Atomic Force Microscopy Application for the Measurement of Infliximab Concentration in Healthy Donors and Pediatric Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. AFM-Based Nanoassay
2.2. Infliximab Patients’ Cohort
2.3. ELISA Assay
2.4. Statistical Analysis
3. Results
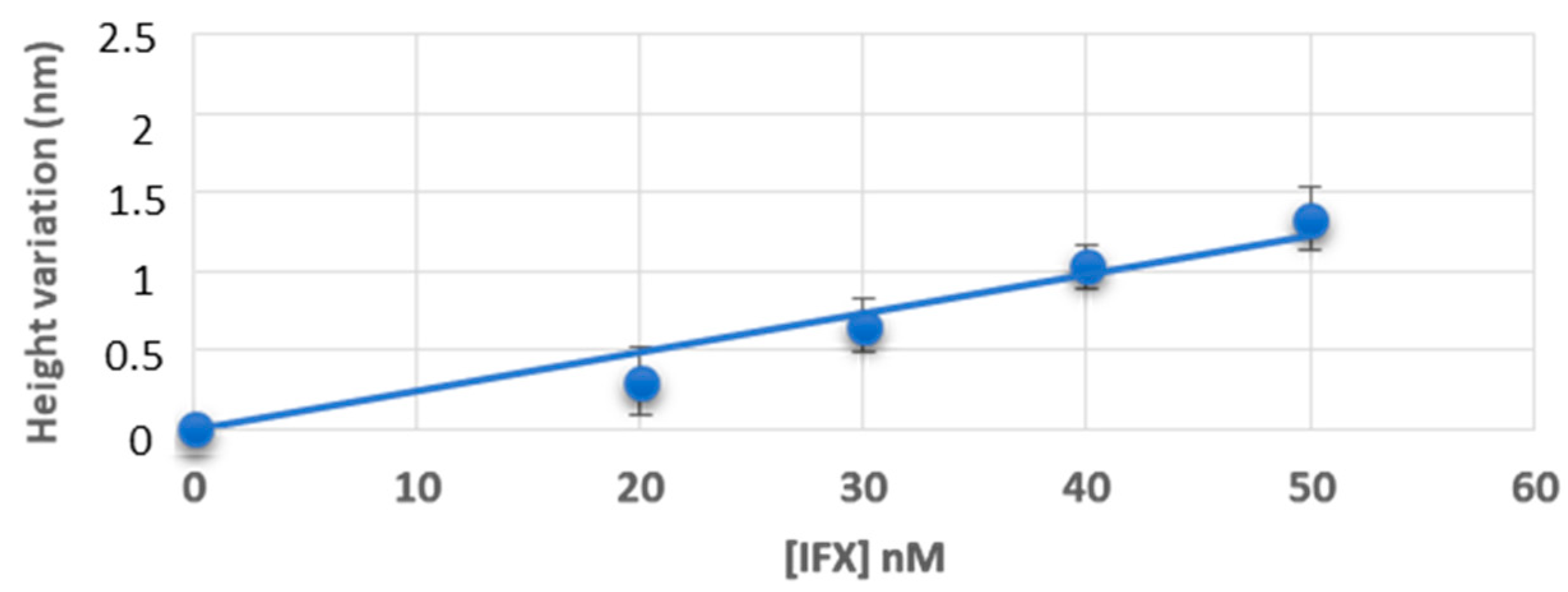
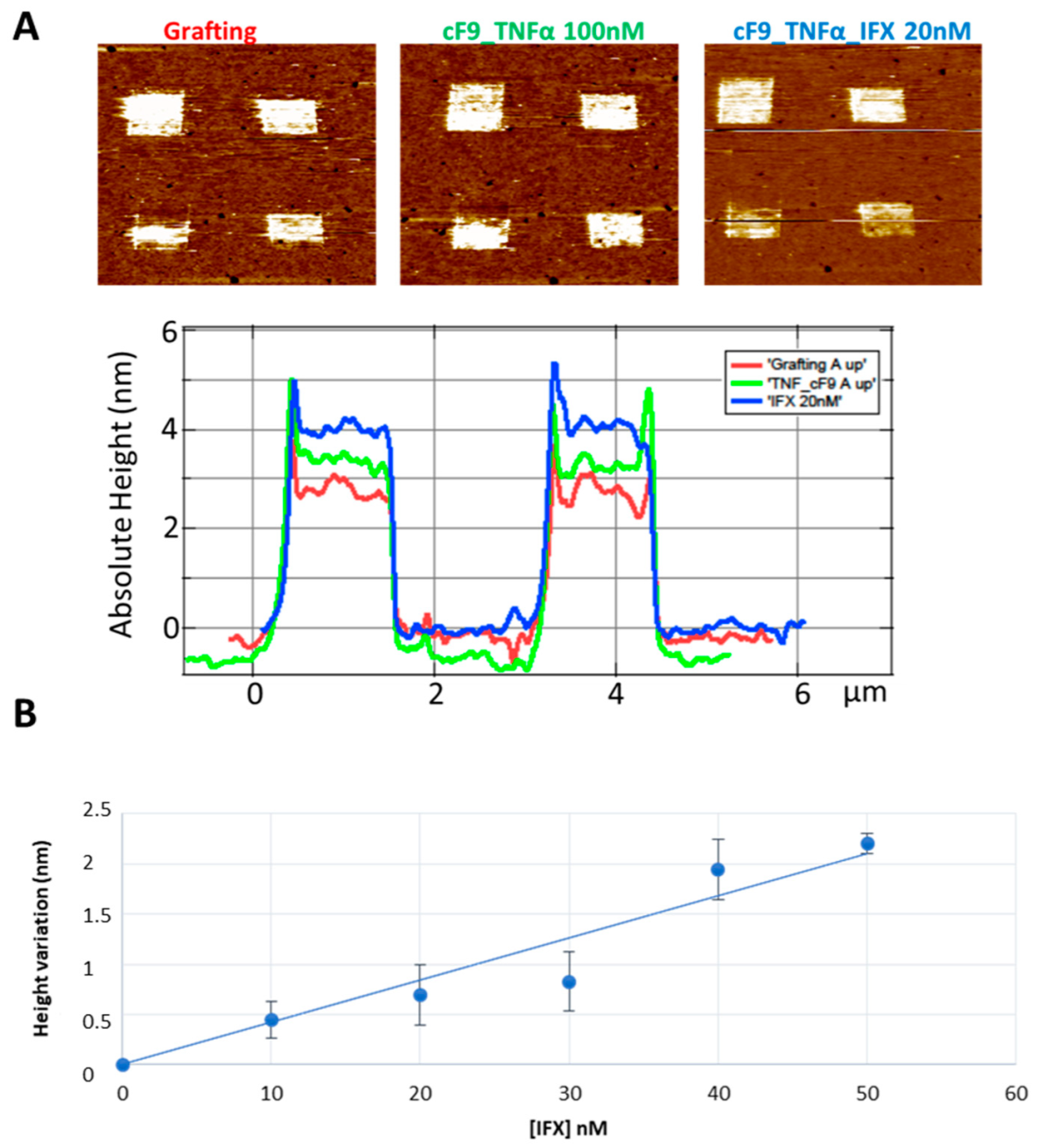
3.1. Measurement of Infliximab Concentration, in Buffer and Healthy Control Sera, by Atomic Force Microscopy
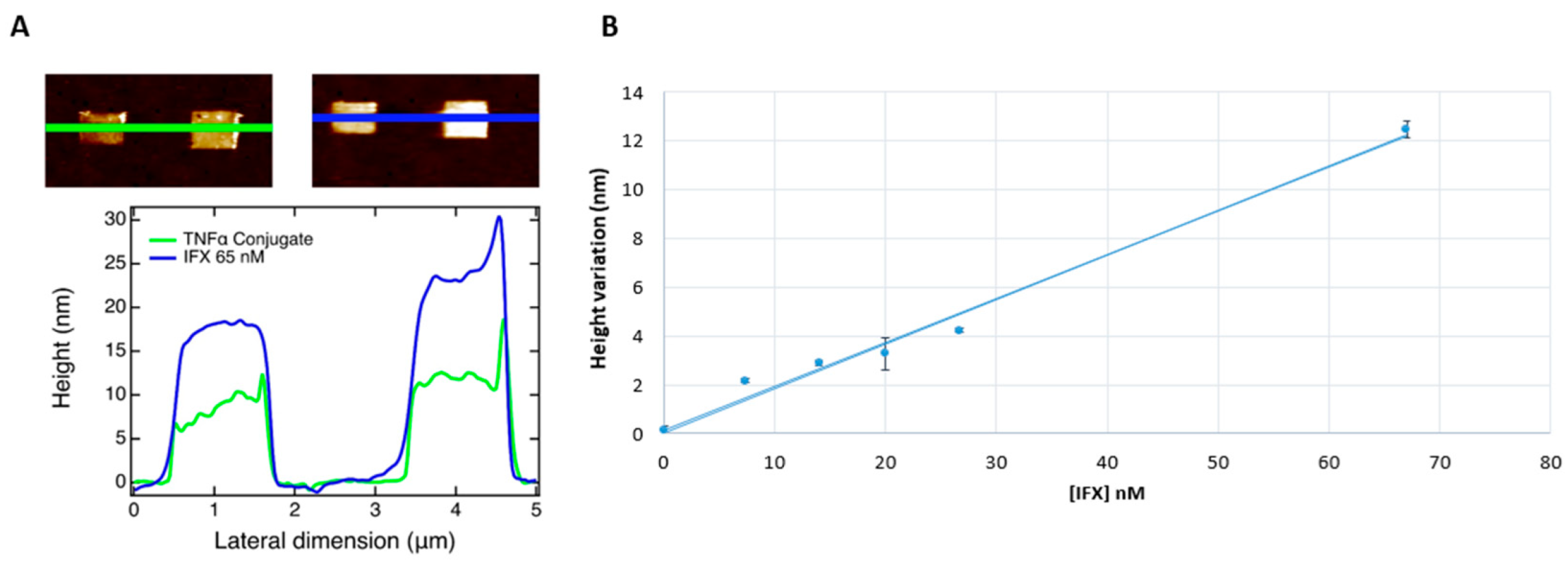
3.2. Measurement of Infliximab Concentration in Pediatric IBD Patients’ Sera, by Atomic Force Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, D.R.; Colombel, J.-F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019, 10, 2040622319838443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Coliti 2021, 15, 171–194. [Google Scholar] [CrossRef]
- Franca, R.; Curci, D.; Lucafò, M.; Decorti, G.; Stocco, G. Therapeutic drug monitoring to improve outcome of anti-TNF drugs in pediatric inflammatory bowel disease. Expert Opin. Drug Metab. Toxicol. 2019, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Labetoulle, R.; Harzallah, I.; Berger, A.-E.; Roblin, X.; Paul, S. Comparison of Point-of-Care and Classical Immunoassays for the Monitoring Infliximab and Antibodies Against Infliximab in IBD. Am. J. Dig. Dis. 2018, 63, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Casteele, N.V. Assays for measurement of TNF antagonists in practice. Front. Gastroenterol. 2016, 8, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tüdős, A.J.; Besselink, G.A.J.; Schasfoort, R.B.M. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab Chip 2001, 1, 83–95. [Google Scholar] [CrossRef]
- Huang, S.; Wang, W.; Li, J.; Zhang, T.; Liang, Y.; Wang, Q.; Jiang, Z. Multifunctional DNA mediated spatially confined assembly for antibody orientation: Surpassing sensitivity and accuracy for rituximab detection. Chem. Eng. J. 2021, 419, 129613. [Google Scholar] [CrossRef]
- Imaeda, H.; Andoh, A.; Fujiyama, Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J. Gastroenterol. 2011, 47, 136–143. [Google Scholar] [CrossRef]
- Lu, J.; Spasic, D.; Delport, F.; Van Stappen, T.; Detrez, I.; Daems, D.; Vermeire, S.; Gils, A.; Lammertyn, J. Immunoassay for Detection of Infliximab in Whole Blood Using a Fiber-Optic Surface Plasmon Resonance Biosensor. Anal. Chem. 2017, 89, 3664–3671. [Google Scholar] [CrossRef]
- Muneer, S.; Ayoko, G.A.; Islam, N.; Izake, E.L. Utilizing the thiol chemistry of biomolecules for the rapid determination of anti-TNF-α drug in blood. Talanta 2019, 208, 120411. [Google Scholar] [CrossRef] [PubMed]
- Thoren, K.L.; Pasi, B.; Delgado, J.C.; Wu, A.H.; Lynch, K.L. Quantitation of Infliximab and Detection of Antidrug Antibodies in Serum by Use of Surface Plasmon Resonance. J. Appl. Lab. Med. 2018, 2, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeni, L.; Perri, C.; Cennamo, N.; Arcadio, F.; D’Agostino, G.; Salmona, M.; Beeg, M.; Gobbi, M. A portable optical-fibre-based surface plasmon resonance biosensor for the detection of therapeutic antibodies in human serum. Sci. Rep. 2020, 10, 11154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2012, 65, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Alsteens, D.; Gaub, H.E.; Newton, R.; Pfreundschuh, M.; Gerber, C.; Muller, D.J. Atomic force microscopy-based characterization and design of biointerfaces. Nat. Rev. Mater. 2017, 2, 17008. [Google Scholar] [CrossRef]
- Menotta, M.; Biagiotti, S.; Streppa, L.; Rossi, L.; Magnani, M. Label-free quantification of Tacrolimus in biological samples by atomic force microscopy. Anal. Chim. Acta 2015, 884, 90–96. [Google Scholar] [CrossRef]
- Steffens, C.; Leite, F.L.; Bueno, C.C.; Manzoli, A.; Herrmann, P.S.D.P. Atomic Force Microscopy as a Tool Applied to Nano/Biosensors. Sensors 2012, 12, 8278–8300. [Google Scholar] [CrossRef]
- Bano, F.; Fruk, L.; Sanavio, B.; Glettenberg, M.; Casalis, L.; Niemeyer, C.M.; Scoles, G. Toward Multiprotein Nanoarrays Using Nanografting and DNA Directed Immobilization of Proteins. Nano Lett. 2009, 9, 2614–2618. [Google Scholar] [CrossRef]
- Castronovo, M.; Scaini, D. The Atomic Force Microscopy as a Lithographic Tool: Nanografting of DNA Nanostructures for Biosensing Applications. Methods Mol. Biol. 2011, 749, 209–221. [Google Scholar] [CrossRef]
- Ambrosetti, E.; Paoletti, P.; Bosco, A.; Parisse, P.; Scaini, D.; Tagliabue, E.; De Marco, A.; Casalis, L. Quantification of Circulating Cancer Biomarkers via Sensitive Topographic Measurements on Single Binder Nanoarrays. ACS Omega 2017, 2, 2618–2629. [Google Scholar] [CrossRef] [Green Version]
- Ganau, M.; Bosco, A.; Palma, A.; Corvaglia, S.; Parisse, P.; Fruk, L.; Beltrami, A.P.; Cesselli, D.; Casalis, L.; Scoles, G. A DNA-based nano-immunoassay for the label-free detection of glial fibrillary acidic protein in multicell lysates. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T.; et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatric Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef]
- Turner, D.; Otley, A.R.; Mack, D.; Hyams, J.; de Bruijne, J.; Uusoue, K.; Walters, T.D.; Zachos, M.; Mamula, P.; Beaton, D.E.; et al. Development, Validation, and Evaluation of a Pediatric Ulcerative Colitis Activity Index: A Prospective Multicenter Study. Gastroenterology 2007, 133, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, J.G.; Dumitru, A.C.; Herruzo, E.T.; Mendieta-Moreno, J.I.; Garcia, R.; Serena, P.A.; Pérez, R. Adsorption orientations and immunological recognition of antibodies on graphene. Nanoscale 2016, 8, 13463–13475. [Google Scholar] [CrossRef]
- Bendtzen, K. Immunogenicity of Anti-TNF-α Biotherapies: II. Clinical Relevance of Methods Used for Anti-Drug Antibody Detection. Front. Immunol. 2015, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Tomečková, V.; Tóth, Š.; Tóth, T.; Komanický, V.; Krajčíková, K.; Široká, M.; Glinská, G.; Pella, D.; Mašlanková, J.; Tomečko, M.; et al. Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques. Open Chem. 2018, 16, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.; Gunasekaran, S.; Eom, K. Atomic force microscopy-based cancer diagnosis by detecting cancer-specific biomolecules and cells. Biochim. Biophys. Acta 2019, 1871, 367–378. [Google Scholar] [CrossRef]
- Husale, S.; Persson, H.H.J.; Sahin, O. DNA nanomechanics allows direct digital detection of complementary DNA and microRNA targets. Nature 2009, 462, 1075–1078. [Google Scholar] [CrossRef] [Green Version]

| Overall (n = 6) | Crohn’s Disease (n = 3) | Ulcerative Colitis (n = 3) | |
|---|---|---|---|
| Age-years (IQR) | 14.88 (12.7–16.1) | 13.13 (10.8–15.7) | 15.33 (13.1–16.1) |
| Disease duration—months (IQR) | 39.4 (25.2–51.6) | 43.18 (28.4–56.6) | 22.6 (17.3–29.5) |
| Disease activity index at inclusion | |||
| PCDAI (IQR) | 14.2 (7–19) | - | |
| PUCAI (IQR) | - | 15.1 (8–21.3) | |
| Paris Classification: | |||
| Localization E3/E4 (%) | - | 0 | 3 (100) |
| Localization L3/L4 (%) | - | 3 (100) | 0 |
| Behavior B1 (%) | - | 3 (100) | 3 (100) |
| Maintenance treatment | 6 | 3 | 3 |
| Other biologics before infliximab (yes/no) | no | no | no |
| Gender | |||
| Female (%) | 3 (50) | 1 (33.3) | 2 (66.7) |
| Male (%) | 3 (50) | 2 (66.7) | 1 (33.3) |
| Pediatric IBD Patient | Infliximab (nM) | Infliximab (µg/mL) |
|---|---|---|
| 1 | 0 | 0.0 |
| 2 | 6.7 | 1.0 |
| 3 | 13.4 | 2.0 |
| 4 | 20 | 3.0 |
| 5 | 28 | 4.2 |
| 6 | 65 | 9.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curci, D.; Lucafò, M.; Parisse, P.; Decorti, G.; Bramuzzo, M.; Casalis, L.; Stocco, G. Atomic Force Microscopy Application for the Measurement of Infliximab Concentration in Healthy Donors and Pediatric Patients with Inflammatory Bowel Disease. J. Pers. Med. 2022, 12, 948. https://doi.org/10.3390/jpm12060948
Curci D, Lucafò M, Parisse P, Decorti G, Bramuzzo M, Casalis L, Stocco G. Atomic Force Microscopy Application for the Measurement of Infliximab Concentration in Healthy Donors and Pediatric Patients with Inflammatory Bowel Disease. Journal of Personalized Medicine. 2022; 12(6):948. https://doi.org/10.3390/jpm12060948
Chicago/Turabian StyleCurci, Debora, Marianna Lucafò, Pietro Parisse, Giuliana Decorti, Matteo Bramuzzo, Loredana Casalis, and Gabriele Stocco. 2022. "Atomic Force Microscopy Application for the Measurement of Infliximab Concentration in Healthy Donors and Pediatric Patients with Inflammatory Bowel Disease" Journal of Personalized Medicine 12, no. 6: 948. https://doi.org/10.3390/jpm12060948
APA StyleCurci, D., Lucafò, M., Parisse, P., Decorti, G., Bramuzzo, M., Casalis, L., & Stocco, G. (2022). Atomic Force Microscopy Application for the Measurement of Infliximab Concentration in Healthy Donors and Pediatric Patients with Inflammatory Bowel Disease. Journal of Personalized Medicine, 12(6), 948. https://doi.org/10.3390/jpm12060948







