Abstract
Diffuse intrinsic pontine glioma (DIPG) is a type of intrinsic brainstem glial tumor that occurs primarily in the pediatric population. DIPG is initially diagnosed based on clinical symptoms and the characteristic location on imaging. Histologically, these tumors are characterized by a heterogenous population of cells with multiple genetic mutations and high infiltrative capacity. The most common mutation seen in this group is a lysine to methionine point mutation seen at position 27 (K27M) within histone 3 (H3). Tumors with the H3 K27M mutation, are considered grade 4 and are now categorized within the H3 K27-altered diffuse midline glioma category by World Health Organization classification. Due to its critical location and aggressive nature, DIPG is resistant to the most eradicative treatment and is universally fatal; however, modern advances in the surgical techniques resulting in safe biopsy of the lesion have significantly improved our understanding of this disease at the molecular level. Genomic analysis has shown several mutations that play a role in the pathophysiology of the disease and can be targeted therapeutically. In this review, we will elaborate on DIPG from general aspects and the evolving molecular landscape. We will also review innovative therapeutic options that have been trialed along with new promising treatments on the horizon.
1. Introduction
Diffuse intrinsic pontine glioma (DIPG) is a primary central nervous system (CNS) malignancy that grows within the pons and predominantly affects the pediatric population. Filbin et al. found that these gliomas primarily contain oligodendrocyte precursor cells with a greater potential for proliferation and tumorigenesis than other similar cells or origin [1]. The tumor is characterized by its highly infiltrative nature and typical location within the brain. Although these tumors generally arise in the brainstem, invasion into the cerebellum and thalamus via white matter tracts and leptomeningeal dissemination is also seen. This led to the re-classification of DIPG encompassed within the diffuse midline glioma (DMG) category [2]. DIPG shares features with HGG (grade 4) or anaplastic astrocytoma (grade 3), and these gliomas clinically behave as high-grade malignant lesions with a dismal prognosis [3]. The current treatment approach to DIPG is radiation therapy; however, this is only palliative [4,5].
Historically, biopsy was typically not performed, due to the location; however, recent advances in surgical adjunct technology have made this safer, leading to a deeper understanding of this disease at a molecular level. DIPG was previously thought to resemble adult high-grade gliomas (HGG), but recent advancements have shown this not to be true. In 2021, the World Health Organization (WHO) updated the original brain tumor classifications, taking markers into consideration and classifying pediatric gliomas with a K27M mutation in histone H3 (3.1 or 3.3) as diffuse midline gliomas (DMG), H3 K27-altered [6,7]. Due to the poor prognosis of tumors with this mutation, they were assigned a grade 4 designation regardless of conventional histologic features [8]. This new classification now includes the majority of DIPGs as more than 80% of these tumors harbor this mutation [9].
In this review, we provide an overview of the epidemiology, clinical presentation, diagnosis and treatment of DIPG, with the primary focus on recent advances in the understanding of the molecular landscape related to disease initiation and prognosis, along with the pathways that are driving the next generation of therapeutic development. Finally, we outline promising current clinical trials that have been targeting molecular players important for the development and progression of DIPG.
2. Methods
We sought to write a comprehensive review article about DIPG with a focus on its molecular landscape along with current and upcoming treatment options. Articles utilized were predominantly from the last 25 years, with those dating beyond this excluded. However, older landmark DIPG studies along with published results from prior clinical trials were also included. Current ongoing clinical trials for the treatment of DIPG were assessed from www.clinicaltrials.gov and included those registered as of 28 January 2022.
3. Epidemiology
DIPG arises mainly in children, with a peak incidence at 6 to 9 years of age and with a similar distribution in males and females [4,10,11]. The exact incidence within the adult population is not known but is rare [12]. In the pediatric population, it accounts for about 15–20% of all brain tumors and represents 80% of those within the brainstem [13]. It is ranked as the second most common malignant brain tumor in children, with approximately 150–400 children per year being diagnosed within the United States [10]. The median survival for patients ranges from 8 to 12 months, with less than 10% of patient surviving past 2 years from their time of diagnosis [14].
4. Clinical Presentation
The clinical presentation of patients is wide varying and results from the compression or dysfunction of critical white matter tracks in the brainstem. Cerebellar dysfunction (dysarthria, ataxia, dysmetria), myelopathy (motor deficits, Babinsky sign, hyperreflexia) and cranial nerve deficits (diplopia and facial weakness) are the most common presenting symptoms [15,16]. Cranial nerve VI and VII are most commonly affected and specific to DIPG as they originate where the tumor typically infiltrates [17]. This leads to diplopia and a conjugate gaze palsy (i.e., abducens palsy) which is a common initial sign and is a poor prognostic factor [16]. Children present with these symptoms in less than a months’ time prior to their diagnosis [18]. Increased intracranial pressure and hydrocephalus occurs infrequently at disease presentation but can occur during the latter part of progression [2,19].
5. Imaging
The diagnosis is made based upon the patient’s clinical presentation along with radiographic findings of the tumor within the pons. Magnetic resonance imaging (MRI) with and without contrast is the imaging modality of choice for the diagnosis [20,21]. The classic MRI findings include T1-hypointensity and T2-hyperintensity with ill-defined margins along with minimal contrast enhancement around areas of necrosis or inflammation [21]. The tumor is generally centered within the pons and at the time of diagnosis can occupy greater than 50% of the axial diameter [20,21] (Figure 1).
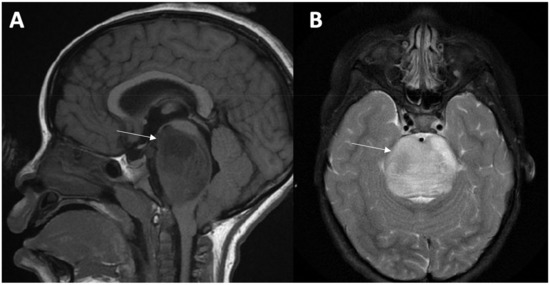
Figure 1.
DIPG Imaging Findings. MRI of the brain with and without gadolinium in a child with an H3 K27-altered, DIPG. The imaging demonstrates a sagittal T1 hypointense (A) and axial T2 hyperintense (B) lesion with homogeneous enhancement along with obstruction within the fourth ventricle of the brain occupying almost half of the axial diameter.
Advanced MRI sequences have also been studied to see if they may play a role in aiding the diagnosis or management. One of these techniques is diffusion tensor imaging (DTI), which has been considered to aid in defining cortical white matter tracts from brain neoplasms and may even help differentiate DIPG from other neoplasms [22,23,24]. Along with this, a recent report suggested that the apparent diffuse coefficient (ADC) may play a prognostic role as patients with a higher value showed improved survival [23].
Positron emission tomography (PET) is also considered to aid in the evaluation. In the preclinical setting, a group at Memorial Sloan Kettering characterized a PET probe targeting PARP1, which is highly expressed in DIPG compared to normal tissue in a murine model [25]. They demonstrated that this mode of imaging can quantify the tumor burden in a murine model and could aid in the diagnosis and future monitoring of treatment response in clinical trials [25]. New approaches using PET tracers can potentially identify relevant biomarkers without the need for invasive procedures, allowing for more personalized treatment and improved quality of life [25].
6. Histologic Characteristics
Historically, biopsy was not undertaken, due to the tumor location and increased morbidity associated with surgical intervention. However, increasing advances to better understand the molecular environment along with improvements in surgical techniques have helped move this towards the standard approach [26,27,28].
Histologically, DIPG has conventionally been characterized as a heterogeneous disease with a varied spectrum of morphologies and grades [2,29] (Figure 2). Yoshimura et al. reviewed the autopsies of 33 pediatric patients with brainstem gliomas and reported that 29 of them had glioblastoma (GBM, WHO grade 4) histology and 2 anaplastic astrocytoma (AA, WHO grade 3) histology [2]. An even larger autopsy-based assessment demonstrated 42 cases of GBM, 18 AA, 8 low-grade glioma (LGG, WHO grade 1 and 2) and 2 with histology consistent with primitive neuroectodermal tumor (PNET, WHO grade 4) [30]. The 2018 collaborative report from the International and European society for Pediatric Oncology (SIOP) DIPG Registries showed a very similar histologic variation in their review of 288 biopsy and 76 autopsy samples [29].

Figure 2.
Histology of H3 K27-Altered DIPG. Histology of Diffuse Midline Glioma, H3 K27-Altered, WHO Grade 4, the most common tumor comprising DIPG. (A) Microscopy demonstrates a high grade, infiltrative glioma with large, atypical, and hyperchromatic nuclei in a fibrillary background. Though high-grade features, including mitotic figures (inset, arrows), are not requisite for the diagnosis, they are generally easy to detect. Microvascular proliferation and necrosis may be variably present and may not be represented on small biopsies. (B) Another tumor from a different patient, demonstrating somewhat variable histology, in this case with particularly hyperchromatic, round, and atypical nuclei with frequent multinucleation (arrows). (C) Immunohistochemistry demonstrates diffuse nuclear positivity with an antibody detecting the H3 K27M mutation, which is diagnostic for this tumor. Note adjacent negativity within blood vessels and infiltrated non-neoplastic brain parenchyma (arrows), serving as a negative internal control. (D) H3K27Me3 immunostaining, which detects trimethylation at the K27 residue, is always lost secondary to the K27M mutation and should be used as a confirmatory stain in making the diagnosis, though DNA sequencing is the diagnostic gold standard. Adjacent non-neoplastic tissue retains trimethylation, and stains positive (arrows). TP53 and/or ATRX mutations are frequent within these neoplasms, which generally manifest as diffuse nuclear positivity for the TP53 protein by immunostaining (E), or loss of nuclear positivity for the ATRX protein (F). Note negative and positive non-neoplastic tissue, respectively, serving as internal controls (arrows).
However, these studies found no difference in overall survival based on the histological grade of the tumor. Both the studies conducted by Buczkowicz et al. and the SIOP DIPG Registries showed that patients with a low-grade lesion do just as poorly as those with a high-grade [29,31,32]. More recent studies analyzing the molecular profiles of DIPG found that most harbor a mutation in histone H3 and that they are more aggressive and have worse outcomes compared to their non-mutant counterparts, regardless of histology [33]. This finding has helped lead to the new WHO classification of H3 K27-altered brainstem and midline tumors as a separate, grade 4 pathologic entity that has become the clinical standard for the diagnosis.
7. Molecular Pathways
Due to the high rates of therapeutic failure, DIPG was initially thought to have similar genetic profiles as adult high-grade gliomas, and thus were treated in a similar manner [34]. However, recent genome-wide analysis has shown unique molecular entities which have been linked with these gliomas (Figure 3). The tumor is primarily categorized as being H3 K27M-mutant or non-mutant, with the majority harboring the mutation. The new 2021 WHO classification of CNS tumors has listed these under the H3 K27-altered category with four distinct primary subtypes including H3.3 K27M-mutant, H3.1 K27M-mutant, H3-wildtype and EGFR-mutant [35]. Other pathways are also hypothesized to play a role in tumor development [15]. Here, we outline molecular alterations found within DIPG and their occurrences within the H3 K27M-mutant or non-mutant groups.
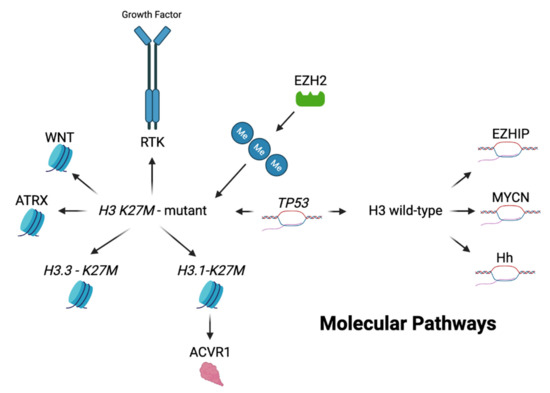
Figure 3.
Genetic landscape of DIPG. Here outlines a brief overview of the genetic landscape and molecular pathways of H3 K27M-mutant and non-mutant pediatric gliomas. The H3 K27M mutation can be divided into two particular genetic alterations—H3.3 on the H3F3A gene and the H3.1 on the HIST1H3B gene. Of these, the H3.3 mutation is more commonly altered in H3 K27M-mutant pediatric gliomas. Enhancer of zeste homolog 2 (EZH2) is closely associated with H3 K27M-mutant gliomas leading to hypermethylation, increased mutant gene expression and tumor formation. The receptor tyrosine kinase (RTK) pathway is also closely associated with H3 K27M-mutant gliomas. They play an integral role in tumor development and cell proliferation via their affects primarily on platelet derived, epidermal and fibroblast growth factor receptors. Mutations within the WNT pathway lead to increased levels of β-catenin, causing an over proliferation of malignant cells and has shown to occur concurrently with the H3 K27M mutation in a preclinical murine model. Histone chaperone alpha-thalassemia/mental retardation syndrome X-linked (ATRX) has a high co-occurrence with H3 K27M-mutant gliomas and contributes to tumor development by destabilizing telomeres and altering gene expression at a molecular level. ACVR1 mutated gliomas are more prominently found with the H3.1 K27M mutation, leading to increased tumor proliferation via dysregulation of the BMP/SMAD pathway. This mutation tends to lead to tumor formation at an earlier age along with increased overall survival. TP53 alterations have been shown to occur in high frequency with the H3 K27M-mutation but are also present on H3 wild-type tumors. Abnormal TP53 function leads to increased DNA and protein instability causing decreased apoptosis and increased tumor development. Alterations to the MYCN and Hedgehog (Hh) signaling pathways occur more frequently in H3 wild-type tumors. MYCN alterations lead to overexpression of this transcriptional factor resulting in tumor formation via DNA hypermethylation and chromosomal rearrangement. Disturbances to the Hh pathway led to ventral pontine hyperplasia in a murine preclinical DIPG model and are proposed to play a role in tumor formation independently of the H3 K27M mutation. Enhancer of zeste homologs inhibitory protein (EZHIP) overexpression is present in the majority of H3 wild-type tumors and is characterized by the overexpression of the CXorf67 gene. This overexpression mimics the amino acid sequence present within H3 K27M-mutant gliomas, leading to global hypomethylation. Direct inhibitors of EZHIP have not yet been found but are currently being studied as a primary target for H3 wild-type gliomas. Image created with BioRender.
7.1. H3 K27M
The H3 K27M-mutant subgroup exhibits a missense mutation in histone 3 with the substitution of lysine (K) for methionine (M) at position 27 (Figure 4). Two variations of this mutation include H3F3A (H3.3) or HIST1H3B/C (H3.1) [36,37]. This genetic alteration is present in approximately 80% of all DIPG [28]. The H3 K27M mutation has also been associated with midline gliomas. In 2016, the WHO classified these tumors into the pathologic entity “diffuse midline glioma, H3 K27M-mutant”, a grade 4 malignancy regardless of conventional histologic criteria [36]. The 2021 WHO classification of tumors of the CNS reclassified H3 K27M-mutant DIPGs under the “diffuse midline glioma, H3 K27-altered” category [35]. Although the exact role of the H3 K27M mutation in tumor development is not fully understood, the mutation is linked to a worse prognosis [36,38]. DIPG itself is universally fatal given the location, but studies show that those that harbor the H3 K27M mutation have an even shorter overall survival (OS) with those harboring H3.3 faring worse than H3.1 [36]. A meta-analysis performed by Mackay and colleagues in 2017 showed that the survival time for H3 K27M-mutant DIPG was 2.5 months less that the wild type [36]. Among the H3 K27M-mutated midline gliomas, survival is also affected by the neuroanatomical location. In 2018, Karreman et al. showed that pediatric patients with thalamic H3 K27M-mutated midline gliomas survived longer than those with DIPG or tumors within the spinal cord [39].

Figure 4.
Significance of H3 K27M. Overall, 80% of all DIPG exhibit a missense mutation at histone H3 K27M. This mutation is a substitution of lysine with methionine in the position 27 (K27M). Histone H3 is a checkpoint control of transcriptional regulation in the synthesis phase of DNA. When mutated, H3 K27 trimethylation is ablated resulting in a repression of the polycomb repressive complex 2 target genes, which can lead to chromatin disaggregation and cellular aneuploidy. The prognosis for these H3 K27M-mutant pediatric gliomas is very poor and universally fatal. Image created with BioRender.
7.2. Histone Chaperone Alpha-Thalassemia/Mental Retardation Syndrome X-Linked (ATRX)
The ATRX complex mutation has a high co-occurrence with the H3 K27M-mutant tumors [35,40]. The depletion of the complex is hypothesized to contribute to tumor development by causing destabilization of telomeres and altering gene expression in conjunction with the H3 K27M mutation [33,35,40]. There are also strong co-occurrences of the TP53, platelet-derived growth factor receptor (PDGFRA) amplification and ACVR1 genetic alterations within the H3 K27M-mutated category [35]. The clinical significance of these co-occurrences remains unknown, with further research ongoing to better understand the varying genetic landscape of H3 K27M-mutant tumors.
7.3. Enhancer of Zeste Homolog 2 (EZH2)
Recent data suggests that EZH2 activity plays a role in the growth of H3 K27M-mutant DIPG cells in in vivo mouse models [41]. EZH2 is involved in many cellular processes including cell cycle progression, proliferation and apoptosis [41,42]. The mutant H3 K27M binds to EZH2, which interferes with the methyltransferase activity resulting in hypermethylation and increased tumor formation [41,42]. EZH2 inhibitors have shown promising results in preclinical models in combination with other targets for treatment of DIPG, but more research is needed to better translate this data into human applicability [42].
7.4. Receptor Tyrosine Kinase (RTK)
RTKs are transmembrane protein receptors that contain intrinsic enzymatic activity, and they play a critical role in signaling pathways that include cell proliferation, differentiation and survival. RTKs include platelet-derived growth factor receptors (PDGFR), epidermal growth factor receptors (EGFR) and fibroblast growth factor receptors (FGFR). Various amplifications and mutations to components within the RTK-RAS-PI3K pathway are seen in up to 60% of DIPG and occur frequently with the H3 K27M-mutant group [43]. EGFR-mutant gliomas have more recently been classified per the WHO as a distinct subtype of H3 K27-altered gliomas with primary abnormalities occurring within the EGFR oncogene on chromosome band 7 [35]. It is thought that potential treatments targeting this specific EGFR alteration may benefit these patients, though further research is needed. Histone H3.3 glycine 34 to arginine/valine (G34R/V) mutations encompass another subset of pediatric HGG with about half of these bearing activating PDGFR mutations [44]. Unlike the H3 K27M-mutant gliomas, these primarily occur within the cortex and other superficial areas within the brain with a GABAergic inhibitory interneuron cell of origin [44].
7.5. WNT
The WNT pathway is known to promote cell growth, survival and decreased apoptosis [45,46]. Mutations in this pathway results in increased levels of β-catenin, which is a key protein in WNT signaling and leads to an over proliferation of cells [45]. In an orthotopically xenografted H3 K27M-mutant murine model, the DIPG cells demonstrated active WNT signaling suggesting their co-occurrence [45]. The oncogenesis and severity of those with DIPG and mutations altering the WNT pathway have not been well described but is hypothesized to have a less severe phenotype as seen in the WNT subgroup of pediatric medulloblastoma and other HGGs [47,48].
7.6. Activin A Receptor, Type 1 (ACVR1)
ACVR1 is a bone morphogenic protein (BMP) receptor which belongs to the TGF-beta signaling family [32,49,50,51]. It binds to many ligands and is crucial for signaling resulting in phosphorylation and activation of growth-promoting genes through SMAD transcription factors [52]. Certain mutations within ACVR1 are present in the molecular make-up of DIPG. Somatic mutations R206H, R258G, G328E/V/W and G356D within ACVR1 have been found in up to 25% of DIPG in retrospective analysis [50]. In normal conditions, ACVR1 helps with myelination within the CNS [53]. When mutated, it encodes a serine/threonine kinase (ALK2) receptor with enhanced sensitivity to the ligand activin A, resulting in dysregulation of the BMP/SMAD pathway and increased tumor proliferation [49,53]. There is also a co-occurrence with the H3 K27M mutation with both being present in up to 22% of DIPG and the majority of these associated with the H3.1 variety [50,51]. The mutation also tends to an earlier age of tumor development with a median of 5 years at diagnosis along with a slightly increased OS of 15 months [53].
7.7. Tumor Protein p53 (TP53)
TP53 is a tumor suppressor gene that controls cellular functions including cell cycle senescence, apoptosis and metabolism. Abnormal TP53 function in cancer cells can lead to increased DNA and protein instability causing decreased apoptosis [54,55]. This mutation is present in up to 75% of DIPG samples along with an increased co-occurrence with the H3 K27M mutation [54,55,56]. In a retrospective analysis, H3 K27M-mutant and TP53-mutant DIPG had increased RT resistance, enhanced tumor aggressiveness and worse OS in comparison to patients without the mutations or with only one mutation present [55]. Though the H3 K27M mutation is likely the primary oncogenic driver, the presence of both mutations led to worse clinical outcomes and proposes possible synergism with a more aggressive phenotype. This shows the multifactorial molecular mechanisms that make up DIPG and contribute to its fatal nature.
7.8. MYCN
The transcriptional factor MYCN and its protein target PVT1 are also overexpressed and amplified, resulting in tumor initiation, progression and recurrence [57]. The DIPG MYCN molecular subtype is characterized by DNA hypermethylation and chromosomal rearrangement resulting in aneuploidy [58,59]. It is proposed that the MYCN pathway may be induced by the H3 K27M mutation, but the pathways are otherwise independent [33,60,61]. The overall prognosis of MYCN mutated DIPG in comparison to others is not completely well understood at this time. Given MYCN’s association with other adult and pediatric high-grade gliomas, it is proposed that the mutation carries a more severe phenotype [62].
7.9. Hedgehog (Hh) Signaling
Hh pathways play a major role in the regulation of processes such as cell proliferation and stem cell maintenance [6,63]. Abnormal signaling in the Hh pathway has shown ventral pontine hyperplasia within pre-clinical murine models [7,63]. Monje et al. showed that the upregulation of the Hh pathway may in fact play a role in DIPG tumor formation in a portion of DIPG patients independently of the presence of H3 K27M, though it is possible that a second stimulus may still be needed for tumor development [63].
7.10. Enhancer of Zeste Homologs Inhibitory Protein (EZHIP)
H3 wild-type glioma are characterized predominantly by the overexpression of the CXorf67 gene which encodes EZHIP [64]. Given its high rate of occurrence, EZHIP-overexpressing DMG has been given its own classification in the latest WHO classification system. The c-terminal peptide in EZHIP mirrors that seen in the H3 K27M mutation leading to hypomethylation via reduced histone methyltransferase activity [64]. This alteration tends to lead to a slightly prolonged OS, though remains fatal [35]. Unfortunately, there is not yet a direct inhibitor that has been identified for this alteration, but further studies are ongoing to further address this.
8. Clinical Management
8.1. Surgical Intervention
Surgical management has been a topic of debate for many years with the initial thought to forego biopsy or further interventions given the tenuous location and limited therapeutic options [26,28,41]. However, as molecular analysis has continued to progress, so has the mindset of obtaining a biopsy at the time of diagnosis. Conventional needle biopsies had been avoided in the past, due to the difficulty in determining a safe trajectory [65]. Stereotactic biopsy is now being utilized more commonly along with newer navigation technologies and high-resolution imaging modalities [28]. A meta-analysis conducted by Hamisch et al. in 2017 retrospectively reviewed 735 brainstem tumor patients who underwent stereotactic biopsy and found a diagnostic yield of 96.1% with only a 0.6% mortality rate [26]. This shows that stereotactic biopsy is a safer procedure than previously thought. Additionally, others have similarly shown that brainstem biopsies can be obtained safely [66,67,68]. A meta-analysis of 13 studies that performed stereotactic biopsies of brainstem lesions in 381 patients showed a 96% diagnostic yield with a low rate of morbidity and mortality [68]. This highlights that pontine biopsy can be carried out safely at experienced medical centers.
8.2. Liquid Biopsy
Liquid biopsy is a newer technique that has been trialed for various malignancies. This is a non-invasive technique that involves obtaining biofluids (i.e., blood, CSF, urine or saliva) to detect circulating tumor DNA (ctDNA) or the tumor cells themselves [69]. Huang et al. used this approach in a cohort of patients that included pediatric DIPG to detect H3 mutations within the CSF via tumor derived cells [70]. Though this appeared to be a viable option to detect the molecular rearrangement, the sensitivity was poor [70]. Pages et al. prospectively collected blood, urine and CSF samples from 258 pediatric patients with various brain tumors and found that CSF contained the most ctDNA, though again showed the overall sensitivity to be poor [71]. Currently, obtaining a lumbar puncture at the time of diagnosis is not the standard of care, but the ability to test for ctDNA that has the ability to detect the H3 K27M mutation does appear to be promising with further validation needed [40].
8.3. Radiation Therapy
Radiation therapy (RT) is the standard treatment of DIPG; however, it is only palliative [65]. RT is expected to increase survival for patients by about 3 months on average [72,73]. The current standard radiation scheme involves 54–60 Gy to be given in fractionated doses (typically 1.8–2 Gy daily) over a 6-week span [74]. This scheme was first developed in 1988 by Freeman et al., and many further studies have looked to see if this could be improved upon [4]. A recent systematic review of the role of RT in the management of DIPG was done by Gallitto et al. This showed that the median OS of patients who received either the standard or modified hyper- or hypo-fractionated RT remained unchanged between 1988 and 2017 [75].
All the reviewed studies used photon beam RT. Proton beam RT is a newer modality that has the ability to more precisely target the tumor and thus reduces radiation exposure and potential damage to healthy tissue. It has been hypothesized to possibly reduce radiation toxicities by delivering therapy more precisely to the affected areas, though it has shown no increased survival for DIPG [75,76]. Additionally, proton beam RT is not widely available and has shown similar rates of radiation necrosis to that of conventional photon therapy [76].
Janssens et al. evaluated a hypo-fractionated RT compared to the conventional and found no impact on OS [77]. However, giving the RT at hypo-fractionated dosing (39 Gy in 13–16 fractions) did decrease the total number of sessions, which had an impact on the amount of time the patient spent in the hospital along with the associated social and emotional burdens [77]. A hyper-fractionated approach (78 Gy) was not advantageous without any survival benefit [14].
Following RT, tumor recurrence is universal and occurs approximately 6 months after treatment [78]. For patient with recurrent or progressive tumors, salvage re-irradiation is an option to help with controlling tumor progression and neurologic sequalae [78]. Gallitto et al. showed that reirradiation prolonged survival by approximately 10 months, though this came with significant neurologic deficits [75]. A multi-collaborative analysis lead by SIOP showed similar results that reirradiation slowed tumor progression but had significant risks and burdens [79]. Based on these studies, reirradiation is not typically recommended (Table 1).

Table 1.
Active clinical trials for children with diffuse intrinsic pontine glioma involving radiation therapy as found on www.Clinicaltrials.gov as of 12 January 2022.
8.4. Chemotherapy
Many chemotherapeutic agents have been tested as either a single modality treatment or in combination with radiation. Unfortunately, very few have led to any significant improvement in survival beyond what has been shown with radiotherapy alone [13].
As with many other malignant gliomas, DIPG does not show a significant response to the oral chemotherapy agent temozolomide (TMZ) which can cross the blood–brain barrier (BBB). A recent study by Izzuddeen et al. in 2019 tested the use of TMZ concurrently and following conventional RT versus RT alone in 33 patients [80]. The median OS for the experimental group was 12 months, with only a month extension over the conventional treatment group and significant hematologic toxicities associated with TMZ [80]. In 2011, Cohen et al. explored the use of TMZ as concomitant therapy to radiation in patients with newly diagnosed DIPG and found very similar results with no significant improvement in event-free survival (EFS) or OS [81]. The results of a United Kingdom phase II trial (CNS 200704) involving 43 patients also showed no benefits with prolonged regimens of TMZ given along with RT to patients who received RT alone [82]. It is hypothesized that the lack of BBB disruption in DIPG compared to other gliomas may account for the limited penetration of this agent and thus the lack of response to treatment [83].
Other chemotherapeutic approaches with proven successes in other neuro-oncological malignancies have also been tested with limited success. These agents include, but are not limited to, etoposide, carboplatin, vincristine, methotrexate, busulfan and bevacizumab. The majority of these agents are associated with poor clinical benefits along with significant adverse toxicities [84]. A trial by Frappaz et al. looked at the utility of administering pre-radiation cisplatin and methotrexate [72]. This showed an increased median survival up to 17 months with the addition but came with higher toxicity and infection rates that resulted in prolonged hospitalizations in the chemotherapy-treated group compared to RT alone [72]. Hargrave et al. conducted a meta-analysis of 29 clinical trials involving 973 patients and concluded that there is no improvement of outcomes with chemo-radiotherapy before, during or after conventional RT [74].
The synergistic effects of multiple chemotherapeutics have also been tested to target multiple pathways. Crotty et al. retrospectively examined the survival of patients treated with a combination regimen of TMZ, irinotecan and bevacizumab at Seattle Children’s Hospital from 2009 to 2018 [85]. This showed an increased 1-year OS in those treated with bevacizumab (80%) over the historical group (45.3%) but also suffered significant toxicities following systemic chemotherapy [85]. The Children’s Oncology Group (COG) ACNS0423 clinical trial looked at TMZ and radiation followed by lomustine and showed marginal benefits in the 3-year EFS [86]. The COG ACNS0822 trial compared the treatment outcomes of vorinostat, bevacizumab or TMZ given concomitantly to conventional RT followed by bevacizumab and TMZ for maintenance therapy [87]. This showed a 1-year EFS of 36.5%, 10.1% and 10.2% for vorinostat, bevacizumab or TMZ [87]. Significant hematologic and systemic toxicities were seen along with increased incidences of intracranial hemorrhage [87]. The HERBY clinical trial (NCT01390948) evaluated the addition of bevacizumab to conventional RT plus TMZ [88]. This showed that the addition of bevacizumab to RT and TMZ did not improve EFS [88].
Overall, chemotherapeutic agents do not appear to reach the tumor in high enough concentrations to be effective because the BBB appears well preserved [11,89]. A trial of high-dose myeloablative chemotherapy with autologous stem cell rescue was attempted to overcome this, but it was unsuccessful [84]. The outcomes of this study only showed a median survival time of 10 months for its patients without any significant benefit compared to RT alone [84]. This suggests that DIPG lacks response to both traditional and highly cytotoxic chemotherapeutic options (Table 2).

Table 2.
Active clinical trials for children with diffuse intrinsic pontine glioma involving chemotherapy as found on www.Clinicaltrials.gov as of 12 January 2022.
9. Ongoing Investigations and Future Considerations
Many ongoing investigations are looking into other avenues of treatment for DIPG. Currently, there are 99 clinical trials that are identified and available for view within the portal of ClinicalTrials.gov. Of these, only 10 trials have results readily available for review (Table 3). In review of these trials, none showed significant clinical benefits in OS, progression-free survival (PFS) and objective response. The modalities of treatment included immunotherapeutic options and chemotherapeutic regimens with or without RT [90]. Despite this, it is promising to see the various treatment modalities being trialed and researched.

Table 3.
Clinical trials for children with diffuse intrinsic pontine glioma with results available as found on www.Clinicaltrials.gov as of 12 January 2022.
9.1. Immunotherapy
Immunotherapeutic options have shown promising results in the treatment of various CNS malignancies, including other HGGs. These agents include chimeric antigen receptor (CAR) T cells, checkpoint blockade, vaccine and oncolytic viral therapies. The thought is to utilize immunotherapeutic options to either boost or alter a patient’s immune system to combat the tumor (Table 4).

Table 4.
Active clinical trials for children with diffuse intrinsic pontine glioma involving immunotherapeutic options as found on www.Clinicaltrials.gov as of 12 January 2022.
The tumor microenvironment has been evaluated in many recent studies and is neutral in regard to an inflammatory or immunosuppressive state [97]. With common neoantigens that have been found, the goal is to target them with treatments that will drive T cells to halt tumor progression. CAR T cell therapy has been promising in this respect because of the neutral microenvironment, which would hinder the inflammatory or anticancer effects [98,99,100]. In preclinical studies, CAR T cells targeting GD2, a disialoganglioside highly expressed in H3 K27M-mutated gliomas, have shown promising results by eliminating the tumor in a xenograft model [98,99]. Currently, clinical trials utilizing GD2 specific CAR T-cell therapy are ongoing to determine its efficacy and safety. Majzner et al. recently published preliminary results of their phase 1 clinical trial utilizing a GD2 CAR approach for H3 K27M-mutant DIPG/DMG with three out of four patients showing initial radiographic and clinical benefits, though all did succumb to their disease [101]. These results highlight the possibility of CAR T therapy as a viable approach to target this malignancy. Additionally, B7H3 has been an additional target for therapy with ongoing trials also investigating its efficacy [100]. A limiting factor is the inherent inflammation associated with the anti-tumor effect of this therapy and the location within the CNS that would be affected. Further research continues to be done to find additional targets and neoantigens to be used. Although promising, the safety of CAR T therapy must be considered along with insights on how to prevent any associated toxicity.
Viral therapy has also emerged as a promising option. An oncolytic adenovirus has shown promise in the treatment of HGGs including DIPG in children. A phase I trial by Lang et al. in 2018 aimed to provoke an immune response by injecting DNX-2401 oncolytic adenovirus directly into 25 patient tumors [102]. The study observed that 18 of these patients, including those with DIPG, showed tumor shrinkage and 5 of these patients survived for more than three years after treatment [102].
ONC201 is an immunostimulatory small molecule antagonist dopamine receptors (DRD2/3) that has been reported in a range of malignancies including H3 K27M-mutant DIPG/DMG [103]. In preclinical murine models, this has been shown to double the OS [104]. Currently, there is an ongoing phase 2 clinical trial that looks to assess its efficacy further in pediatric H3 K27M-mutant gliomas.
Although it is too early to say if immunotherapeutic options will be successful, it does represent an innovative approach with potential to improve current outcomes. Immunotherapy-induced inflammation remains a concern with ongoing concurrent treatments to combat this. [99].
9.2. Epigenetic Modifying Agents
Epigenetic changes play a role in tumor development. Modifiers such as histone deacetylase (HDAC) inhibitor, panobinostat, has been utilized as a multi-HDAC inhibitor to increase H3 acetylation and H3 K27M methylation to reduce oncogenesis and tumor formation [105,106]. Panobinostat has shown promising results in pre-clinical H3 K27M-mutant glioma models. It does have a narrow therapeutic index that can cause dose limiting toxicities which has limited its efficacy [60]. Currently, panobinostat is being tested in clinical trials to determine its efficacy as a monotherapy or in combination with other treatments [90]. EZH2 is also highly expressed on H3 K27M-mutant gliomas [42,107]. Mohammed et al. showed that tazemetostat, an EZH2 inhibitor, does have anti-tumor properties in a pre-clinical DIPG model and could be efficacious in treatment but further studies are needed [107]. Bromodomain and extraterminal (BET) family proteins regulate expression of various oncogenes and are involved in cell cycle arrest that can lead to tumor proliferation [108]. JQ1 is a BET inhibitor that has shown inhibition of DIPG growth in preclinical models when combined with other agents such as EZH2 and other small molecule antagonists, though has yet to be translated into clinical trials [42,109]. Studies are also ongoing to find ways to limit the impact of ACVR1 mutations that are observed. An ALK2/BMP type 1 receptor kinase inhibitor has been studied in pre-clinical models for other disease processes (i.e., fibrodysplasia ossificans progressiva) associated with ACVR1 with good results. The effects of this for DIPG development remain unknown, with further research needed [27]. Finally, a JMJD3 inhibitor (GSKJ4) has also shown anti-tumor properties in pre-clinical H3 K27M-mutant studies [21,110]. JMJD3 is as a key enzyme in H3 K27M demethylation which leads to suppression of the PCR2 target genes, causing chromatin disaggregation, cellular aneuploidy and tumorigenesis [110,111]. Utilizing GSKJ4 treatment, up to 50% growth inhibition of H3 K27M-mutant cells was seen in pre-clinical studies with further translational work needing to be done to asses this as a viable treatment [21].
9.3. Convection Enhanced Delivery (CED)
CED is a methodology to overcome the difficulty of delivering drugs through the BBB. It is a relatively low-risk method of distributing therapeutic drugs directly into and around the brain tumor stereotactically via hydraulic pressure through catheters [112]. It is designed to direct drugs to a specific region of the brain in effective concentrations. Various agents are being tested for stability and effectiveness when used with CED. A phase I trial used convection enhanced delivery to deliver a radioimmunotherapy agent, omburtamab, that targets the B7-H3 antigen to 28 children [113]. The first results found an OS of 15.3 months with three of the children surviving for over three years [113]. In another recent trial by Szychot et al., nine children were treated with CED infusions of carboplatin and sodium valproate [114]. This study also showed a prolonged survival for patients [114]. Because CED concentrates drugs in a specific region in the brain, systemic toxicity is limited [115]. There are limitations with backflow, so new cannula designs are being studied to allow for higher flow rates without increased reflux [116]. Newer capabilities of PET are also being studied to better monitor the distribution of the drugs administered [117]. CED is only performed in a single session currently, but additional research is needed to determine the efficacy of delivering multiple/continuous doses of chemotherapy [112].
10. Conclusions
In summary, DIPG continues to have a poor prognosis, but advances are ongoing. While the diagnosis is typically solely made based upon radiographic evidence on MRI, the need for biopsy sampling has become more evident to advance our understanding of the disease. Radiation therapy continues to remain the standard treatment option. The current environment points towards epigenetic modifiers and immunotherapy being the next wave of therapies along with advancing surgical and therapeutic barriers to bypass the BBB. Future therapeutic approaches should account for the molecular subgroups, specifically the H3 K27-altered that has been given its own designation and comprises the majority of DIPG.
Author Contributions
S.D. and M.D. conceived the project, supervised, and wrote the manuscript. M.L.-V. and B.C.W. wrote the manuscript and prepared Figure 3 and Figure 4. J.H. wrote the pathology section of the manuscript and prepared Figure 2. M.D. provided overall supervision for the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NIH K08NS092895 grant (MD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Filbin, M.G.; Tirosh, I.; Hovestadt, V.; Shaw, M.L.; Escalante, L.E.; Mathewson, N.D.; Neftel, C.; Frank, N.; Pelton, K.; Hebert, C.M.; et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 2018, 360, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, J. Clinicopathological study of diffuse type brainstem gliomas: Analysis of 40 autopsy cases. Neurol. Med. Chir. 2003, 43, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Baker, S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 2014, 14, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.R.; Farmer, J.P. Pediatric brain stem gliomas: A review. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 265–271. [Google Scholar] [CrossRef]
- Leach, J.L.; Roebker, J.; Schafer, A.; Baugh, J.; Chaney, B.; Fuller, C.; Fouladi, M.; Lane, A.; Doughman, R.; Drissi, R.; et al. MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: Report from the International DIPG Registry. Neuro. Oncol. 2020, 22, 1647–1657. [Google Scholar] [CrossRef]
- Nazarian, J.; Mason, G.E.; Ho, C.Y.; Panditharatna, E.; Kambhampati, M.; Vezina, L.G.; Packer, R.J.; Hwang, E.I. Histological and molecular analysis of a progressive diffuse intrinsic pontine glioma and synchronous metastatic lesions: A case report. Oncotarget 2016, 7, 42837–42842. [Google Scholar] [CrossRef][Green Version]
- Welby, J.P.; Kaptzan, T.; Wohl, A.; Peterson, T.E.; Raghunathan, A.; Brown, D.A.; Gupta, S.K.; Zhang, L.; Daniels, D.J. Current Murine Models and New Developments in H3K27M Diffuse Midline Gliomas. Front. Oncol. 2019, 9, 92. [Google Scholar] [CrossRef]
- Lober, R.M.; Cho, Y.J.; Tang, Y.; Barnes, P.D.; Edwards, M.S.; Vogel, H.; Fisher, P.G.; Monje, M.; Yeom, K.W. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J. Neurooncol. 2014, 117, 175–182. [Google Scholar] [CrossRef]
- Harutyunyan, A.S.; Krug, B.; Chen, H.; Papillon-Cavanagh, S.; Zeinieh, M.; De Jay, N.; Deshmukh, S.; Chen, C.C.L.; Belle, J.; Mikael, L.G.; et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 2019, 10, 1262. [Google Scholar] [CrossRef]
- Johung, T.B.; Monje, M. Diffuse Intrinsic Pontine Glioma: New Pathophysiological Insights and Emerging Therapeutic Targets. Curr. Neuropharmacol. 2017, 15, 88–97. [Google Scholar] [CrossRef]
- Warren, K.E. Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Eschbacher, K.L.; Ida, C.M.; Johnson, D.R.; Alvi, M.A.; Jenkins, S.M.; Ruff, M.W.; Kerezoudis, P.; Neth, B.J.; Pasion, R.M.; Daniels, D.J.; et al. Diffuse Gliomas of the Brainstem and Cerebellum in Adults Show Molecular Heterogeneity. Am. J. Surg. Pathol. 2021, 45, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen van Zanten, S.E.; Jansen, M.H.; Sanchez Aliaga, E.; van Vuurden, D.G.; Vandertop, W.P.; Kaspers, G.J. A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Expert. Rev. Anticancer Ther. 2015, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Boyett, J.M.; Zimmerman, R.A.; Albright, A.L.; Kaplan, A.M.; Rorke, L.B.; Selch, M.T.; Cherlow, J.M.; Finlay, J.L.; Wara, W.M. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer 1994, 74, 1827–1834. [Google Scholar] [CrossRef]
- Hennika, T.; Becher, O.J. Diffuse Intrinsic Pontine Glioma: Time for Cautious Optimism. J. Child Neurol. 2016, 31, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Fisher, P.G. Neurological complications following treatment of children with brain tumors. J. Pediatr. Rehabil. Med. 2011, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Santa-Maria Lopez, V.; Hendrikse, N.H.; Kaspers, G.J.L.; Loizos, G.; Sumerauer, D.; Nysom, K.; Pruunsild, K.; Pentikainen, V.; et al. Diagnostics and treatment of diffuse intrinsic pontine glioma: Where do we stand? J. Neurooncol. 2019, 145, 177–184. [Google Scholar] [CrossRef]
- Donaldson, S.S.; Laningham, F.; Fisher, P.G. Advances toward an understanding of brainstem gliomas. J. Clin. Oncol. 2006, 24, 1266–1272. [Google Scholar] [CrossRef]
- Caretti, V.; Bugiani, M.; Freret, M.; Schellen, P.; Jansen, M.; van Vuurden, D.; Kaspers, G.; Fisher, P.G.; Hulleman, E.; Wesseling, P.; et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014, 128, 605–607. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Krischer, J.; Kun, L.E.; Packer, R.; Zimmerman, R.A.; Freeman, C.R.; Wara, W.M.; Albright, L.; Allen, J.C.; Hoffman, H.J. Brain stem gliomas: A classification system based on magnetic resonance imaging. Pediatr. Neurosurg. 1990, 16, 73–83. [Google Scholar] [CrossRef]
- Lewis, P.W.; Allis, C.D. Poisoning the “histone code” in pediatric gliomagenesis. Cell Cycle 2013, 12, 3241–3242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Long, W.; Yi, Y.; Chen, S.; Cao, Q.; Zhao, W.; Liu, Q. Potential New Therapies for Pediatric Diffuse Intrinsic Pontine Glioma. Front. Pharmacol. 2017, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Mekkaoui, C.; Metellus, P.; Kostis, W.J.; Martuzzi, R.; Pereira, F.R.; Beregi, J.P.; Reese, T.G.; Constable, T.R.; Jackowski, M.P. Diffusion Tensor Imaging in Patients with Glioblastoma Multiforme Using the Supertoroidal Model. PLoS ONE 2016, 11, e0146693. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.M. The role of diffusion tensor imaging metrics in the discrimination between cerebellar medulloblastoma and brainstem glioma. Pediatr. Blood Cancer 2020, 67, e28468. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Carney, B.; Schweitzer, M.; Carlucci, G.; Miloushev, V.Z.; Maachani, U.B.; Rajappa, P.; Keshari, K.R.; Pisapia, D.; Weber, W.A.; et al. Biomarker-Based PET Imaging of Diffuse Intrinsic Pontine Glioma in Mouse Models. Cancer Res. 2017, 77, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, C.; Kickingereder, P.; Fischer, M.; Simon, T.; Ruge, M.I. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J. Neurosurg. Pediatr. 2017, 20, 261–268. [Google Scholar] [CrossRef]
- Mohedas, A.H.; Wang, Y.; Sanvitale, C.E.; Canning, P.; Choi, S.; Xing, X.; Bullock, A.N.; Cuny, G.D.; Yu, P.B. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J. Med. Chem. 2014, 57, 7900–7915. [Google Scholar] [CrossRef]
- Williams, J.R.; Young, C.C.; Vitanza, N.A.; McGrath, M.; Feroze, A.H.; Browd, S.R.; Hauptman, J.S. Progress in diffuse intrinsic pontine glioma: Advocating for stereotactic biopsy in the standard of care. Neurosurg. Focus 2020, 48, E4. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Veldhuijzen van Zanten, S.E.M.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Angelini, P.; Hawkins, C.; Laperriere, N.; Bouffet, E.; Bartels, U. Post mortem examinations in diffuse intrinsic pontine glioma: Challenges and chances. J. Neurooncol. 2011, 101, 75–81. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hawkins, C. Pathology, Molecular Genetics, and Epigenetics of Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2015, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U.; et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Khuong-Quang, D.A.; Buczkowicz, P.; Rakopoulos, P.; Liu, X.Y.; Fontebasso, A.M.; Bouffet, E.; Bartels, U.; Albrecht, S.; Schwartzentruber, J.; Letourneau, L.; et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Bender, S.; Jones, D.T.; Lichter, P.; Grill, J.; Becher, O.; Hawkins, C.; Majewski, J.; Jones, C.; Costello, J.F.; et al. Paediatric and adult glioblastoma: Multiform (epi)genomic culprits emerge. Nat. Rev. Cancer 2014, 14, 92–107. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef]
- Luo, J.; Xu, X.; Hall, H.; Hyland, E.M.; Boeke, J.D.; Hazbun, T.; Kuo, M.H. Histone h3 exerts a key function in mitotic checkpoint control. Mol. Cell Biol. 2010, 30, 537–549. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Castel, D.; Philippe, C.; Calmon, R.; Le Dret, L.; Truffaux, N.; Boddaert, N.; Pagès, M.; Taylor, K.R.; Saulnier, P.; Lacroix, L.; et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015, 130, 815–827. [Google Scholar] [CrossRef]
- Karremann, M.; Gielen, G.H.; Hoffmann, M.; Wiese, M.; Colditz, N.; Warmuth-Metz, M.; Bison, B.; Claviez, A.; van Vuurden, D.G.; von Bueren, A.O.; et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro. Oncol. 2018, 20, 123–131. [Google Scholar] [CrossRef]
- Saratsis, A.M.; Yadavilli, S.; Magge, S.; Rood, B.R.; Perez, J.; Hill, D.A.; Hwang, E.; Kilburn, L.; Packer, R.J.; Nazarian, J. Insights into pediatric diffuse intrinsic pontine glioma through proteomic analysis of cerebrospinal fluid. Neuro. Oncol. 2012, 14, 547–560. [Google Scholar] [CrossRef]
- Mohanty, A. Biopsy of brain stem gliomas: Changing trends? J. Neurosci. Rural Pract. 2014, 5, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, W.; Zhu, J.; Wang, L.; Wu, X.; Shan, H. Combination of EZH2 inhibitor and BET inhibitor for treatment of diffuse intrinsic pontine glioma. Cell Biosci. 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Chen, C.C.L.; Deshmukh, S.; Jessa, S.; Hadjadj, D.; Lisi, V.; Andrade, A.F.; Faury, D.; Jawhar, W.; Dali, R.; Suzuki, H.; et al. Histone H3.3G34-Mutant Interneuron Progenitors Co-opt PDGFRA for Gliomagenesis. Cell 2020, 183, 1617–1633. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Pedersen, H.; Schmiegelow, K.; Hamerlik, P. Radio-Resistance and DNA Repair in Pediatric Diffuse Midline Gliomas. Cancers 2020, 12, 2813. [Google Scholar] [CrossRef]
- Meel, M.H.; Schaper, S.A.; Kaspers, G.J.L.; Hulleman, E. Signaling pathways and mesenchymal transition in pediatric high-grade glioma. Cell Mol. Life Sci. 2018, 75, 871–887. [Google Scholar] [CrossRef]
- Huang, S.Y.; Yang, J.Y. Targeting the Hedgehog Pathway in Pediatric Medulloblastoma. Cancers 2015, 7, 2110–2123. [Google Scholar] [CrossRef]
- Hoeman, C.M.; Cordero, F.J.; Hu, G.; Misuraca, K.; Romero, M.M.; Cardona, H.J.; Nazarian, J.; Hashizume, R.; McLendon, R.; Yu, P.; et al. ACVR1 R206H cooperates with H3.1K27M in promoting diffuse intrinsic pontine glioma pathogenesis. Nat. Commun. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Taylor, K.R.; Vinci, M.; Bullock, A.N.; Jones, C. ACVR1 mutations in DIPG: Lessons learned from FOP. Cancer Res. 2014, 74, 4565–4570. [Google Scholar] [CrossRef]
- Vanan, M.I.; Eisenstat, D.D. DIPG in Children—What Can We Learn from the Past? Front. Oncol. 2015, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Haupt, J.; Xu, M.; Shore, E.M. Variable signaling activity by FOP ACVR1 mutations. Bone 2018, 109, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Taylor, K.R.; Olaciregui, N.G.; Molinari, V.; Clarke, M.; Mackay, A.; Ruddle, R.; Henley, A.; Valenti, M.; Hayes, A.; et al. ALK2 inhibitors display beneficial effects in preclinical models of. Commun. Biol. 2019, 2, 156. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Strasser, A.; Kelly, G.L. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026062. [Google Scholar] [CrossRef] [PubMed]
- Werbrouck, C.; Evangelista, C.C.S.; Lobón-Iglesias, M.J.; Barret, E.; Le Teuff, G.; Merlevede, J.; Brusini, R.; Kergrohen, T.; Mondini, M.; Bolle, S.; et al. TP53 Pathway Alterations Drive Radioresistance in Diffuse Intrinsic Pontine Gliomas (DIPG). Clin. Cancer Res. 2019, 25, 6788–6800. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.; Ruggiero, A.; Martini, M.; Rizzo, V.; Maurizi, P.; Riccardi, R. Molecular Biology in Pediatric High-Grade Glioma: Impact on Prognosis and Treatment. Biomed. Res. Int. 2015, 2015, 215135. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Bolin, S.; Weishaupt, H.; Swartling, F.J. Modeling and Targeting MYC Genes in Childhood Brain Tumors. Genes 2017, 8, 107. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Qing, G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther. 2018, 3, 5. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef]
- Grasso, C.S.; Tang, Y.; Truffaux, N.; Berlow, N.E.; Liu, L.; Debily, M.A.; Quist, M.J.; Davis, L.E.; Huang, E.C.; Woo, P.J.; et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 2015, 21, 827. [Google Scholar] [CrossRef]
- Saratsis, A.M.; Kambhampati, M.; Snyder, K.; Yadavilli, S.; Devaney, J.M.; Harmon, B.; Hall, J.; Raabe, E.H.; An, P.; Weingart, M.; et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuropathol. 2014, 127, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Duchatel, R.J.; Jackson, E.R.; Alvaro, F.; Nixon, B.; Hondermarck, H.; Dun, M.D. Signal Transduction in Diffuse Intrinsic Pontine Glioma. Proteomics 2019, 19, e1800479. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Mitra, S.S.; Freret, M.E.; Raveh, T.B.; Kim, J.; Masek, M.; Attema, J.L.; Li, G.; Haddix, T.; Edwards, M.S.; et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl. Acad. Sci. USA 2011, 108, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Castel, D.; Kergrohen, T.; Tauziede-Espariat, A.; Mackay, A.; Ghermaoui, S.; Lechapt, E.; Pfister, S.M.; Kramm, C.M.; Boddaert, N.; Blauwblomme, T.; et al. Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol. 2020, 139, 1109–1113. [Google Scholar] [CrossRef]
- Langmoen, I.A.; Lundar, T.; Storm-Mathisen, I.; Lie, S.O.; Hovind, K.H. Management of pediatric pontine gliomas. Childs Nerv. Syst. 1991, 7, 13–15. [Google Scholar] [CrossRef]
- Cage, T.A.; Samagh, S.P.; Mueller, S.; Nicolaides, T.; Haas-Kogan, D.; Prados, M.; Banerjee, A.; Auguste, K.I.; Gupta, N. Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv. Syst. 2013, 29, 1313–1319. [Google Scholar] [CrossRef]
- Gupta, N.; Goumnerova, L.C.; Manley, P.; Chi, S.N.; Neuberg, D.; Puligandla, M.; Fangusaro, J.; Goldman, S.; Tomita, T.; Alden, T.; et al. Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro. Oncol. 2018, 20, 1547–1555. [Google Scholar] [CrossRef]
- Pfaff, E.; El Damaty, A.; Balasubramanian, G.P.; Blattner-Johnson, M.; Worst, B.C.; Stark, S.; Witt, H.; Pajtler, K.W.; van Tilburg, C.M.; Witt, R.; et al. Brainstem biopsy in pediatric diffuse intrinsic pontine glioma in the era of precision medicine: The INFORM study experience. Eur. J. Cancer 2019, 114, 27–35. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Huang, T.Y.; Piunti, A.; Lulla, R.R.; Qi, J.; Horbinski, C.M.; Tomita, T.; James, C.D.; Shilatifard, A.; Saratsis, A.M. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol. Commun. 2017, 5, 28. [Google Scholar] [CrossRef]
- Pages, M.; Rotem, D.; Gydush, G.; Reed, S.; Rhoades, J.; Ha, G.; Lo, C.; Fleharty, M.; Duran, M.; Jones, R.; et al. Liquid biopsy detection of genomic alterations in pediatric brain tumors from cell-free DNA in peripheral blood, CSF, and urine. Neuro. Oncol. 2022, noab299. [Google Scholar] [CrossRef] [PubMed]
- Frappaz, D.; Schell, M.; Thiesse, P.; Marec-Bérard, P.; Mottolese, C.; Perol, D.; Bergeron, C.; Philip, T.; Ricci, A.C.; Galand-Desme, S.; et al. Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: Final results of BSG 98 prospective trial. Neuro. Oncol. 2008, 10, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.L.; Lee, J.; Thomale, U.W.; Noggle, J.C.; Cohen, K.J.; Jallo, G.I. Treatment of diffuse intrinsic brainstem gliomas: Failed approaches and future strategies. J. Neurosurg. Pediatr. 2009, 3, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, D.; Bartels, U.; Bouffet, E. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet Oncol. 2006, 7, 241–248. [Google Scholar] [CrossRef]
- Gallitto, M.; Lazarev, S.; Wasserman, I.; Stafford, J.M.; Wolden, S.L.; Terezakis, S.A.; Bindra, R.S.; Bakst, R.L. Role of Radiation Therapy in the Management of Diffuse Intrinsic Pontine Glioma: A Systematic Review. Adv. Radiat. Oncol. 2019, 4, 520–531. [Google Scholar] [CrossRef]
- Muroi, A.; Mizumoto, M.; Ishikawa, E.; Ihara, S.; Fukushima, H.; Tsurubuchi, T.; Sakurai, H.; Matsumura, A. Proton therapy for newly diagnosed pediatric diffuse intrinsic pontine glioma. Childs Nerv. Syst. 2020, 36, 507–512. [Google Scholar] [CrossRef]
- Janssens, G.O.; Jansen, M.H.; Lauwers, S.J.; Nowak, P.J.; Oldenburger, F.R.; Bouffet, E.; Saran, F.; Kamphuis-van Ulzen, K.; van Lindert, E.J.; Schieving, J.H.; et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: A matched-cohort analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 315–320. [Google Scholar] [CrossRef]
- Ermoian, R.; MacDonald, S.; Laack, N.N.I.; Baldini, E.; Breneman, J. Reirradiation in Pediatric Patients With Recurrent Brain Tumors: A Last Hope, But One With Greatly Feared Consequences. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1–4. [Google Scholar] [CrossRef]
- Janssens, G.O.; Gandola, L.; Bolle, S.; Mandeville, H.; Ramos-Albiac, M.; van Beek, K.; Benghiat, H.; Hoeben, B.; Morales La Madrid, A.; Kortmann, R.D.; et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur. J. Cancer 2017, 73, 38–47. [Google Scholar] [CrossRef]
- Izzuddeen, Y.; Gupta, S.; Haresh, K.P.; Sharma, D.; Giridhar, P.; Rath, G.K. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: A randomized controlled trial. J. Neurooncol. 2020, 146, 91–95. [Google Scholar] [CrossRef]
- Cohen, K.J.; Heideman, R.L.; Zhou, T.; Holmes, E.J.; Lavey, R.S.; Bouffet, E.; Pollack, I.F. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children’s Oncology Group. Neuro. Oncol. 2011, 13, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Howman, A.; Wheatley, K.; Wherton, D.; Boota, N.; Pizer, B.; Fisher, D.; Kearns, P.; Picton, S.; Saran, F.; et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04). Eur. J. Cancer 2013, 49, 3856–3862. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Declèves, X.; Taghi, M.; Menet, M.C.; Lacombe, J.; Varlet, P.; Olaciregui, N.G.; Carcaboso, A.M.; Cisternino, S. Characterization of the Blood-Brain Barrier Integrity and the Brain Transport of SN-38 in an Orthotopic Xenograft Rat Model of Diffuse Intrinsic Pontine Glioma. Pharmaceutics 2020, 12, 399. [Google Scholar] [CrossRef]
- Main, C.; Wilson, J.S.; Stevens, S.P.; Houlton, A.E.; English, M.; Kearns, P.R.; Phillips, B.; Pizer, B.; Wilne, S.; Wheatley, K. The role of high-dose myeloablative chemotherapy with haematopoietic stem cell transplantation (HSCT) in children with central nervous system (CNS) tumours: Protocol for a systematic review and meta-analysis. Syst. Rev. 2015, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Crotty, E.E.; Leary, S.E.S.; Geyer, J.R.; Olson, J.M.; Millard, N.E.; Sato, A.A.; Ermoian, R.P.; Cole, B.L.; Lockwood, C.M.; Paulson, V.A.; et al. Children with DIPG and high-grade glioma treated with temozolomide, irinotecan, and bevacizumab: The Seattle Children’s Hospital experience. J. Neurooncol. 2020, 148, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Jakacki, R.I.; Cohen, K.J.; Buxton, A.; Krailo, M.D.; Burger, P.C.; Rosenblum, M.K.; Brat, D.J.; Hamilton, R.L.; Eckel, S.P.; Zhou, T.; et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: A report of the Children’s Oncology Group ACNS0423 study. Neuro. Oncol. 2016, 18, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Geller, J.; Leach, J.; Fouladi, M. A Feasibility and Randomized Phase II Study of Vorinostat, Bevacizumab, or Temozolomide During Radiation Followed by Maintenance Chemotherapy in Newly-Diagnosed Pediatric High-Grade Glioma: Children’s Oncology Group ACNS0822. Neuro Oncol. 2015, 17, iii39–iii40. [Google Scholar] [CrossRef]
- Grill, J.; Massimino, M.; Bouffet, E.; Azizi, A.A.; McCowage, G.; Cañete, A.; Saran, F.; Le Deley, M.C.; Varlet, P.; Morgan, P.S.; et al. Phase II, Open-Label, Randomized, Multicenter Trial (HERBY) of Bevacizumab in Pediatric Patients With Newly Diagnosed High-Grade Glioma. J. Clin. Oncol. 2018, 36, 951–958. [Google Scholar] [CrossRef]
- Warren, K.; Bent, R.; Wolters, P.L.; Prager, A.; Hanson, R.; Packer, R.; Shih, J.; Camphausen, K. A phase 2 study of pegylated interferon α-2b (PEG-Intron(®)) in children with diffuse intrinsic pontine glioma. Cancer 2012, 118, 3607–3613. [Google Scholar] [CrossRef]
- Rechberger, J.S.; Lu, V.M.; Zhang, L.; Power, E.A.; Daniels, D.J. Clinical trials for diffuse intrinsic pontine glioma: The current state of affairs. Childs Nerv. Syst. 2020, 36, 39–46. [Google Scholar] [CrossRef]
- DeWire, M.; Fuller, C.; Hummel, T.R.; Chow, L.M.L.; Salloum, R.; de Blank, P.; Pater, L.; Lawson, S.; Zhu, X.; Dexheimer, P.; et al. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neurooncol. 2020, 149, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Kilburn, L.B.; Mansur, D.B.; Krailo, M.; Buxton, A.; Adekunle, A.; Gajjar, A.; Adamson, P.C.; Weigel, B.; Fox, E.; et al. Phase 1/2 Trial of Vorinostat and Radiation and Maintenance Vorinostat in Children with Diffuse Intrinsic Pontine Glioma: A Children’s Oncology Group Report. Neuro. Oncol. 2021, 24, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.A.; Su, J.M.; Onar-Thomas, A.; Billups, C.A.; Li, X.N.; Poussaint, T.Y.; Smith, E.R.; Thompson, P.; Adesina, A.; Ansell, P.; et al. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: A Pediatric Brain Tumor Consortium study. Neuro. Oncol. 2020, 22, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Murray, J.C.; McNall-Knapp, R.Y.; Bowers, D.C.; Shah, S.; Adesina, A.M.; Paulino, A.C.; Jo, E.; Mo, Q.; Baxter, P.A.; et al. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer 2020, 67, e28283. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; Hummel, T.R.; Kumar, S.S.; Dorris, K.; Li, S.; Lin, T.; Daryani, V.M.; Stewart, C.F.; Miles, L.; Poussaint, T.Y.; et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: A pediatric brain tumor consortium study. J. Neurooncol. 2016, 129, 443–451. [Google Scholar] [CrossRef]
- Fangusaro, J.; Mitchell, D.A.; Kocak, M.; Robinson, G.W.; Baxter, P.A.; Hwang, E.I.; Huang, J.; Onar-Thomas, A.; Dunkel, I.J.; Fouladi, M.; et al. Phase 1 study of pomalidomide in children with recurrent, refractory, and progressive central nervous system tumors: A Pediatric Brain Tumor Consortium trial. Pediatr. Blood Cancer 2021, 68, e28756. [Google Scholar] [CrossRef]
- Lieberman, N.A.P.; DeGolier, K.; Kovar, H.M.; Davis, A.; Hoglund, V.; Stevens, J.; Winter, C.; Deutsch, G.; Furlan, S.N.; Vitanza, N.A.; et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: Implications for development of immunotherapy. Neuro. Oncol. 2019, 21, 83–94. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Patterson, J.D.; Henson, J.C.; Breese, R.O.; Bielamowicz, K.J.; Rodriguez, A. CAR T Cell Therapy for Pediatric Brain Tumors. Front. Oncol. 2020, 10, 1582. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Malhotra, J.; Tarapore, R.S.; Malhotra, U.; Silk, A.W.; Chan, N.; Rodriguez, L.; Aisner, J.; Aiken, R.D.; Mayer, T.; et al. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J. Immunother. Cancer 2019, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Duchatel, R.J.; Mannan, A.; Woldu, A.S.; Hawtrey, T.; Hindley, P.A.; Douglas, A.M.; Jackson, E.R.; Findlay, I.J.; Germon, Z.P.; Staudt, D.; et al. Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma. Neurooncol. Adv. 2021, 3, vdab169. [Google Scholar] [CrossRef]
- Perla, A.; Fratini, L.; Cardoso, P.S.; Nör, C.; Brunetto, A.T.; Brunetto, A.L.; de Farias, C.B.; Jaeger, M.; Roesler, R. Histone Deacetylase Inhibitors in Pediatric Brain Cancers: Biological Activities and Therapeutic Potential. Front. Cell. Dev. Biol. 2020, 8, 546. [Google Scholar] [CrossRef]
- New, M.; Olzscha, H.; La Thangue, N.B. HDAC inhibitor-based therapies: Can we interpret the code? Mol. Oncol. 2012, 6, 637–656. [Google Scholar] [CrossRef]
- Mohammad, F.; Weissmann, S.; Leblanc, B.; Pandey, D.P.; Højfeldt, J.W.; Comet, I.; Zheng, C.; Johansen, J.V.; Rapin, N.; Porse, B.T.; et al. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat. Med. 2017, 23, 483–492. [Google Scholar] [CrossRef]
- French, C.A. Small-Molecule Targeting of BET Proteins in Cancer. Adv. Cancer Res. 2016, 131, 21–58. [Google Scholar] [CrossRef]
- Wiese, M.; Hamdan, F.H.; Kubiak, K.; Diederichs, C.; Gielen, G.H.; Nussbaumer, G.; Carcaboso, A.M.; Hulleman, E.; Johnsen, S.A.; Kramm, C.M. Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells. Cell Death Dis. 2020, 11, 673. [Google Scholar] [CrossRef]
- Hashizume, R.; Andor, N.; Ihara, Y.; Lerner, R.; Gan, H.; Chen, X.; Fang, D.; Huang, X.; Tom, M.W.; Ngo, V.; et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 2014, 20, 1394–1396. [Google Scholar] [CrossRef]
- Nichol, J.N.; Dupéré-Richer, D.; Ezponda, T.; Licht, J.D.; Miller, W.H. H3K27 Methylation: A Focal Point of Epigenetic Deregulation in Cancer. Adv. Cancer Res. 2016, 131, 59–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; Carrasquillo, J.A.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef]
- Szychot, E.; Collins, P.; Cassidy, H.; Walker, D. New trial of convection enhanced drug delivery (CED) in DIPG- applying the SIOPe DIPG survival prediction model for power calculation. Neuro. Oncol. 2019, 21, iv11. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Forsayeth, J.R.; Dickinson, P.J.; Bankiewicz, K.S. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics 2008, 5, 123–127. [Google Scholar] [CrossRef]
- Pagani, M.; Stone-Elander, S.; Larsson, S.A. Alternative positron emission tomography with non-conventional positron emitters: Effects of their physical properties on image quality and potential clinical applications. Eur. J. Nucl. Med. 1997, 24, 1301–1327. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).