Abstract
Glioblastoma, as the most aggressive brain tumor, is associated with a poor prognosis and outcome. To optimize prognosis and clinical therapy decisions, there is an urgent need to stratify patients with increased risk for recurrent tumors and low therapeutic success to optimize individual treatment. Radiogenomics establishes a link between radiological and pathological information. This review provides a state-of-the-art picture illustrating the latest developments in the use of radiogenomic markers regarding prognosis and their potential for monitoring recurrence. Databases PubMed, Google Scholar, and Cochrane Library were searched. Inclusion criteria were defined as diagnosis of glioblastoma with histopathological and radiological follow-up. Out of 321 reviewed articles, 43 articles met these inclusion criteria. Included studies were analyzed for the frequency of radiological and molecular tumor markers whereby radiogenomic associations were analyzed. Six main associations were described: radiogenomic prognosis, MGMT status, IDH, EGFR status, molecular subgroups, and tumor location. Prospective studies analyzing prognostic features of glioblastoma together with radiological features are lacking. By reviewing the progress in the development of radiogenomic markers, we provide insights into the potential efficacy of such an approach for clinical routine use eventually enabling early identification of glioblastoma recurrence and therefore supporting a further personalized monitoring and treatment strategy.
1. Introduction
Despite well-established multimodal therapeutic regimens, glioblastoma (GBM) is the most lethal primary brain tumor with low survival rates [1] that have not significantly improved over the last few years. Notably, more than half of patients will undergo tumor progression after six months following surgery [2,3], and the median survival is between ten and twelve months [4,5]. Magnetic resonance imaging (MRI) plays an essential role in diagnosing and monitoring tumor progression and therapy. Thus, in addition to preoperative diagnostic information, the extent of resection can be assessed early after surgery. Even though comprehensive imaging modalities for MRI are available, there is still no standard method that can reliably detect early tumor progression. A commonly used marker for tumor progression in higher grade gliomas is contrast enhancement [6]. However, parameters such as flow rates from perfusion measurements [7] also appear to be related to tumor progression and angiogenesis. In addition, there are parameters from MR spectroscopy to distinguish recurrence/progression and necrosis during therapy [8].
When assessing prognosis and selecting an individually appropriate treatment strategy, prognostic and predictive molecular markers play an increasingly significant role [9] and have prompted significant changes to the 2016 World Health Organization (WHO) classification of central nervous system (CNS) tumors and refinements over the last years [10,11]. According to the new WHO classification published in 2021, only isocitrate dehydrogenase wildtype and H3 wildtype (IDHwtH3wt) astrocytomas are now classified as the GBM WHO grade 4 [11].
GBMs with similar imaging characteristics may exhibit different clinical courses, treatment responses, and outcomes [12] whilst genetically similar GBM tumors may present varying radiological characteristics morphologically distinct on MRI [13,14]. Additionally, investigations on the molecular level can provide little information regarding macroscopic characteristics, such as vascularization or space-occupying effects. In an era of precision medicine, intensive research has been conducted on imaging markers derived from routine clinical MR images to match with observations regarding molecular features of GBM tumors in a non-invasive manner. By taking a personalized view of the disease, precision medicine approaches aim to enable individualized decision making about the diagnostic and therapeutic approaches by using multiple data sources—from genomics to radiological sequences [15,16]. However, despite those numerous efforts in recent years, there still seems to have been no significant breakthrough in determining interrelated molecular and radiological characteristics that can be accurately used for individual prognostic stratification in GBM patients.
1.1. Prognosis of Disease Progression in Glioblastoma
Multiple factors have been shown to be relevant for the prognosis of disease progression. At the individual patient level, the pre-operative Karnofsky Performance Score (KPS) plays an important role, along with age and sex [17,18,19,20,21,22]. From a surgical point of view, location and accessibility are essential for maximally safe resection. Clinical studies have shown that the extent of maximum safe resection in glioblastoma therapy correlates with the progression-free interval (PFS) and overall survival (OS) [23,24,25]. To improve the extent of resection, the role of neuronavigation and intraoperative MRI could be demonstrated [26].
The prognosis in GBM is highly influenced by tumor biological characteristics. O6-methylguanine-DNA methyl-transferase (MGMT) promoter methylation is considered the most important predictive tool in adjuvant treatment with alkylating chemotherapeutic agents such as temozolomide, thus influencing treatment response and prognosis [12]. A methylated MGMT promoter correlates with increased survival time and recurrence pattern [27,28,29]. IDH mutations possess the greatest prognostic significance in gliomas and are associated with longer OS and PFS [9]. IDH1 and IDH2 mutations have been shown to correlate with a two- to three-fold increase in survival [30]. However, these mutations occur in only up to 12% of GBMs [31]. Increasingly, IDH mutation and malignant transformation are being linked to numerous alterations at the cellular level [32], e.g., cellular epigenetics, DNA repair pathways, and redox homeostasis [33]. This could provide potential opportunities for targeting these pathways [34] or complementing currently available approved therapeutic protocols [33,35,36]. The epidermal growth factor receptor (EGFR) belongs to the family of epidermal growth factor receptors with tyrosine kinase activity [37]. Dysregulation of the epidermal growth factor signaling pathway can be observed in approximately 80% of high-grade gliomas, resulting either from aberrant expression of EGFR or an EGFR variant (EGFRvIII) [38,39]. Generally, EGFR amplifications are associated with a poorer prognosis [40,41,42].
Of note, various molecular classifications of GBM exist. A frequently used classification is the one described by Verhaak et al. based on gene signatures thereby discriminating four main GBM subtypes: proneural, neural, classical, and mesenchymal [43]. The proneural subtype is more common in younger patients and is associated with a better prognosis [44]. The role of tumor location also appears to play a prognostic role. In particular, the subventricular zone (SVZ) is discussed as an associated site in gliomagenesis and resistance to treatment. Thereby, the SVZ seems to be an independent prognostic factor in glioblastoma [45]. In addition, tumor size (e.g., diameter greater than 5 cm [46,47]), tumors crossing midline, and central tumor locations appear to have a poorer prognosis [46,47,48,49]. Table 1 summarizes various factors associated with the prognosis of glioblastoma.

Table 1.
Factors influencing the prognosis in GBM.
Besides traditionally well-described correlations, the question of recurrence and progression-free survival remains unresolved. There is still no standard follow-up and no individualization depending on the patient’s risk. Therefore, efforts have been made to combine imaging and tumor characteristics for follow-up and prognostic assessment.
1.2. Radio(geno)mics of Glioblastoma
Radiogenomics can be understood as a synthesis of two basic concepts. Possible molecular correlations can be related to a specific radiological phenotype, whereas, on the other hand, it can be shown how a particular genomic variation affects tumor imaging properties [16,58,59]. Radiogenomics defines relationships between image features and molecular markers [16]. Those investigations can be divided into either exploratory or hypothesis-driven types of studies [58]. Exploratory studies compare imaging features with different genomic alterations, whereas hypothesis-driven studies evaluate radiation phenotypes relevant to a particular genetic alteration [60]. Especially in recent years, the application of artificial intelligence (AI) in medicine has increased considerably and is affecting multiple medical specialties [61]. Machine learning (ML) approaches have become a popular and nowadays widespread used technique to extract multiple features by converting for example medical images into mineable high-dimensional feature sets [62], selecting and determining relevant features for further reduction of data complexity, and finally more precise classification and outcome prediction [63,64]. Beyond the scope of manually extracted features, ML allows for an automated extraction of, e.g., first-order statistics, shape-based/textural/wavelet/geodesic features, or tissue probability maps [65]. As high dimensionality may lead to increased model complexity and overfitting issues, reducing dimensionality by feature selection is an essential step that can be performed by methods such as least absolute shrinkage and selection operator (LASSO) or random forests (RF) [66,67]. Based on selected features, various ML approaches can be applied for classification and outcome prediction, e.g., support vector machines (SVM), decision trees (DT), RFs as an extension of DTs, artificial neural networks (ANN), logistic regression (LR), Naïve Bayes (NB), or K-nearest neighbors (KNN). For further details and comparison of those methods, see [68].
1.3. Rationale
Supporting interdisciplinary therapy of glioblastoma requires a comprehensive understanding of the patient’s individual risk of tumor progression and recurrence formation to discuss early therapeutic considerations in a comprehensive manner. In that case, noninvasive routine MR clinical studies may provide insight into a likely response to treatment modalities and could aid in decision making for adjusting an individual follow-up regime. However, numerous studies were conducted over a decade that demonstrated numerous correlations of varying degrees. Thus, to provide a comprehensive view of the connection between tumor-related parameters and imaging characteristics, a systematic literature review was conducted to summarize the current status of radiogenomic associations and their prognostic potential that can be considered for optimization of the patient-specific monitoring and treatment strategy.
2. Materials and Methods
2.1. Eligibility Criteria
The inclusion of registered articles was limited to those written in English, conducted on human subjects, and published in peer-reviewed journals. The period was limited to the years 2011 to 2021. Inclusion criteria for full review were: (1) confirmed diagnosis of GBM (WHO 2016 definition) by a certified neuropathologist; (2) tumor resection without evidence of residual tumor; (3) evaluation of tumor markers or histopathology; (4) evaluation of magnetic resonance imaging sequences. Exclusion criteria were: (1) low-grade glioma and WHO III anaplastic glioma; (2) subtotal tumor resection with residual tumor; (3) animal studies; (4) reviews.
2.2. Information Sources and Search Strategy
An extensive web-based search was conducted following the guidelines provided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The study was registered in PROSPERO; registration number CRD42022300803. The literature search was conducted using the databases PubMed, Google Scholar, and Cochrane Library. Keywords used were “glioma”, “glioblastoma”, “imaging genomics” and “radiogenomics”. Search terms were combined with two Boolean operators: AND, OR. The search strategy was peer-reviewed by M.B. using the Peer Review of Electronic Search Strategies (PRESS) checklist. The search strategies can be found in the Appendix A (see Table A1).
2.3. Selection and Data Collection Process
An extensive web-based search was conducted following the guidelines provided by PRISMA [69] (see Appendix A, Table A2 and Table A3). The PubMed search yielded 121 results, the Google Scholar search 196, and the Cochrane Library 4 results. In total, 321 articles from databases were identified (as of 2 July 2021). As an additional search strategy, references of the selected papers and other reviews were scanned. This led to the identification of additional 30 articles. Titles and abstracts were screened for the inclusion and exclusion criteria by two independent authors (F.C. and D.G.). To increase the consistency among the authors, all authors screened the same publications and discussed the results. If these matched the inclusion criteria, the full-text article was screened for quality using the GRADE criteria and Critical Appraisal Skills Program (CASP) qualitative research checklist. If the quality was judged to be good (meeting three out of four inclusion criteria), the articles were included for the data extraction process. A third author was consulted in case of disagreement between the other two initial authors. Potentially relevant articles were therefore collected in a data table and examined again in consultation with the reviewers. The following information was collected in the consequent data extraction process: (1) author name; (2) publication year; (3) study design; (4) sample size; (5) tumor type; (6) tumor markers; (7) radiological sequences; (8) outcome measure; (9) results. Duplicates were removed. At the end of the selection process, a total of 43 articles was included in this review. A qualitative systematic review was conducted in this setting as numerical data were unavailable in the primarily qualitative studies. Mainly nominal data from the available articles were analyzed.
3. Results
In total, 321 studies were initially identified. Duplicates (n = 3) were removed. Thus, the remaining 318 articles were screened. Reasons for a preliminary exclusion were mainly titles that did not address the research question or were written in a language other than English. A total of 67 articles were excluded. The remaining 251 articles were screened first by abstract, then by full text. Studies were excluded based on the exclusion criteria, e.g., reviews, low-grade gliomas, other tumor types, or animal experiments. Available reviews on this topic were primarily excluded but were searched in a secondary step for valuable literature in the references. Thus, an additional 30 articles of potential relevance were found. These articles went through the same screening process so that a total of 22 more articles were identified here. A total of 43 articles were included in this systematic review. The flow chart of data selection is shown in Figure 1.

Figure 1.
PRISMA flow chart for the systematic review detailing the database searches, the number of records screened, and the studies included.
Articles were published between 2011 and 2021. The study’s authors, year of publication, molecular parameters, radiological sequences, feature types, and utilization of machine learning are presented in Table 2.

Table 2.
Literature review table.
A retrospective study design was predominant (88.37%, n = 38). In total, 9176 patients were involved. A heterogeneous, polarizing pattern of cohort sizes, ranging from 6 to 3800 (mean value 382.3, SD 1360.53) was evident. The most evaluated parameters were MGMT (n = 22), IDH (n = 19), EGFR (n = 14), and GBM molecular subgroups (classic, cystic, mesenchymal, and proneural subtype, n = 6). Other tumor markers included phosphatase and tensin homolog (PTEN, n = 5), platelet-derived growth factor receptor A (PDGFRA, n = 5), tumor protein 53 (TP53, n = 3), telomerase reverse transcriptase (TERT, n = 3), mouse double minute 2 (MDM2, n = 3), retinoblastoma protein 1 (RB1, n = 3), Ki (Kiel)-antigen Nr. 67 (Ki-67, n = 2), mRNA (n = 2), signaling pathways (n = 2), cyclin dependent kinase 4 (CDK4, n = 2), Cyclin Dependent Kinase Inhibitor 2A (CDKN2A, n = 2), neurofibromatosis type 1 gene (NF1, n = 2), epithelial–mesenchymal transition (EMT) pathway activation (n = 1), and mechanistic target of rapamycin (mTOR, n = 1).
In addition, the frequency of the radiological MRI sequences used was evaluated. Here, the most frequent sequences were T1 weighted with (n = 37) and without contrast agent (n = 31), as well as T2-weighted MR sequences (n = 33) and fluid-attenuated inversion recovery (FLAIR) sequences (n = 26). Other methods included diffusion-weighted imaging (DWI, n = 13), incorporating in part parameters such as apparent diffusion coefficient (ADC), fractional anisotropy (FA), radial and axial diffusivity (AD/RD), dynamic susceptibility imaging (DSC, n = 12), partially using cerebral blood volume (CBV), cerebral blood flow (CBF), peak height (PH), percentage signal recovery (PSR). Less commonly used modalities were dynamic contrast-enhanced imaging (DCE, n = 4), MR spectroscopy (MRS, n = 2), arterial spin labeling (ASL, n = 1), susceptibility weighted imaging (SWI, n = 1), proton density (PD, n = 1), spoiled gradient recalled (SPGR) acquisition (n = 1) and α-[¹¹C]-methyl-l-tryptophan PET (AMT-PET, n = 1).
3.1. Studies Assessing the Radiogenomic Prognosis
Five of 43 articles were identified as prospective studies with a total of 321 patients [71,85,88,92,95]. In addition to these articles, other authors also pointed out prognostic implications. [70,73,76,89,105,109]. Evaluations of a retrospective and prospective cohort of patients with newly diagnosed glioblastoma showed that older age, the volume of contrast-enhancing tumor, edema volume, and relatively short distance between the tumor and ventricular system were predictive of shorter survival. In addition, survival was shortened with a relatively high number of voxels of high T1 contrast enhancement intensity, low T2 intensity, high peak height (PH), and low trace (TR) [85]. Heiland et al. demonstrated a strong link between fractional anisotropy (FA) and the epithelial-to-mesenchymal transition (EMT) pathway using network analysis. A high FA correlated with a worse clinical outcome. In contrast, high mean diffusivity (MD) correlated with more prolonged survival [88]. Furthermore, a correlation between a high AMT tumor/cortex uptake ratio on PET and prolonged survival could be observed [92]. Concerning gene and mRNA expression in glioblastoma, Kaplan–Meier curves showed that periostin (POSTN) expression resulted in significantly shorter survival and time to progression [70]. Approaching of the tumor to ADIFFI (analysis of differential involvement)-classified regions in the left temporal lobe significantly prolonged the survival rate [73]. The contrast-enhancing region of the tumor and longest axis length of the tumor were also associated with poor survival [76]. Perfusion imaging showing a high ratio of peri-enhancing tumor area (rCBVperi-tumor) was found in patients with overall survival of less than 14 months. Moreover, the strongest predictors of overall survival were rCBVperi-tumor and age [89].
3.2. Studies Assessing the MGMT Methylation Status
Twenty-two out of 43 articles (including 3688 patients) assessed the MGMT methylation status in connection with specific radiological characteristics [72,73,77,78,80,83,84,86,87,91,92,96,98,99,100,101,103,104,105,108,109,111]. In T2/FLAIR images, MGMT promoter-methylated tumors were shown to have a lower hyperintense tumor volume, in contrast to unmethylated tumors [73]. Additionally, elevated transfer constant (Ktrans) levels in MGMT-methylated tumors were observed in MR perfusion [78]. In diffusion imaging, increased minimum ADC values were more likely in MGMT-methylated tumors. A significant reduction in the mean measure of the low ADC value in the two-mixture model histogram seemed to be associated with methylated tumors as well [80]. Metabolic volume and tumor/cortex AMT unidirectional uptake ratios were lower in MGMT-methylated tumors [92]. A significant association between MGMT-methylated tumors and grade of radiographic necrosis was furthermore demonstrated [105].
3.3. Studies Assessing the IDH Mutation Status
Nineteen out of 43 articles (including 2875 patients) examined the IDH status in relation to radiologic characteristics [66,71,72,77,83,84,89,92,93,96,98,99,101,103,104,105,108,109,111]. Using MR spectroscopy, increased levels of 2-hydroxyglutarate (2-HG), the product of the neomorphic IDH enzyme activity, were found in IDH1 mutant tumors. The analysis also showed an increase in measured choline and decreased glutathione levels in IDH1 mutant tumors [71]. There was evidence that IDH1 mutant tumors were non-contrast enhancing tumor (nCET) positive. Tumor size and nCET could be used to determine IDH1 mutated tumors with an accuracy of 97.5% [72]. In contrast to a correlation of MGMT methylation and minimum ADC maps [80], Yamashita et al. did not find a significant difference in differentiation between wild-type IDH1 tumor and mutant IDH1 status in their study. However, a significant increase in absolute tumor blood flow, relative tumor blood flow, necrosis area, and percentage of cross-sectional necrosis area inside the enhancing lesion was observed [84]. Moreover, a larger tumor volume in T2-weighted sequences and a higher volume ratio between T2w and T1 sequences with contrast agents were observed in IDH mutant tumors. Additionally, higher mean normalized apparent diffusion coefficient (ADC) values were seen in these tumors [98].
3.4. Studies Assessing the EGFR Status
Fourteen out of 43 articles (including 2265 patients) assessed EGFR or epidermal growth factor receptor variant III (EGFRvIII) status in GBM patients [77,81,82,83,86,89,90,92,95,96,97,101,103,106]. A positive EGFRvIII status, an increased relative plasma volume (rVP), and increased relative contrast transfer coefficient parameters were revealed [81]. Furthermore, higher median relative cerebral blood volume (rCBV) and lower percentage signal recovery (PSR) levels were seen in MR perfusion, which was associated with high levels of EGFR amplification. In addition, higher median relative peak height (rPH) levels were associated with EGFRvIII mutation [82]. Bosnyák et al. described lower T1 contrast-enhancing tumor volume, lower T1 contrast/T2 volume, and T1 contrast/PET volume ratios associated with EGFR amplification [92]. Similarly, higher levels of relative contrast enhancement were seen in the presence of EGFR mutations at alanine 289 (EGFRA289D/T/V). Lower T1 and increased T2 signals were also detected in the enhancing tumor region [103].
3.5. Studies Assessing the Molecular Subtypes
Overall, seven out of 43 articles involving 775 patients investigated radiogenomic associations related to glioblastoma molecular subtypes [70,75,76,79,85,86,101]. High POSTN and low miR-219 expression could be significantly associated with the mesenchymal GBM subtype [70]. Differences in image morphology were evident with respect to the volume of contrast enhancement, the volume of central necrosis, the combined volume of contrast enhancement and central necrosis, and necrosis concerning the distinction of the mesenchymal and non-mesenchymal subtypes. The volume ratio of T2-weighted hyperintensity to contrast enhancement and central necrosis was significantly lower in the mesenchymal glioblastoma subtype [75]. Similarly, the proneural class showed significantly lower levels of contrast uptake compared to other subtypes. The mesenchymal subtype showed low levels of non-enhanced tumor [76].
3.6. Studies Assessing the Tumor Location
Seven of 43 articles (involving 1557 patients) were able to provide an additional link between radiogenomic markers and tumor location [72,73,77,86,95,96,99]. There was a correlation between nCET positive IDH1 mutant tumors, 79% localized in the frontal lobe [72]. MGMT-methylated tumors having a lower T2/FLAIR volume were localized in the left hemisphere. In contrast, MGMT-unmethylated tumors appeared to be localized in the right hemisphere. T2/FLAIR hyperintense signaling increases frequently occurred in the posterior subventricular zone [73]. Another study showed frequent localization of MGMT methylated tumors in the left temporal lobe. For example, localization in the frontal lobe was seen with younger age, IDH1 mutated tumors, and loss of PTEN. Moreover, in the frontal lobe, localized in the left hemisphere, MGMT-methylated, IDH1-mutated glioblastomas appeared [77]. EGFR amplified, and EGFRvIII-expressing tumors were most common in the left temporal lobe. EGFRvIII-positive tumors occurred more frequently in the frontal and parietal lobe areas than EGFRvIII-negative tumors [95]. Altieri et al. showed a predictive value for left hemisphere occurrence at low Ki-67 levels and IDH1 mutations occurring in the right hemisphere. IDH wild-type glioblastomas appeared to be localized in the temporal lobe. MGMT-methylated tumors occurred mainly in the parietal lobe, whereas unmethylated glioblastomas were localized in the insula [99]. Figure 2 illustrates radiogenomic tumor localizations.
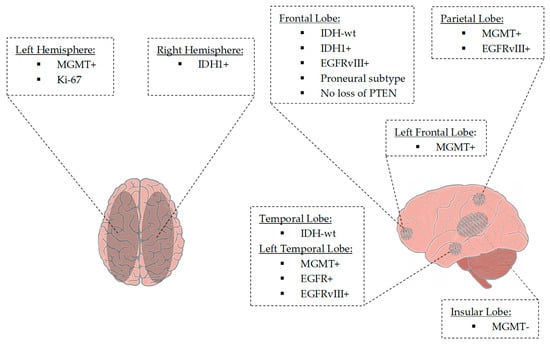
Figure 2.
Tumor localization based on tumor-associated parameters. MGMT-methylated tumors and tumors with a lower Ki-67 value were lateralized to the left hemisphere. In contrast, MGMT-unmethylated tumors and tumors with mutation of IDH1 were found to be lateralized to the right hemisphere. IDH1-mutant tumors were located in the frontal lobe, whereas IDH wild-type tumors were located in the frontal and temporal lobes. Tumors with the proneural gene expression subtype and tumors with an absent loss of PTEN frequently occurred in the frontal lobe. MGMT-methylated tumors were located in the (left) frontal and left temporal lobes. In contrast, MGMT-unmethylated tumors were located in the insular lobe. Tumors revealing EGFR amplification and EGFRvIII-mutated tumors involved the frontal, left temporal and parietal regions. Temporal location was associated with IDH wild-type tumors. Abbreviations: EGFR+, EGFR amplification; EGFRvIII+, EGFRvIII mutation; IDH1+, IDH1 mutation; IDH-wt, IDH wildtype; Ki-67, Kiel-antigen Nr.67; MGMT-, MGMT promotor unmethylated; MGMT+, MGMT promotor methylated.
4. Discussion
4.1. Summary of Findings
Numerous tumor-related molecular markers are used to monitor the treatment of patients with GBM, thereby playing a role in improving diagnostic accuracy, assessing prognosis, and predicting treatment response. The newly established WHO CNS tumor classification 2021 places higher importance on molecular markers than all other previous classifications [11]. In this review, the recent advantages in correlating tumor-associated molecular parameters with imaging phenotypes were highlighted, thereby exhibiting correlative prognostic capabilities of radiogenomics.
MGMT promoter methylation has clinical relevance in the prediction of chemotherapy responsiveness to TMZ [112,113]. MGMT-methylated tumors showed less T2/FLAIR hyperintense volume [73,77] and lower volume contrast enhancement [77] which was associated with the extent of radiographic necrosis [105]. However, Kickingereder et al. described a higher ratio of contrast-enhancing tumor volume (T1CE) to the complete volume (T1w) [86]. Further associations included higher Ktrans [78], higher relative CBV [86], lower metabolic volume, lower tumor/cortex AMT unidirectional uptake ratios on PET imaging [92], and elevated minimum ADC [80].
IDH1 mutation represents a significant independent factor in predicting longer OS and PFS in patients with GBM [114]. IDH1-mutant tumors revealed elevated levels of 2-HG, a decreased glutathione, and elevated choline in MRS [71]. IDH-mutated tumors revealed higher nADC values, larger volumes in T2w imaging, and a higher ratio between T2w and T1CE [98]. In contrast, IDH wild-type tumors demonstrated larger volumes of contrast enhancement [77], higher blood flow, increased necrotic areas, and a higher percentage of cross-sectional necrosis inside the enhancing lesion [84].
Amplification and alteration of EGFR are frequently observed in GBM, resulting in overexpression of several mutations, including EGFRvIII [41], and was shown to be a predictor of poor prognosis in OS [40,41]. EGFR amplifications were associated with a higher T1CE and T2/FLAIR hyperintense volume [77]. Conversely, Bosnyák et al. found lower T1CE contrast volumes, lower T1 contrast/T2 volume, and T1 contrast/PET volume ratios [92]. EGFR amplification correlated with a higher median rCBV and lower PSR [82], increased rCBF and rCBV [86,89], and a higher Ki-67 labeling index [92]. EGFRvIII expression of tumors was associated with increased rVP [81], relative ktrans [81], higher median rPH [82,101], higher rCBV [95,101], FA [101], elevated mean PH within the enhancing tumor [101], and lower ADC signals [95].
Identification of GBM subtypes is of high importance for prognosis as they exhibit different clinical outcomes and molecular features [115]. The mesenchymal subtype was characterized by a high FLAIR signal associated with upregulated levels of POSTN [70], lower ratios of volume of contrast enhancement, and volume of central necrosis [75], lower levels of non-enhanced tumor [76], and lower rCBV [86]. The classical subtype revealed associations concerning T2/FLAIR intensity, enhancing tumor size, and PH signal for edema [79,85] whereas the proneural subtype demonstrated lower levels of contrast enhancement [76] and associations of T1w and T2/FLAIR intensity in the enhancing tumor region [85].
Besides individual biological tumor parameters exhibiting prognostic potential, we identified radiological sequences and tumor locations that were found to be associated with prognosis individually. A high T1CE signal, low T2w intensity, high PH, and low trace (TR) were associated with a shortened survival [85]. FA correlated with the activation of the EMT pathway, thus being associated with a worse clinical outcome [88]. High FLAIR intensity signal was found to correlate with an upregulated POSTN expression level and showed a significantly shortened survival [70]. In contrast, a high MD [88] and a high AMT tumor/cortex ratio on PET corresponded with prolonged survival [92]. A strong predictor for OS was rCBV in the peritumor region [89]. Concerning their location in the CNS, tumors in the left temporal lobe were associated with longer OS independent of treatment and MGMT status [73]. Furthermore, these regions were associated with a more favorable response to radiochemotherapy and increased survival [77].
4.2. Limitations
Even though all included studies related to molecular and radiological markers focusing on tumor progression in GBM, one major drawback is the methodological heterogeneity found across the studies. Besides varying the selection of molecular and radiological markers, there are several aspects to consider. Due to the lack of standardization in imaging routines and system-related differences across MRI systems and used sequences, one limitation can be seen in the analysis of the variability and inconsistency in radiological parameters and features. Whereas the used imaging sequences varied across the study, scanner-related variabilities need to be considered when comparing those studies or pooling data in multicenter approaches [116]. In this way, besides other factors, e.g., the magnetic field strength is known to influence image contrast, noise, and tumor-related measures such as lesion contrast [117,118]. Furthermore, significant differences in the methodology could be observed across the included studies, and especially recent ones used AI and ML approaches. In addition, different software with different algorithms was used.
Radiomics and radiogenomics have been associated with AI and ML, especially in recent years. Radio(geno)mic studies can be divided into classical (conventional) and novel approaches with ML [63], representing an essential methodological difference in evaluating and interpreting the study types, as summarized here. The classical approach contrasts to machine learning and deep learning radio(geno)mic pipelines, where images are processed, and features are automatically extracted and related. In the classical approach, the observer’s regions of interest are manually or automatically delineated, and hand-granted features are extracted [63]. Image-derived features are processed, and statistical models like univariate or multivariate analyses are used to calculate mathematical relationships between variables and outcomes [63]. Several of our included studies feature ML and AI algorithms, of which the results cannot be extrapolated for the observer in contrast to the classical radiogenomic approach. Therefore, the results of the individual studies in this review must be interpreted with caution.
4.3. Clinical Relevance
In the future, prospective patient cohorts may provide systemic data acquisition concerning radiologic and histopathologic markers. Information on genetic profiles with various biomarkers, structural and functional MR imaging characteristics, and clinical responses related to the type of surgery, could be analyzed. Correlations of these will be analyzed to obtain predictive radiogenomic markers for the prognosis of patients with GBMs with regard to progression, recurrence, and malignancy, enabling them to be used in the future for more personalized and improved treatment planning. This includes a multi-dimensional multi-omic characterization of tumors, i.e., by integrating genomic, proteomic, and radiomic data. To optimize prognosis as well as clinical therapy decisions, there is a need to identify patients at an early stage who are at increased risk for recurrent tumors or low therapeutic success. Relevant radiogenomic characteristics of gliomas can be determined, intratumoral heterogeneity in imaging can be compared with intratumoral genetic heterogeneity, and the differences between primary and secondary glioblastomas as well as markers of tumor angiogenesis can be elaborated. In addition to determining relevant predictive radiogenomic markers for the progression and recurrence of brain-derived tumors, determining prognostically relevant radiogenomic markers, and the formulation of a standard protocol for imaging follow-up concerning the biological characteristics of the tumor, this data collection could allow further analyses. A broad-spectral database thereby created, in conjunction with patients systematically followed up to recurrence, could provide radiogenomic information to identify early tumor recurrence. Such high-risk patients could be monitored more closely in the future, and therapeutic interventions could be considered earlier. Furthermore, a statistically evaluated database of radiogenomic associations can be used for a prognostic scoring system that will be implemented clinically. Similarly, stem cells could be isolated, and different therapy arms (e.g., chemotherapy with temozolomide and radiation) could be analyzed in this regard to achieve improved prognosis and therapy decisions for each patient individually on a broad basis.
4.4. Future Directions
Glioblastoma remains in its position as a clinically difficult tumor to treat, yet recent years have demonstrated a steady improvement in imaging modalities, especially in MRI. Attempts to incorporate increasing autonomy into imaging processing programs using AI to create initial prognostic determinations are rising. Radio(geno)mics offers a strong upward trend in recent years and may offer a personalized revolutionary approach in GBM. Likewise, advances in machine learning and deep learning approaches could be observed. New reviews in this field have focused on ML and deep learning approaches [67]. However, in contrast to other reviews, we focused on both classical to machine learning approaches. Our primary objective was to provide a comprehensive summary of radiological parameters and molecular tumor characteristics, thus enabling risk stratification rather than focusing on other aspects such as therapeutic options.
Machine learning algorithms for program-controlled, non-invasive detection of radiogenomic markers in IDH and EGFR in low-grade gliomas and glioblastomas showed success rates of over 80% [101]. Similarly, experiments using anomaly detection analytics detected IDH mutations in glioblastomas using preoperative T1-weighted MR sequences [119]. A neural network-based approach using high-dimensional gene expression data to perform non-linear mapping to imaging traits also showed that imaging features of the tumor exhibited specific transcriptional patterns [120]. Using a hypercolumn-based convolutional network to segment tumor regions from MRI images and extract radiological features such as geometry, shape, and histogram, and finally to fuse them with gene expression profiling data represents another attempt to predict patient survival rates. In this context, the most essential genes identified were, for example, interleukin-1β, KLHL4, ATP1A2, IQGAP2, and TMSL8, which strongly contribute to prognostic analysis [121]. To predict progression-free survival and recurrence, the Cancer Imaging Phenomics Toolkit (CaPTk) software suite was further used to analyze standard clinical multiparametric MRI scans of the brain. Predictive signatures based on various classification schemes were evaluated. These predictors also generated high predictability of the timing and location of recurrence [122]. At the tumor microenvironment level, machine learning-based magnetic resonance radiomodeling was developed to classify immune phenotypes in glioblastoma, which assessed the enrichment levels of four immune subsets. Five immunophenotypes were identified that could also predict patient prognosis [123]. Using a deep learning pipeline to predict MGMT status in glioblastoma patients automatically showed good predictive performance. Using FLAIR images, better status prediction and tumor segmentation could be reached [124]. An essential goal of radiogenomics is to provide prognostic information regarding invasiveness, recurrence, and survival early in the clinical patient course. Many studies can be found in the literature using multicenter databases, for example, The Cancer Genome Atlas (TCGA) and The Cancer Imaging Archive (TCIA). In the future, there is still a lack of clinical, prospective studies that follow up patients with fixed examination times until recurrence, collecting different radiological and tumor biological characteristics at the respective follow-up times, which are finally evaluated by biostatistics using an extensive database.
5. Conclusions
The present review provides a broad and informative state-of-the-art picture and illustrates the latest developments in radiogenomic markers with regard to prognosis and their potential monitoring for GBM recurrence. By linking tumor biology parameters with phenotypic characteristics in MR imaging on a patient-specific basis, a significant trend towards a personalized approach is emerging. However, prospective studies analyzing radiogenomic features of glioblastoma are lacking. New information providing answers to prognosis and recurrence early in the course of the disease could provide new clinical implications for individual management and treatment strategies in GBM patients, who are up to now still faced with a poor prognosis and outcome.
Author Contributions
Conceptualization, F.C. and D.G.; methodology, F.C. and M.H.A.B.; software, M.H.A.B.; validation, M.H.A.B., D.G. and C.N.; formal analysis, B.S., M.P., B.C., F.C. and D.G.; investigation, F.C. and M.H.A.B.; resources, J.W.B. and C.N.; data curation, F.C. and D.G.; writing—original draft preparation, F.C.; writing—review and editing, M.H.A.B., J.W.B., C.N., D.G. and B.S.; visualization, F.C. and M.H.A.B.; supervision, M.H.A.B.; project administration, M.H.A.B. and C.N.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Results of the present work are part of the bachelor thesis of F.C. Open Access funding was provided by the Open Acess Publication Fund of Philipps-Universität Marburg with support of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) The Authors would like to thank Noa-Lena Hillenbrand for the illustrative artwork in this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
PubMed, Google Scholar, and Cochrane Library search strategies.
Table A1.
PubMed, Google Scholar, and Cochrane Library search strategies.
| Database: PubMed | |
| The search strategy for Title/Abstract terms used a combination of subject headings (MeSH terms) and keywords: | |
| Search Strategy: | |
| #1 | “GBM”[All fields] |
| #2 | “Glioblastoma”[Mesh] |
| #3 | “Glioma *”[Mesh] |
| #4 | “glioblastoma *”[All fields] |
| #5 | “glioblastoma multiforme”[All Fields] |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | “Imaging Genomic *” [Mesh] |
| #8 | “Radiogenomic *”[All Fields] |
| #9 | #7 OR #8 |
| #10 | #6 AND #9 |
| Database: Google Scholar | |
| Search Strategy with keywords: | |
| 1 | “glioma” |
| 2 | “glioblastoma” |
| 3 | “imaging genomics” |
| 4 | “radiogenomics” |
| Full search: “glioma” “glioblastoma” “imaging genomics” “radiogenomics | |
| Database: Cochrane Register. | |
| Search Strategy | |
| #1 | Radiogenomic * |
| #2 | Glioma * |
| #3 | #1 AND #2 |

Table A2.
PRISMA 2020 for Abstracts Checklist.
Table A2.
PRISMA 2020 for Abstracts Checklist.
| Section and Topic | Item # | Checklist Item | Reported (Yes/No) |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Yes |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses. | Yes |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | Yes |
| Information sources | 4 | Specify the information sources (e.g., databases, registers) used to identify studies and the date when each was last searched. | Yes |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | No |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Yes |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Yes |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (i.e., which group is favoured). | No |
| Discussion | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g., study risk of bias, inconsistency and imprecision). | Yes |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Yes |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review. | No |
| Registration | 12 | Provide the register name and registration number. | No |

Table A3.
PRISMA 2020 Checklist.
Table A3.
PRISMA 2020 Checklist.
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 4 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Pages 1–4 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 4 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Page 4 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Page 4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Page 4 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Page 4–5 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Page 4–5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | n.a. |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | n.a. |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 5 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | n.a. | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | n.a. | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | n.a. | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | n.a. | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | n.a. | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | n.a. |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | n.a. |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 5 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | n.a. | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Pages 6, 9 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | n.a. |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Pages 9–12 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | n.a. |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | n.a. | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | n.a. | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | n.a. | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | n.a. |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | n.a. |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 12 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 13 | |
| 23c | Discuss any limitations of the review processes used. | Page 13 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Pages 13–14 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Page 4 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | n.a. | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | n.a. | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Page 16 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 16 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | n.a. |
References
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, K.; Li, M.; Cui, Y.; Ren, X.; Yang, C.; Zhao, X.; Lin, S. Classification of Progression Patterns in Glioblastoma: Analysis of Predictive Factors and Clinical Implications. Front. Oncol. 2020, 10, 590648. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009. Neuro-Oncology 2016, 18, v1–v75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korja, M.; Raj, R.; Seppä, K.; Luostarinen, T.; Malila, N.; Seppälä, M.; Mäenpää, H.; Pitkäniemi, J. Glioblastoma survival is improving despite increasing incidence rates: A nationwide study between 2000 and 2013 in Finland. Neuro-Oncology 2018, 21, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chukwueke, U.N.; Wen, P.Y. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019, 8, CNS28. [Google Scholar] [CrossRef] [Green Version]
- Sanghvi, D. Post-treatment imaging of high-grade gliomas. Indian J. Radiol. Imaging 2015, 25, 102–108. [Google Scholar] [CrossRef]
- Weybright, P.; Sundgren, P.C.; Maly, P.; Hassan, D.G.; Nan, B.; Rohrer, S.; Junck, L. Differentiation Between Brain Tumor Recurrence and Radiation Injury Using MR Spectroscopy. AJR Am. J. Roentgenol. 2005, 185, 1471–1476. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemée, J.-M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Aum, D.J.; Kim, D.H.; Beaumont, T.L.; Leuthardt, E.C.; Dunn, G.P.; Kim, A.H. Molecular and cellular heterogeneity: The hallmark of glioblastoma. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhoon, N. Precision Medicine: A New Paradigm in Therapeutics. Int. J. Prev. Med. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.D.; Jamshidi, N. Behind the Numbers: Decoding Molecular Phenotypes with Radiogenomics—Guiding Principles and Technical Considerations. Radiology 2014, 270, 320–325. [Google Scholar] [CrossRef]
- Tian, M.M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of gender on the survival of patients with glioblastoma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro-Oncology 2018, 20, 576–577. [Google Scholar] [CrossRef]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; Berens, M.E.; Lathia, J.; et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neuro-Oncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef]
- Smrdel, U.; Vidmar, M.S.; Smrdel, A. Glioblastoma in patients over 70 years of age. Radiol. Oncol. 2018, 52, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Lamborn, K.R.; Chang, S.M.; Prados, M.D. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro-Oncology 2004, 6, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibetseder, A.; Ackerl, M.; Flechl, B.; Woehrer, A.; Widhalm, G.; Dieckmann, K.; Kreinecker, S.-S.; Pichler, J.; Hainfellner, J.; Preusser, M.; et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: A study of the Society of Austrian Neurooncology (SANO). Neuro-Oncology 2013, 15, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichlmeier, U.; Bink, A.; Schackert, G.; Stummer, W. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro-Oncology 2008, 10, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wang, S.; Song, C.; Zha, Y.; Li, L. The prognostic value of MGMT promoter status by pyrosequencing assay for glioblastoma patients’ survival: A meta-analysis. World J. Surg. Oncol. 2016, 14, 261. [Google Scholar] [CrossRef] [Green Version]
- Brandes, A.A.; Tosoni, A.; Franceschi, E.; Sotti, G.; Frezza, G.; Amistà, P.; Morandi, L.; Spagnolli, F.; Ermani, M. Recurrence Pattern After Temozolomide Concomitant with and Adjuvant to Radiotherapy in Newly Diagnosed Patients with Glioblastoma: Correlation with MGMT Promoter Methylation Status. J. Clin. Oncol. 2009, 27, 1275–1279. [Google Scholar] [CrossRef] [Green Version]
- Gerstner, E.R.; Yip, S.; Wang, D.L.; Louis, D.N.; Iafrate, A.J.; Batchelor, T.T. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology 2009, 73, 1509–1510. [Google Scholar] [CrossRef] [Green Version]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate Dehydrogenase 1 Codon 132 Mutation Is an Important Prognostic Biomarker in Gliomas. J. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Turcan, S. From Laboratory Studies to Clinical Trials: Temozolomide Use in IDH-Mutant Gliomas. Cells 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lang, F.; Chou, F.-J.; Zaghloul, K.A.; Yang, C. Isocitrate Dehydrogenase Mutations in Glioma: Genetics, Biochemistry, and Clinical Indications. Biomedicines 2020, 8, 294. [Google Scholar] [CrossRef]
- Kayabolen, A.; Yilmaz, E.; Bagci-Onder, T. IDH Mutations in Glioma: Double-Edged Sword in Clinical Applications? Biomedicines 2021, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Isocitrate Dehydrogenase Mutations in Defining the Biology of and Supporting Clinical Decision Making in Glioblastoma. 23 January 2022. Available online: https://elitmed.hu/en/publications/clinical-neuroscience/isocitrate-dehydrogenase-mutations-in-defining-the-biology-of-and-supporting-clinical-decision-making-in-glioblastoma (accessed on 23 January 2022).
- Mirchia, K.; Richardson, T.E. Beyond IDH-Mutation: Emerging Molecular Diagnostic and Prognostic Features in Adult Diffuse Gliomas. Cancers 2020, 12, 1817. [Google Scholar] [CrossRef]
- Linggi, B.; Carpenter, G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006, 16, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; LeBrun, D.G.; Yang, J.; Zhu, V.F.; Li, M. Deregulated Signaling Pathways in Glioblastoma Multiforme: Molecular Mechanisms and Therapeutic Targets. Cancer Investig. 2012, 30, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, R.; Song, C.; Xiang, Y.; Liu, Y. Prognostic significance of epidermal growth factor receptor expression in glioma patients. OncoTargets Ther. 2018, 11, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Shinojima, N.; Tada, K.; Shiraishi, S.; Kamiryo, T.; Kochi, M.; Nakamura, H.; Makino, K.; Saya, H.; Hirano, H.; Kuratsu, J.; et al. Prognostic value of epidermal growth factor re-ceptor in patients with glioblastoma multiforme. Cancer Res. 2003, 63, 6962–6970. [Google Scholar]
- Tripathy, K.; Das, B.; Singh, A.K.; Misra, A.; Misra, S.; Misra, S.S. Prognostic Significance of Epidermal Growth Factor Receptor in Patients of Glioblastoma Multiforme. J. Clin. Diagn. Res. 2017, 11, EC05–EC08. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, S.; Van Bodegraven, E.; Seute, T.; Spliet, W.G.M.; Geurts, M.; Hendrikse, J.; Schoysman, L.; Huiszoon, W.B.; Varkila, M.; Rouss, S.; et al. Adverse prognosis of glioblastoma contacting the subventricular zone: Biological correlates. PLoS ONE 2019, 14, e0222717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellor, S.V.; Pagano-Young, T.A.; Avgeropoulos, N.G. Glioblastoma: Background, Standard Treatment Paradigms, and Supportive Care Considerations. J. Law Med. Ethic 2014, 42, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, G.; Stupp, R.; van den Bent, M.V.D.; Hegi, M.E.; Tonn, J.C.; Wick, W.; Weller, M. Molecular diagnostics of gliomas: The clinical perspective. Acta Neuropathol. 2010, 120, 585–592. [Google Scholar] [CrossRef]
- Fyllingen, E.H.; Bø, L.E.; Reinertsen, I.; Jakola, A.S.; Sagberg, L.M.; Berntsen, E.M.; Salvesen, Ø.; Solheim, O. Survival of glioblastoma in relation to tumor location: A statistical tumor atlas of a population-based cohort. Acta Neurochir. 2021, 163, 1895–1905. [Google Scholar] [CrossRef]
- Liu, T.T.; Achrol, A.S.; Mitchell, L.A.; Du, W.A.; Loya, J.J.; Rodriguez, S.A.; Feroze, A.; Westbroek, E.M.; Yeom, K.W.; Stuart, J.M.; et al. Computational Identification of Tumor Anatomic Location Associated with Survival in 2 Large Cohorts of Human Primary Glioblastomas. AJNR Am. J. Neuroradiol. 2016, 37, 621–628. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; Demonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Kiwit, J.C.; Floeth, F.W.; Bock, W.J. Survival in malignant glioma: Analysis of prognostic factors with special regard to cytoreductive surgery. Zentralbl Neurochir. 1996, 57, 76–88. [Google Scholar]
- Chaudhry, N.S.; Shah, A.H.; Ferraro, N.; Snelling, B.M.; Bregy, A.; Madhavan, K.; Komotar, R.J. Predictors of Long-Term Survival in Patients with Glioblastoma Multiforme: Advancements from the Last Quarter Century. Cancer Investig. 2013, 31, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.V.D.; Dubbink, H.J.; Marie, Y.; Brandes, A.; Taphoorn, M.J.; Wesseling, P.; Frenay, M.; Tijssen, C.C.; Lacombe, D.; Idbaih, A.; et al. IDH1 and IDH2 Mutations Are Prognostic but not Predictive for Outcome in Anaplastic Oligodendroglial Tumors: A Report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin. Cancer Res. 2010, 16, 1597–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular Predictors of Progression-Free and Overall Survival in Patients with Newly Diagnosed Glioblastoma: A Prospective Translational Study of the German Glioma Network. J. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro-Oncology 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nonoguchi, N.; Ohta, T.; Oh, J.-E.; Kim, Y.-H.; Kleihues, P.; Ohgaki, H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Labussière, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.-L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef]
- Pinker, K.; Shitano, F.; Sala, E.; Do, R.K.; Young, R.J.; Wibmer, A.G.; Hricak, H.; Sutton, E.J.; Morris, E.A. Background, current role, and potential applications of radiogenomics. J. Magn. Reson. Imaging 2018, 47, 604–620. [Google Scholar] [CrossRef]
- Zinn, P.O.; Mahmood, Z.; Elbanan, M.G.; Colen, R.R. Imaging Genomics in Gliomas. Cancer J. 2015, 21, 225–234. [Google Scholar] [CrossRef]
- Fathi Kazerooni, A.; Bakas, S.; Saligheh Rad, H.; Davatzikos, C. Imaging signatures of glioblastoma molecular characteristics: A radiogenomics review. J. Magn. Reson. Imaging 2020, 52, 54–69. [Google Scholar] [CrossRef]
- Briganti, G.; Le Moine, O. Artificial Intelligence in Medicine: Today and Tomorrow. Front. Med. 2020, 7, 27. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.L.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging imaging and genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Nuechterlein, N.; Li, B.; Feroze, A.; Holland, E.C.; Shapiro, L.; Haynor, D.; Fink, J.; Cimino, P.J. Radiogenomic modeling predicts survival-associated prognostic groups in glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab004. [Google Scholar] [CrossRef]
- Aftab, K.; Aamir, F.B.; Mallick, S.; Mubarak, F.; Pope, W.B.; Mikkelsen, T.; Rock, J.P.; Enam, S.A. Radiomics for precision medicine in glioblastoma. J. Neuro-Oncol. 2022, 156, 217–231. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zinn, P.O.; Mhajan, B.; Majadan, B.; Sathyan, P.; Singh, S.K.; Majumder, S.; Jolesz, F.A.; Colen, R.R. Radiogenomic Mapping of Edema/Cellular Invasion MRI-Phenotypes in Glioblastoma Multiforme. PLoS ONE 2011, 6, e25451. [Google Scholar] [CrossRef]
- Pope, W.B.; Prins, R.M.A.; Thomas, M.A.; Nagarajan, R.; Yen, K.E.; Bittinger, M.A.; Salamon, N.; Chou, A.P.; Yong, W.H.; Soto, H.; et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J. Neuro-Oncol. 2012, 107, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.A.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.; et al. Relationship between Tumor Enhancement, Edema, IDH1Mutational Status, MGMTPromoter Methylation, and Survival in Glioblastoma. AJNR Am. J. Neuroradiol. 2012, 33, 1349–1355. [Google Scholar] [CrossRef] [Green Version]
- Ellingson, B.M.; Cloughesy, T.F.; Pope, W.B.; Zaw, T.M.; Phillips, H.; Lalezari, S.; Nghiemphu, P.L.; Ibrahim, H.; Naeini, K.M.; Harris, R.J.; et al. Anatomic localization of O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated tumors: A radiographic study in 358 de novo human glioblastomas. NeuroImage 2012, 59, 908–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidi, N.; Diehn, M.; Bredel, M.; Kuo, M.D. Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology 2014, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naeini, K.M.; Pope, W.B.; Cloughesy, T.F.; Harris, R.J.; Lai, A.; Eskin, A.; Chowdhury, R.; Phillips, H.S.; Nghiemphu, P.L.; Behbahanian, Y.; et al. Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. Neuro-Oncology 2013, 15, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Gutman, D.A.; Cooper, L.A.D.; Hwang, S.N.; Holder, C.A.; Gao, J.; Aurora, T.D.; Dunn, W.D.; Scarpace, L.; Mikkelsen, T.; Jain, R.; et al. MR Imaging Predictors of Molecular Profile and Survival: Multi-institutional Study of the TCGA Glioblastoma Data Set. Radiol. 2013, 267, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Lai, A.; Harris, R.J.; Selfridge, J.M.; Yong, W.H.; Das, K.; Pope, W.; Nghiemphu, P.; Vinters, H.; Liau, L.; et al. Probabilistic Radiographic Atlas of Glioblastoma Phenotypes. AJNR Am. J. Neuroradiol. 2013, 34, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.S.; Shin, N.-Y.; Chang, J.H.; Kim, S.H.; Kim, E.H.; Kim, D.W.; Lee, S.-K. Prediction of methylguanine methyltransferase promoter methylation in glioblastoma using dynamic contrast-enhanced magnetic resonance and diffusion tensor imaging. J. Neurosurg. 2014, 121, 367–373. [Google Scholar] [CrossRef]
- Gevaert, O.; Mitchell, L.A.; Achrol, A.S.; Xu, J.; Echegaray, S.; Steinberg, G.K.; Cheshier, S.H.; Napel, S.; Zaharchuk, G.; Plevritis, S.K. Glioblastoma Multiforme: Exploratory Radiogenomic Analysis by Using Quantitative Image Features. Radiology 2014, 273, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Rundle-Thiele, D.; Day, B.; Stringer, B.; Fay, M.; Martin, J.; Jeffree, R.L.; Thomas, P.; Bell, C.; Salvado, O.; Gal, Y.; et al. Using the apparent diffusion coefficient to identifying MGMT promoter methylation status early in glioblastoma: Importance of analytical method. J. Med. Radiat. Sci. 2015, 62, 92–98. [Google Scholar] [CrossRef]
- Arevalo-Perez, J.; Thomas, A.A.; Kaley, T.; Lyo, J.; Peck, K.; Holodny, A.; Mellinghoff, I.; Shi, W.; Zhang, Z.; Young, R. T1-Weighted Dynamic Contrast-Enhanced MRI as a Noninvasive Biomarker of Epidermal Growth Factor Receptor vIII Status. AJNR Am. J. Neuroradiol. 2015, 36, 2256–2261. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Young, R.J.; Shah, A.D.; Schweitzer, A.D.; Graber, J.J.; Shi, W.; Zhang, Z.; Huse, J.; Omuro, A.M.P. Pretreatment Dynamic Susceptibility Contrast MRI Perfusion in Glioblastoma: Prediction of EGFR Gene Amplification. Clin. Neuroradiol. 2015, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Itakura, H.; Achrol, A.S.; Mitchell, L.A.; Loya, J.J.; Liu, T.; Westbroek, E.M.; Feroze, A.H.; Rodriguez, S.; Echegaray, S.; Azad, T.D.; et al. Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci. Transl. Med. 2015, 7, 303ra138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Hatae, R.; Yoshimoto, K.; Mizoguchi, M.; Suzuki, S.; Yoshiura, T.; Honda, H. MR Imaging–Based Analysis of Glioblastoma Multiforme: Estimation ofIDH1Mutation Status. Am. J. Neuroradiol. 2015, 37, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macyszyn, L.; Akbari, H.; Pisapia, J.M.; Da, X.; Attiah, M.A.; Pigrish, V.; Bi, Y.; Pal, S.; Davuluri, R.V.; Roccograndi, L.; et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro-Oncology 2016, 18, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.-P.; Radbruch, A.; et al. Radiogenomics of Glioblastoma: Machine Learning–based Classification of Molecular Characteristics by Using Multiparametric and Multiregional MR Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korfiatis, P.; Kline, T.L.; Coufalova, L.; Lachance, D.H.; Parney, I.F.; Carter, R.E.; Buckner, J.C.; Erickson, B.J. MRI texture features as biomarkers to predict MGMT methylation status in glioblastomas. Med. Phys. 2016, 43, 2835–2844. [Google Scholar] [CrossRef]
- Heiland, D.H.; Simon-Gabriel, C.P.; Demerath, T.; Haaker, G.; Pfeifer, D.; Kellner, E.; Kiselev, V.; Staszewski, O.; Urbach, H.; Weyerbrock, A.; et al. Integrative Diffusion-Weighted Imaging and Radiogenomic Network Analysis of Glioblastoma multiforme. Sci. Rep. 2017, 7, 43523. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mangla, R.; Tian, W.; Qiu, X.; Li, D.; Walter, K.A.; Ekholm, S.; Johnson, M.D. The preliminary radiogenomics association between MR perfusion imaging parameters and genomic biomarkers, and their predictive performance of overall survival in patients with glioblastoma. J. Neuro-Oncol. 2017, 135, 553–560. [Google Scholar] [CrossRef]
- Hu, L.S.; Ning, S.; Eschbacher, J.M.; Baxter, L.C.; Gaw, N.; Ranjbar, S.; Plasencia, J.; Dueck, A.C.; Peng, S.; Smith, K.A.; et al. Radiogenomics to characterize regional genetic heterogeneity in glioblastoma. Neuro-Oncology 2017, 19, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Kickingereder, P.; Neuberger, U.; Bonekamp, D.; Piechotta, P.L.; Götz, M.; Wick, A.; Sill, M.; Kratz, A.; Shinohara, R.T.; Jones, D.T.W.; et al. Radiomic subtyping improves disease stratification beyond key molecular, clinical, and standard imaging characteristics in patients with glioblastoma. Neuro-Oncology 2018, 20, 848–857. [Google Scholar] [CrossRef]
- Bosnyák, E.; Michelhaugh, S.K.; Klinger, N.V.; Kamson, D.O.; Barger, G.R.; Mittal, S.; Juhász, C. Prognostic Molecular and Imaging Biomarkers in Primary Glioblastoma. Clin. Nucl. Med. 2017, 42, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Zhang, R.; Liang, D.; Song, T.; Ai, T.; Xia, C.; Xia, L.; Wang, Y. Multimodal 3D DenseNet for IDH Genotype Prediction in Gliomas. Genes 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beig, N.; Patel, J.; Prasanna, P.; Hill, V.; Gupta, A.; Correa, R.; Bera, K.; Singh, S.; Partovi, S.; Varadan, V.; et al. Radiogenomic analysis of hypoxia pathway is predictive of overall survival in Glioblastoma. Sci. Rep. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, H.; Bakas, S.; Pisapia, J.M.; Nasrallah, M.P.; Rozycki, M.; Martinez-Lage, M.; Morrissette, J.J.D.; Dahmane, N.; O’Rourke, D.M.; Davatzikos, C. In vivoevaluation of EGFRvIII mutation in primary glioblastoma patients via complex multiparametric MRI signature. Neuro-Oncology 2018, 20, 1068–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada Neyra, M.A.; Neuberger, U.; Reinhardt, A.; Brugnara, G.; Bonekamp, D.; Sill, M.; Wick, A.; Jones, D.T.W.; Radbruch, A.; Unterberg, A.; et al. Voxel-wise radiogenomic mapping of tumor location with key molecular alterations in patients with glioma. Neuro-Oncology 2018, 20, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Bakas, S.; Akbari, H.; Pisapia, J.; Martinez-Lage, M.; Rozycki, M.; Rathore, S.; Dahmane, N.; O’Rourke, D.; Davatzikos, C. In Vivo Detection of EGFRvIII in Glioblastoma via Perfusion Magnetic Resonance Imaging Signature Consistent with Deep Peritumoral Infiltration: The φ-Index. Clin. Cancer Res. 2017, 23, 4724–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.K.; Choi, S.H.; Shin, D.J.; Jo, S.W.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; et al. Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur. Radiol. 2018, 28, 4350–4361. [Google Scholar] [CrossRef] [PubMed]
- Altieri, R.; Zenga, F.; Ducati, A.; Melcarne, A.; Cofano, F.; Mammi, M.; Di Perna, G.; Savastano, R.; Garbossa, D. Tumor location and patient age predict biological signatures of high-grade gliomas. Neurosurg. Rev. 2018, 41, 599–604. [Google Scholar] [CrossRef]
- Li, Z.-C.; Bai, H.; Sun, Q.; Li, Q.; Liu, L.; Zou, Y.; Chen, Y.; Liang, C.; Zheng, H. Multiregional radiomics features from multiparametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: A multicentre study. Eur. Radiol. 2018, 28, 3640–3650. [Google Scholar] [CrossRef]
- Rathore, S.; Mohan, S.; Bakas, S.; Sako, C.; Badve, C.; Pati, S.; Singh, A.; Bounias, D.; Ngo, P.; Akbari, H.; et al. Multi-institutional noninvasive in vivo characterization of IDH, 1p/19q, and EGFRvIII in glioma using neuro-Cancer Imaging Phenomics Toolkit (neuro-CaPTk). Neuro-Oncol. Adv. 2020, 2, iv22–iv34. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Sun, Z.; Xu, K.; Fan, X.; Li, S.; Zhang, Z.; Jiang, T.; Liu, X.; Wang, Y. Radiogenomic analysis of PTEN mutation in glioblastoma using preoperative multi-parametric magnetic resonance imaging. Neuroradiology 2019, 61, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Binder, Z.A.; Thorne, A.H.; Bakas, S.; Wileyto, E.P.; Bilello, M.; Akbari, H.; Rathore, S.; Ha, S.M.; Zhang, L.; Ferguson, C.J.; et al. Epidermal Growth Factor Receptor Extracellular Domain Mutations in Glioblastoma Present Opportunities for Clinical Imaging and Therapeutic Development. Cancer Cell 2018, 34, 163–177.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, N.Q.K.; Do, D.T.; Chiu, F.-Y.; Yapp, E.K.Y.; Yeh, H.-Y.; Chen, C.-Y. XGBoost Improves Classification of MGMT Promoter Methylation Status in IDH1 Wildtype Glioblastoma. J. Pers. Med. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.-Z.; Cui, Y.-Y.; Zhang, Z.-Z.; Ma, X.-D. The associations between preoperative conventional MRI features and genetic biomarkers status in newly diagnosed gbms: A clinical summary and prognostic analysis. Turk. Neurosurg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, S.Y.; Nam, S.J.; Chun, S.-M.; Jo, Y.; Kim, J.H. Prediction of Core Signaling Pathway by Using Diffusion- and Perfusion-based MRI Radiomics and Next-generation Sequencing in Isocitrate Dehydrogenase Wild-type Glioblastoma. Radiology 2020, 294, 388–397. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Wu, G.; Xu, G. Noninvasive Prediction of TERT Promoter Mutations in High-Grade Glioma by Radiomics Analysis Based on Multiparameter MRI. BioMed Res. Int. 2020, 2020, 3872314. [Google Scholar] [CrossRef]
- Choi, S.; Cho, H.-H.; Koo, H.; Cho, K.; Nenning, K.-H.; Langs, G.; Furtner, J.; Baumann, B.; Woehrer, A.; Cho, H.; et al. Multi-Habitat Radiomics Unravels Distinct Phenotypic Subtypes of Glioblastoma with Clinical and Genomic Significance. Cancers 2020, 12, 1707. [Google Scholar] [CrossRef]
- Beig, N.; Bera, K.; Prasanna, P.; Antunes, J.; Correa, R.; Singh, S.; Bamashmos, A.S.; Ismail, M.; Braman, N.; Verma, R.; et al. Radiogenomic-Based Survival Risk Stratification of Tumor Habitat on Gd-T1w MRI Is Associated with Biological Processes in Glioblastoma. Clin. Cancer Res. 2020, 26, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Tao, W. Identification of Novel Transcriptome Signature as a Potential Prognostic Biomarker for Anti-Angiogenic Therapy in Glioblastoma Multiforme. Cancers 2021, 13, 1013. [Google Scholar] [CrossRef]
- Beig, N.; Singh, S.; Bera, K.; Prasanna, P.; Singh, G.; Chen, J.; Bamashmos, A.S.; Barnett, A.; Hunter, K.; Statsevych, V.; et al. Sexually dimorphic radiogenomic models identify distinct imaging and biological pathways that are prognostic of overall survival in glioblastoma. Neuro-Oncology 2020, 23, 251–263. [Google Scholar] [CrossRef]
- Newlands, E.S.; Stevens, M.; Wedge, S.R.; Wheelhouse, R.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Stupp, R.; Gander, M.; Leyvraz, S.; Newlands, E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001, 2, 552–560. [Google Scholar] [CrossRef]
- Khan, I.; Waqas, M.; Shamim, M.S. Prognostic significance of IDH 1 mutation in patients with glioblastoma multiforme. JPMA J. Pak. Med. Assoc. 2017, 67, 816–817. [Google Scholar] [PubMed]
- Klughammer, J.; Kiesel, B.; Roetzer, T.; Fortelny, N.; Nemc, A.; Nenning, K.-H.; Furtner, J.; Sheffield, N.C.; Datlinger, P.; Peter, N.; et al. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat. Med. 2018, 24, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-Oncology 2015, 17, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Åkeson, P.; Vikhoff, B.; Ståhlberg, F.; Holtås, S. Brain lesion contrast in MR imaging. Dependence on field strength and concen-tration of gadodiamide injection in patients and phantoms. Acta Radiol. 1997, 38, 14–18. [Google Scholar] [CrossRef]
- Nöbauer-Huhmann, I.-M.; Ba-Ssalamah, A.; Mlynarik, V.; Barth, M.; Schöggl, A.; Heimberger, K.; Matula, C.; Fog, A.; Kaider, A.; Trattnig, S. Magnetic Resonance Imaging Contrast Enhancement of Brain Tumors at 3 Tesla Versus 1.5 Tesla. Investig. Radiol. 2002, 37, 114–119. [Google Scholar] [CrossRef]
- Taha, B.; Li, T.; Boley, D.; Chen, C.C.; Sun, J. Detection of Isocitrate Dehydrogenase Mutated Glioblastomas Through Anomaly Detection Analytics. Neurosurgery 2021, 89, 323–328. [Google Scholar] [CrossRef]
- Smedley, N.F.; El-Saden, S.; Hsu, W. Discovering and interpreting transcriptomic drivers of imaging traits using neural networks. Bioinformatics 2020, 36, 3537–3548. [Google Scholar] [CrossRef]
- Wijethilake, N.; Islam, M.; Ren, H. Radiogenomics model for overall survival prediction of glioblastoma. Med. Biol. Eng. Comput. 2020, 58, 1767–1777. [Google Scholar] [CrossRef]
- Fathi Kazerooni, A.; Akbari, H.; Shukla, G.; Badve, C.; Rudie, J.D.; Sako, C.; Rathore, S.; Bakas, S.; Pati, S.; Singh, A.; et al. Cancer Imaging Phenomics via CaPTk: Multi-Institutional Prediction of Progression-Free Survival and Pattern of Recurrence in Glioblastoma. JCO Clin. Cancer Inform. 2020, 4, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.B.-K.; Lee, G.A.; Chang, T.-H.; Huang, S.-W.; Le, N.Q.K.; Chen, Y.-C.; Kuo, D.-P.; Li, Y.-T.; Chen, C.-Y. Radiomic Immunophenotyping of GSEA-Assessed Immunophenotypes of Glioblastoma and Its Implications for Prognosis: A Feasibility Study. Cancers 2020, 12, 3039. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeng, M.; Tong, Y.; Zhang, T.; Fu, Y.; Li, H.; Zhang, Z.; Cheng, Z.; Xu, X.; Yang, R.; et al. Automatic Prediction of MGMT Status in Glioblastoma via Deep Learning-Based MR Image Analysis. BioMed Res. Int. 2020, 2020, 9258649. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).