, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Seth, V.; Kabra, S.K.; Jain, Y.; Semwal, O.P.; Mukhopadhyaya, S.; Jensen, R.L. Tubercular lymphadenitis: Clinical manifestations. Indian J. Pediatr. 1995, 62, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Trovato, P.; Simonetti, I.; Verde, F.; Lomoro, P.; Vinci, G.; Tarotto, L.; Corvino, F.; Corvino, A. Acute epiploic appendagitis: Ultrasound and computed tomography findings of a rare case of acute abdominal pain and the role of other imaging techniques. Pol. J. Radiol. 2020, 85, e178–e182. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, L.N.; Alexander, A.A.; Rawool, N.M.; Kurtz, A.B.; Maguire, H.C.; Mastrangelo, M.J. Malignant melanoma: Impact of superficial US on management. Radiology 1996, 199, 273–277. [Google Scholar] [CrossRef]
- Lomoro, P.; Simonetti, I.; Nanni, A.; Cassone, R.; Di Pietto, F.; Vinci, G.; Prevedoni, M.S.; Romano, S.; Sammarchi, L. Extrapelvic Sciatic Nerve Endometriosis, the Role of Magnetic Resonance Imaging: Case Report and Systematic Review. J. Comput. Assist. Tomogr. 2019, 43, 976–980. [Google Scholar] [CrossRef]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.R.; He, Y.; Chen, J.Q.; Yang, H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): A comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar] [CrossRef]
- Assadsangabi, R.; Babaei, R.; Songco, C.; Ivanovic, V.; Bobinski, M.; Chen, Y.J.; Nabavizadeh, S.A. Multimodality oncologic evaluation of superficial neck and facial lymph nodes. Radiol. Med. 2021, 126, 1074–1084. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Pozzessere, C.; Boudiaf, M.; Cirigliano, A.; Dohan, A.; Mazzei, M.A.; Barat, M.; Volterrani, L.; Soyer, P. MR-enterography: Role in the assessment of suspected anastomotic recurrence of Crohn disease after ileocolic resection. Radiol. Med. 2022, 127, 238–250. [Google Scholar] [CrossRef]
- Cozzi, D.; Bindi, A.; Cavigli, E.; Grosso, A.M.; Luvarà, S.; Morelli, N.; Moroni, C.; Piperio, R.; Miele, V.; Bartolucci, M. Exogenous lipoid pneumonia: When radiologist makes the difference. Radiol. Med. 2021, 126, 22–28. [Google Scholar] [CrossRef]


| Uren et al. [12] | Hunt et al. [5] | Uren et al. [7] | Roozendaal et al. [9] | Ishihara et al. [6] | Mcmasters et al. [4] | |
|---|---|---|---|---|---|---|
| Year | 1995 | 1998 | 2000 | 2001 | 2003 | 2020 |
| Number of Patients | 450 | 13,139 | 2045 | 379 | 9 | 2000 |
| Tumor location | ||||||
| head and neck | 304 | 35 | 219 | |||
| trunk | 905 | 133 | 901 | |||
| lower extremities | 451 | 457 | ||||
| leg or foot | 153 | |||||
| upper extremities | 385 | 423 | ||||
| arm | 58 | |||||
| upper arm | 2 | |||||
| forearm/elbow | 700 | 1 | ||||
| wrist | ||||||
| hand | 102 | 1 | ||||
| fingers | 5 | |||||
| SLN location | 148 | |||||
| epitrochlear lymph node | 10 | 2 | 15 | |||
| popliteal area | 3 | 8 | ||||
| peri-umbilical area | 10 | |||||
| occipital and postauricular/mastoid areas | 12 | |||||
| lateral axillary nodes | 3 | |||||
| central axillary nodes | 3 | |||||
| triangular inter-muscular space | 5 | |||||
| flank | 4 | |||||
| peri-areolar area | 2 | |||||
| over the deltoid muscle | 1 | |||||
| bicipital sulcus | 1 | |||||
| cubital nodes | 1 | |||||
| subscapular node | 1 | |||||
| internal mammary lymph node | 2 | |||||
| aberrant lymph nodes | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, I.; Trovato, P.; Granata, V.; Picone, C.; Fusco, R.; Setola, S.V.; Mattace Raso, M.; Caracò, C.; Ascierto, P.A.; Sandomenico, F.; et al. Imaging Assessment of Interval Metastasis from Melanoma. J. Pers. Med. 2022, 12, 1033. https://doi.org/10.3390/jpm12071033
Simonetti I, Trovato P, Granata V, Picone C, Fusco R, Setola SV, Mattace Raso M, Caracò C, Ascierto PA, Sandomenico F, et al. Imaging Assessment of Interval Metastasis from Melanoma. Journal of Personalized Medicine. 2022; 12(7):1033. https://doi.org/10.3390/jpm12071033
Chicago/Turabian StyleSimonetti, Igino, Piero Trovato, Vincenza Granata, Carmine Picone, Roberta Fusco, Sergio Venanzio Setola, Mauro Mattace Raso, Corrado Caracò, Paolo A. Ascierto, Fabio Sandomenico, and et al. 2022. "Imaging Assessment of Interval Metastasis from Melanoma" Journal of Personalized Medicine 12, no. 7: 1033. https://doi.org/10.3390/jpm12071033
APA StyleSimonetti, I., Trovato, P., Granata, V., Picone, C., Fusco, R., Setola, S. V., Mattace Raso, M., Caracò, C., Ascierto, P. A., Sandomenico, F., & Petrillo, A. (2022). Imaging Assessment of Interval Metastasis from Melanoma. Journal of Personalized Medicine, 12(7), 1033. https://doi.org/10.3390/jpm12071033






