The Advent of Omics Sciences in Clinical Trials of Motor Neuron Diseases
Abstract
:1. Introduction
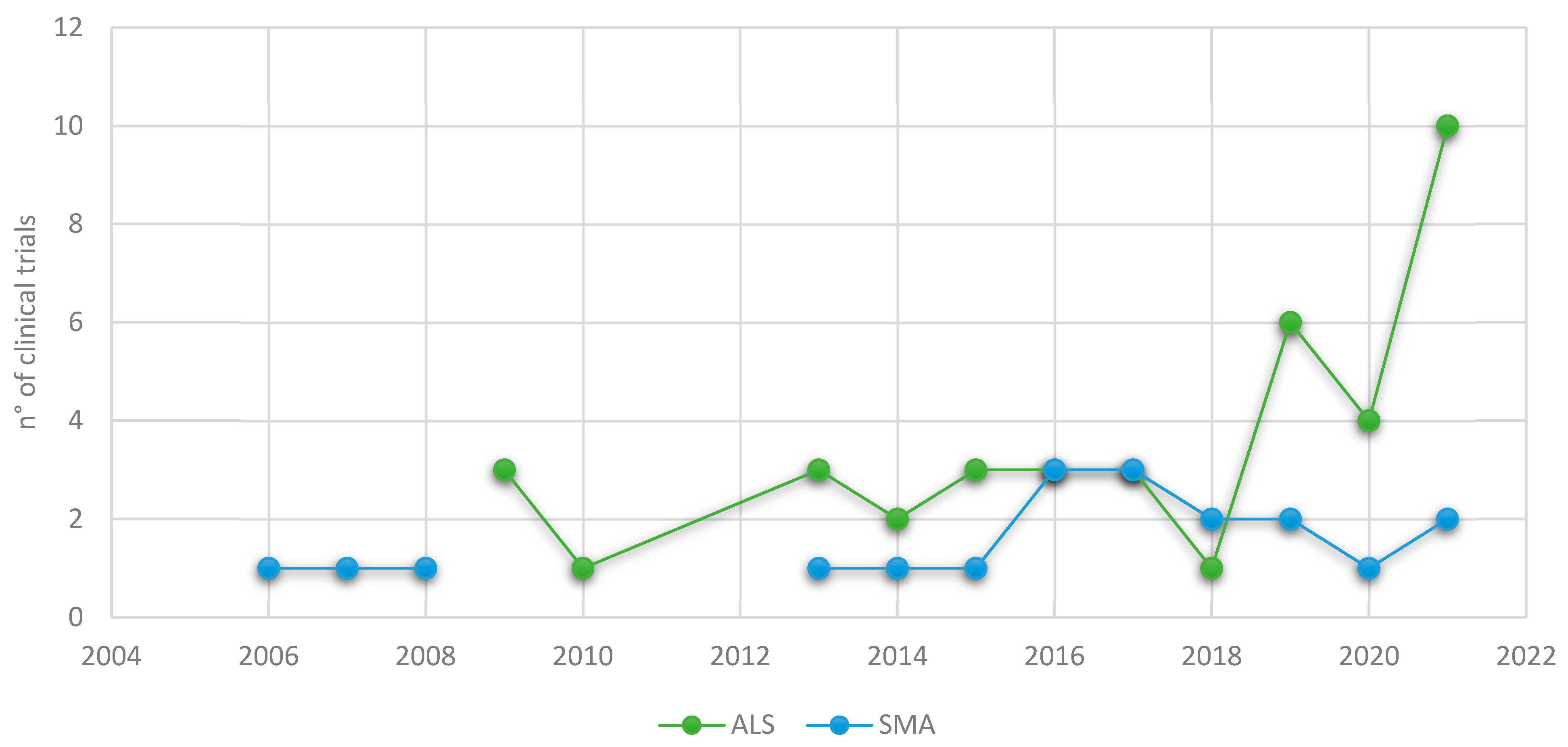
2. Clinical Trials in Motor Neuron Diseases
3. Amyotrophic Lateral Sclerosis
3.1. Drug Development
3.1.1. Omics Approach for Patients Stratifications
3.1.2. Omics Approach for Monitoring
3.1.3. Multi-Omics Approach for Both Stratification and Monitoring
3.2. Not Drug Related Clinical Trials
4. Spinal Muscular Atrophy
4.1. Drug Development
4.1.1. Omics Approach for Patient Stratifications
4.1.2. Omics Approach for Monitoring
4.1.3. Multi-Omics Approach Both for Stratification and Monitoring
4.2. Not Drug Related Clinical Trials
5. Spinal Bulbar Muscular Atrophy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strafella, C.; Caputo, V.; Galota, M.R.; Zampatti, S.; Marella, G.; Mauriello, S.; Cascella, R.; Giardina, E. Application of precision medicine in neurodegenerative diseases. Front. Neurol. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-multi-omics approach: A new frontier in cancer research. Biomed. Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, R.M.; Lipnick, S.; Bateman, J.W.; Chen, L.; Cousins, H.C.; Hubbard, E.G.; Jowett, G.; LaPointe, D.S.; McGredy, M.J.; Odonkor, M.N.; et al. Toward precision medicine for neurological and neuropsychiatric disorders. Cell Stem Cell 2018, 23, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharmadasa, T.; Matamala, J.M.; Huynh, W.; Zoing, M.C.; Kiernan, M.C. Motor neurone disease. Handb. Clin. Neurol. 2018, 159, 345–357. [Google Scholar] [CrossRef]
- Ungaro, C.; Sprovieri, T.; Morello, G.; Perrone, B.; Spampinato, A.G.; Simone, I.L.; Trojsi, F.; Monsurro, M.R.; Spataro, R.; La Bella, V.; et al. Genetic investigation of amyotrophic lateral sclerosis patients in south Italy: A two-decade analysis. Neurobiol. Aging 2021, 99, 99.e7–99.e14. [Google Scholar] [CrossRef]
- Ruffo, P.; Strafella, C.; Cascella, R.; Caputo, V.; Conforti, F.L.; Ando, S.; Giardina, E. Deregulation of ncRNA in neurodegenerative disease: Focus on circRNA, lncRNA and miRNA in amyotrophic lateral sclerosis. Front. Genet. 2021, 12, 784996. [Google Scholar] [CrossRef]
- Morello, G.; Guarnaccia, M.; Spampinato, A.G.; La Cognata, V.; D’Agata, V.; Cavallaro, S. Copy number variations in amyotrophic lateral sclerosis: Piecing the mosaic tiles together through a systems biology approach. Mol. Neurobiol. 2018, 55, 1299–1322. [Google Scholar] [CrossRef] [Green Version]
- Perrone, B.; Conforti, F.L. Common mutations of interest in the diagnosis of amyotrophic lateral sclerosis: How common are common mutations in ALS genes? Expert Rev. Mol. Diagn. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Miller, R.G.; Mitchell, J.D.; Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. 2012, 3, CD001447. [Google Scholar] [CrossRef]
- Yoshino, H. Edaravone for the treatment of amyotrophic lateral sclerosis. Expert Rev. Neurother. 2019, 19, 185–193. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Devenney, E.M.; Strikwerda-Brown, C.; Hodges, J.R.; Piguet, O.; Kiernan, M.C. Phenotypic variability in ALS-FTD and effect on survival. Neurology 2020, 94, e2005–e2013. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, J.H.; Hyman, T.; Dikic, I. Common molecular pathways in amyotrophic lateral sclerosis and frontotemporal dementia. Trends Mol. Med. 2016, 22, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Van Mossevelde, S.; van der Zee, J.; Cruts, M.; Van Broeckhoven, C. Relationship between C9orf72 repeat size and clinical phenotype. Curr. Opin. Genet. Dev. 2017, 44, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Salomone, S.; D’Agata, V.; Conforti, F.L.; Cavallaro, S. From multi-omics approaches to precision medicine in amyotrophic lateral sclerosis. Front. Neurosci. 2020, 14, 577755. [Google Scholar] [CrossRef] [PubMed]
- Morello, G.; Spampinato, A.G.; Cavallaro, S. Molecular Taxonomy of sporadic amyotrophic lateral sclerosis using disease-associated genes. Front. Neurol. 2017, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.M.; Pestronk, A.; David, W.; Rothstein, J.; Simpson, E.; Appel, S.H.; Andres, P.L.; Mahoney, K.; Allred, P.; Alexander, K.; et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: A phase 1, randomised, first-in-man study. Lancet Neurol. 2013, 12, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Atassi, N.; David, W.; Cudkowicz, M.; Schoenfeld, D. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology 2018, 90, e565–e574. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World federation of neurology research group on motor neuron, D. El Escoriall revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot. Neuron. Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Moulignier, A.; Moulonguet, A.; Pialoux, G.; Rozenbaum, W. Reversible ALS-like disorder in HIV infection. Neurology 2001, 57, 995–1001. [Google Scholar] [CrossRef]
- Kindy, M.; Lupinacci, P.; Chau, R.; Shum, T.; Ko, D. A Phase 2A randomized, double-blind, placebo-controlled pilot trial of GM604 in patients with Amyotrophic Lateral Sclerosis (ALS Protocol GALS-001) and a single compassionate patient treatment (Protocol GALS-C). F1000Res 2017, 6, 230. [Google Scholar] [CrossRef] [Green Version]
- Fournier, C.N.; Schoenfeld, D.; Berry, J.D.; Cudkowicz, M.E.; Chan, J.; Quinn, C.; Brown, R.H.; Salameh, J.S.; Tansey, M.G.; Beers, D.R.; et al. An open label study of a novel immunosuppression intervention for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front. Degener. 2018, 19, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.; Atassi, N.; Babu, S.; Barohn, R.J.; Caress, J.B.; Cudkowicz, M.E.; Evora, A.; Hawkins, G.A.; Wosiski-Kuhn, M.; Macklin, E.A.; et al. Tocilizumab is safe and tolerable and reduces C-reactive protein concentrations in the plasma and cerebrospinal fluid of ALS patients. Muscle Nerve 2021, 64, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.A.; Bailey, E.M.; Rybak, M.J. Enoxacin: A new fluoroquinolone. Clin. Pharm 1989, 8, 97–107. [Google Scholar] [PubMed]
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119. [Google Scholar] [CrossRef]
- Lange, D.J.; Shahbazi, M.; Silani, V.; Ludolph, A.C.; Weishaupt, J.H.; Ajroud-Driss, S.; Fields, K.G.; Remanan, R.; Appel, S.H.; Morelli, C.; et al. Pyrimethamine significantly lowers cerebrospinal fluid Cu/Zn superoxide dismutase in amyotrophic lateral sclerosis patients with SOD1 mutations. Ann. Neurol. 2017, 81, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlrab, J.; Stintzing, D.; Schultz, L.; Jugelt, K.; Schroeder, O.H. Influence of janus kinase inhibitors on the neuronal activity as a proof-of-concept model for itch. Ski. Pharm. Physiol. 2022, 35, 94–101. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Querin, G.; Bede, P.; El Mendili, M.M.; Li, M.; Pelegrini-Issac, M.; Rinaldi, D.; Catala, M.; Saracino, D.; Salachas, F.; Camuzat, A.; et al. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: A longitudinal neuroimaging study. Ann. Neurol. 2019, 86, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal muscular atrophy: Mutations, testing, and clinical relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Chen, T.H. New and developing therapies in spinal muscular atrophy: From genotype to phenotype to treatment and where do we stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Lowes, L.P.; Alfano, L.N.; Arnold, W.D.; Shell, R.; Prior, T.W.; McColly, M.; Lehman, K.J.; Church, K.; Sproule, D.M.; Nagendran, S.; et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr. Neurol. 2019, 98, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Pena, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol 2021, 20, 284–293. [Google Scholar] [CrossRef]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef]
- Schwartz, M.; Likhite, S.; Meyer, K. Onasemnogene abeparvovec-xioi: A gene replacement strategy for the treatment of infants diagnosed with spinal muscular atrophy. Drugs Today (Barc) 2021, 57, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Ferraiuolo, L.; Schmelzer, L.; Braun, L.; McGovern, V.; Likhite, S.; Michels, O.; Govoni, A.; Fitzgerald, J.; Morales, P.; et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: A dose-response study in mice and nonhuman primates. Mol. Ther. 2015, 23, 477–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musialek, M.W.; Rybaczek, D. Hydroxyurea—The good, the bad and the ugly. Genes 2021, 12, 1096. [Google Scholar] [CrossRef]
- Harding, A.E.; Thomas, P.K.; Baraitser, M.; Bradbury, P.G.; Morgan-Hughes, J.A.; Ponsford, J.R. X-linked recessive bulbospinal neuronopathy: A report of ten cases. J. Neurol. Neurosurg. Psychiatry 1982, 45, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Breza, M.; Koutsis, G. Kennedy’s disease (spinal and bulbar muscular atrophy): A clinically oriented review of a rare disease. J. Neurol. 2019, 266, 565–573. [Google Scholar] [CrossRef]
- Dolder, C.R. Dutasteride: A dual 5-alpha reductase inhibitor for the treatment of symptomatic benign prostatic hyperplasia. Ann. Pharm. 2006, 40, 658–665. [Google Scholar] [CrossRef]
- Fernandez-Rhodes, L.E.; Kokkinis, A.D.; White, M.J.; Watts, C.A.; Auh, S.; Jeffries, N.O.; Shrader, J.A.; Lehky, T.J.; Li, L.; Ryder, J.E.; et al. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: A randomised placebo-controlled trial. Lancet Neurol. 2011, 10, 140–147. [Google Scholar] [CrossRef] [Green Version]
| Amyotrophic Lateral Sclerosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Trials Identifier | Date | Phase | Status | Treatment | Approach | Title | State | Phase | ||
| Start | Completion | Name | Drug | |||||||
| NCT01041222 | January 2010 | January 2012 | Completed | fALS diagnosis: SOD1 gene mutation carries | ISIS 333611 | ASO designed to inhibit SOD1 expression | Genomics | A Phase 1, Double-Blind, Placebo-Controlled, Dose-Escalation Study of the Safety, Tolerability, and Pharmacokinetics of ISIS 333,611 Administered Intrathecally to Patients with Familial Amyotrophic Lateral Sclerosis Due to Superoxide Dismutase 1 Gene Mutations | United States | I |
| NCT00706147 | January 2009 | December 2014 | Completed | SOD1 genetic mutation | Arimoclomol | HSP response inductor | Genomics | Phase II/III Randomized, Placebo-Controlled Trial of Arimoclomol in SOD1 Positive Familial Amyotrophic Lateral Sclerosis (ALS). | United States | II-III |
| NCT04494256 | 28 September 2020 | Recruiting | Genetic diagnosis (SOD1, FUS, ATXN2) | BIIB105 | ASO is designed to bind ATXN2 mRNA and mediate its degradation | Genomics | A Phase 1 Multiple-Ascending-Dose Study to Assess the Safety, Tolerability, and Pharmacokinetics of BIIB105 Administered Intrathecally to Adults with Amyotrophic Lateral Sclerosis with or without Poly-CAG Expansion in the Ataxin-2 Gene. | United States | I | |
| NCT04632225 | 9 February 2021 | Active, not recruiting | ALS diagnosis according to the El Escorial Criteria | Engensis | Gene therapy using plasmid to deliver the HGF gene directly to nerve cells | Transcriptomics | A Phase 2a, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety of Engensis in Participants with Amyotrophic Lateral Sclerosis | United States | II | |
| NCT03359538 | 19 September 2017 | Active, not recruiting | ALS diagnosis according to the El Escorial Criteria | Rapamycin—Sirolimus | Immunomodulatory effects and improves protein degradation | Transcriptomics | Rapamycin (Sirolimus) Treatment for Amyotrophic Lateral Sclerosis | Italy | II | |
| NCT00875446 | 13 May 2009 | 9 September 2011 | Completed | ALS diagnosis according to the Gold Coast Criteria | GSK1223249- Ozanezumab | Monoclonal antibody targeting NOGO-A protein | Transcriptomics and metabolomics | A Single and Repeat Dose Escalation Study of the Safety, Pharmacokinetics and Pharmacodynamics of GSK1223249 in ALS Patients | United States | I |
| NCT03456882 | 18 November 2016 | 23 November 2020 | Completed | ALS diagnosis according to the El Escorial Criteria | RNS60 | Saline solution with charged oxygenated nanobubbles | Metabolomics and transcriptomics | The Effect of RNS60 on ALS Biomarkers. | Italy | II |
| NCT01854294 | August 2013 | April 2014 | Completed | ALS diagnosis according to the El Escorial Criteria | GM604 | Peptide | Transcriptomics | GM604 Phase 2A Randomized Double-blind Placebo Controlled Pilot Trial in Amyotrophic Lateral Disease (ALS) | United States | II |
| NCT03800524 | 22 February 2019 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Tauroursodeoxycholic | Antiapoptotic and ER stress response damping effects | Transcriptomics | Safety and Efficacy of Tauroursodeoxycholic (TUDCA) as add-on Treatment in Patients Affected by Amyotrophic Lateral Sclerosis (ALS) | Belgium, France and others | III | |
| NCT04505358 | 30 December 2021 | Not yet recruiting | ALS diagnosis according to the El Escorial Criteria | PU-AD— Icapamespib | Brain permeable Hsp90 protein inhibitor | Genomics and transcriptome | A Randomized, Double-blind, Placebo-controlled Pilot Study to Evaluate the Biological Activity, Safety, and Pharmacokinetics of PU-AD in Subjects with Amyotrophic Lateral Sclerosis (ALS) | II | ||
| NCT03693781 | 10 April 2019 | Active, not recruiting | ALS diagnosis according to the El Escorial Criteria | Colchicine | Transcriptomics | Colchicine for Amyotrophic Lateral Sclerosis: A Phase II, Randomized, Double Blind, Placebo Controlled, Multicenter Clinical Trial | Italy | II | ||
| NCT01884571 | October 2013 | January 2016 | Completed | ALS diagnosis according to the El Escorial Criteria | Basiliximab, Methylprednisolone, Prednisone, Tacrolimus, Mycophenolate mofetil | Immunosuppression treatment | Transcriptomics | A Novel Immunosuppression Intervention for the Treatment of Amyotrophic Lateral Sclerosis (ALS) | United States | II |
| NCT02525471 | October 2015 | 21 June 2017 | Completed | ALS diagnosis according to the El Escorial Criteria | RNS60 | Saline solution with charged oxygenated nanobubbles | Transcriptomics | A Pilot Study of RNS60 in Amyotrophic Lateral Sclerosis (ALS) | United States | I |
| NCT02469896 | November 2015 | 11 July 2018 | Completed | ALS diagnosis according to the El Escorial Criteria | Tocilizumab | ASO designed to inhibit interleukin 6 (IL-6) | Transcriptomics | A Phase 2 Randomized, Placebo Controlled Trial of Tocilizumab in ALS Subjects | United States | II |
| NCT05193994 | 18 January 2022 | Not yet recruiting | ALS diagnosis according to the Gold Coast Criteria | Triumeq | Combined treatment of: dolutegravir, abacavir, lamivudine | Genomics and transcriptomics | Randomised Double-Blind Placebo-Controlled Phase 3 Trial of Triumeq in Amyotrophic Lateral Sclerosis | Australia | III | |
| NCT04788745 | 29 June 2021 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Trimetazidine Dihydrochloride | Transcriptomics | Targeting Metabolic Flexibility in ALS (MetFlex); Safety and Tolerability of Trimetazidine for the Treatment of ALS | Australia, United Kindom and others | II | ||
| NCT04840823 | 26 March 2021 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Enoxacin | Quinolone/fluoroquinolone antibiotic | Transcriptomics | A Randomized, Double-blind, Parallel Group, Single Centre, Phase 1b/2 Study to Assess the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Three Orally Administered Doses of Enoxacin (200 mg Twice Daily, 400 mg Twice Daily and 600 mg Twice Daily) in Adults with Amyotrophic Lateral Sclerosis | Canada | I-II | |
| NCT02437110 | 1 April 2019 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Darunavir, Ritonavir, Dolutegravir, Tenofovir alafenamide (TAF) | Darunavir and Ritonavir = protease inhibitor; Dolutegravir = integrase inhibitor; TAF = nucleoside reverse transcriptase inhibitor | Transcriptomics | HERV-K Suppression Using Antiretroviral Therapy in Volunteers with Amyotrophic Lateral Sclerosis (ALS) | United States | I | |
| NCT04066244 | 30 December 2019 | Recruiting | ALS diagnosis according to the El Escorial Criteria | BLZ945 | CSF-1 Inhibitor | Genomics Metabolomics and transcriptomics | An Open-label, Adaptive Design Study in Patients with Amyotrophic Lateral Sclerosis (ALS) to Characterize Safety, Tolerability and Brain Microglia Response, as Measured by TSPO Binding, Following Multiple Doses of BLZ945 Using Positron Emission Tomography (PET) With the Radioligand [11C]-PBR28 | United States | II | |
| NCT03626012 | 10 September 2018 | 17 November 2021 | Completed | C9Orf72 genetic mutation | BIIB078 | ASO designed to target C9Orf72 mRNA | Genomics and metabolomics | A Phase 1 Multiple-Ascending-Dose Study to Assess the Safety, Tolerability, and Pharmacokinetics of BIIB078 Administered Intrathecally to Adults with C9ORF72-Associated Amyotrophic Lateral Sclerosis | United States, Belgium and others | I |
| NCT04288856 | 28 April 2020 | Active, not recruiting | C9Orf72 genetic mutation | BIIB078 | ASO designed to target C9Orf72 mRNA | Genomics and metabolomics | An Extension Study to Assess the Long-Term Safety, Tolerability, Pharmacokinetics, and Effect on Disease Progression of BIIB078 Administered to Previously Treated Adults with C9ORF72-Associated Amyotrophic Lateral Sclerosis | United States | I | |
| NCT05163886 | 23 December 2021 | Recruiting | C9Orf72 genetic mutation | LAM-002A | PIKfyve kinase inhibitor that activates the transcription factor EB (TFEB) | Genomics and transcriptome | A Phase IIa Trial to Evaluate the Safety, Tolerability, and Biological Activity of LAM-002A (Apilimod Dimesylate Capsules) in C9ORF72-Associated Amyotrophic Lateral Sclerosis | United States | II | |
| NCT05053035 | 2 September 2021 | Recruiting | C9Orf72 genetic mutation | LAM-002A | PIKfyve kinase inhibitor that activates the transcription factor EB (TFEB) | Genomics and transcriptome | A Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of AL001 in C9orf72-Associated Amyotrophic Lateral Sclerosis | United States | II | |
| NCT04993755 | 1 October 2021 | Recruiting | C9Orf72 genetic mutation | TPN-101, censavudine | Inhibitor of the reverse transcriptase enzyme | Genomics and metabolomics | A Phase 2a Study of TPN-101 in Patients with Amyotrophic Lateral Sclerosis (ALS) and/or Frontotemporal Dementia (FTD) Associated with Hexanucleotide Repeat Expansion in the C9Orf72 Gene (C9ORF72 ALS/FTD) | United States | II | |
| NCT04931862 | 28 June 2021 | Recruiting | C9Orf72 genetic mutation | WVE-004 | ASO is designed to mediate the degradation of C9ORF72 mRNAs | Genomics | A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 1b/2a Study of WVE-004 Administered Intrathecally to Patients with C9orf72-associated Amyotrophic Lateral Sclerosis (ALS) or Frontotemporal Dementia (FTD) | Australia | I-II | |
| NCT02623699 | 20 January 2016 | 16 July 2021 | Completed | SOD1 genetic mutation | BIIB067—Tofersen | ASO designed to degrade SOD1 mRNA to prevent protein synthesis and reduce levels of harmful proteins. | Genomics and metabolomics | A Study to Evaluate the Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of BIIB067 Administered to Adult Subjects with Amyotrophic Lateral Sclerosis and Confirmed Superoxide Dismutase 1 Mutation | United States | III |
| NCT03070119 | 8 March 2017 | Active, not recruiting | SOD1 genetic mutation | BIIB067—Tofersen | ASO designed to degrade SOD1 mRNA to prevent protein synthesis and reduce levels of harmful proteins. | Genomics | An Extension Study to Assess the Long-Term Safety, Tolerability, Pharmacokinetics, and Effect on Disease Progression of BIIB067 Administered to Previously Treated Adults with Amyotrophic Lateral Sclerosis Caused by Superoxide Dismutase 1 Mutation | United States | III | |
| NCT04856982 | 17 May 2021 | Recruiting | SOD1 genetic mutation | BIIB067—Tofersen | ASO designed to degrade SOD1 mRNA to prevent protein synthesis and reduce levels of harmful proteins. | Genomics and metabolomics | A Phase 3 Randomized, Placebo-Controlled Trial with a Longitudinal Natural History Run-In and Open-Label Extension to Evaluate BIIB067 Initiated in Clinically Presymptomatic Adults with a Confirmed Superoxide Dismutase 1 Mutation | United States | III | |
| NCT01083667 | November 2009 | December 2014 | Completed | SOD1 genetic mutation | Pyrimethamine – Daraprim | Genomics and metabolomics | Phase I/II Study of SOD1 Inhibition by Pyrimethamine in Familial ALS | United States, Germany and others | I-II | |
| NCT04768972 | 14 June 2021 | Recruiting | FUS genetic mutation | ION363—JaciFUS en | ASO designed to reduce the production of a mutated neurotoxic form of the FUS protein | Genomics and metabolomics | A Phase 1–3 Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Intrathecally Administered ION363 in Amyotrophic Lateral Sclerosis Patients with FUS ed in Sarcoma Mutations (FUS -ALS) | United States | III | |
| NCT03707795 | 21 August 2017 | 10 January 2019 | Completed | FUS genetic mutation and fALS diagnosis | Betamethasone | Corticosteroid, reducing inflammation and changing the body’s immune response | Genomics and metabolomics | Treatment of FUS -Related ALS With Betamethasone—The TRANSLATE Study | United States | Early I |
| NCT05189106 | 1 February 2022 | Not yet recruiting | ALS diagnosis according to the El Escorial Criteria | Baricitinib— Olumiant | Immunosuppressant—JAK inhibitors | Genomics, proteomics and metabolomics | Neurodegenerative Alzheimer’s Disease and Amyotrophic Lateral Sclerosis (NADALS) Basket Proof of Concept Trial Including Asymptomatic Individuals Using Baricitinib | United States | I-II | |
| NCT04220021 | 10 January 2020 | Recruiting | C9Orf72 genetic mutation | Metformin | Genomics and metabolomics | A Single-Center, Open Label Study to Assess the Safety and Tolerability of Metformin in Subjects with C9orf72 Amyotrophic Lateral Sclerosis Over 24 Weeks of Treatment | United States | II | ||
| NCT02590276 | 8 October 2015 | 27 October 2020 | Completed | C9Orf72 genetic mutation | Genomics, Metabolomics and transcriptomics | Predict to Prevent Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis | France | Not Applicable | ||
| NCT03984708 | 27 January 2020 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Metabolomics, lipidpomics and transcriptomics | New Therapeutic Strategy in ALS Based on Metabolic Status and Associated Metabolic Pathways. | France | Not Applicable | |||
| NCT01984957 | January 2013 | January 2015 | Completed | ALS diagnosis according to the El Escorial Criteria | Transcriptomics | Differential Study of Muscle Transcriptome in Patients with Neuromuscular Disease and Control Subjects | France | Not Applicable | ||
| NCT02670226 | 29 March 2016 | 9 December 2019 | Completed | ALS diagnosis according to the El Escorial Criteria | Metabolomics and transcriptomics | Metabolomics and Transcriptomics Approaches to Identify Muscular Biomarkers in Amyotrophic Lateral Sclerosis | France | Not Applicable | ||
| NCT03851302 | 28 October 2019 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Transcriptomics | Effects of Remote Ischemic Conditioning on Hand Use in Individuals with Spinal Cord Injury and Amyotrophic Lateral Sclerosis: A Preliminary Study | United States | Not Applicable | |||
| NCT03618966 | 1 November 2014 | 1 May 2016 | Completed | ALS diagnosis according to the El Escorial Criteria | Transcriptomics | Neuromuscular Magnetic Stimulation Counteracts Muscle Decline in ALS Patients | II | |||
| NCT03367650 | 13 May 2014 | Recruiting | ALS diagnosis according to the El Escorial Criteria | Genomics | Epidemiology and Genetics of the Amyotrophic Lateral Sclerosis in the French West Indies | France | Not Applicable | |||
| NCT03573466 | 10 April 2019 | Active, not recruiting | SOD1- C9Orf72 genetic mutation | Genomics | Presymptomatic Neuromuscular Junction Defects and Compensatory Mechanisms in Amyotrophic Lateral Sclerosis (ALS) | France | Not Applicable | |||
| Spinal Muscular Atrophy and Spinal-Bulbar Muscular Atrophy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Trials Identifier | Date | Phase | Status | Treatment | Approach | Title | State | Phase | ||
| Start | Completion | Name | Drug | |||||||
| NCT02122952 | 5 May 2014 | 15 December 2017 | Completed | SMN1–SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Phase I Gene Transfer Clinical Trial for Spinal Muscular Atrophy Type 1 Delivering AVXS-101 | United States | I |
| NCT03306277 | 24 October 2017 | 12 November 2019 | Completed | SMN1–SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Phase 3, Open-Label, Single-Arm, Single-Dose Gene Replacement Therapy Clinical Trial for Patients with Spinal Muscular Atrophy Type 1 With One or Two SMN2 Copies Delivering AVXS-101 by Intravenous InFUS ion | United States | III |
| NCT03461289 | 16 August 2018 | 11 September 2020 | Completed | SMN1–SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Phase 3, Open-Label, Single-Arm, Single-Dose Gene Replacement Therapy Clinical Trial for Patients with Spinal Muscular Atrophy Type 1 With One or Two SMN2 Copies Delivering AVXS-101 by Intravenous InFUS ion | Belgium, France and others | III |
| NCT03837184 | 31 May 2019 | 29 June 2021 | Completed | SMN1–SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Phase 3, Open-Label, Single-Arm, Single-Dose Gene Replacement Therapy Clinical Trial for Patients with Spinal Muscular Atrophy Type 1 With One or Two SMN2 Copies Delivering AVXS-101 by Intravenous InFUS ion | Japan, Korea and Taiwan | III |
| NCT03381729 | 14 December 2017 | 18 November 2021 | Completed | SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Phase I, Open-Label, Dose Comparison Study of AVXS-101 for Sitting but Non-ambulatory Patients with Spinal Muscular Atrophy | United States | I |
| NCT03505099 | 2 April 2018 | 15 June 2021 | Completed | SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | A Global Study of a Single, One-Time Dose of AVXS-101 Delivered to Infants with Genetically Diagnosed and Pre-symptomatic Spinal Muscular Atrophy with Multiple Copies of SMN2 | United States | III |
| NCT04042025 | 10 February 2020 | Enrolling by invitation | SMA clinical and genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | A Long-term Follow-up Study of Patients in the Clinical Trials for Spinal Muscular Atrophy Receiving AVXS-101 | United States | IV | |
| NCT02628743 | 20 January 2016 | 18 December 2018 | Completed | SMN2 genetic diagnosis | AVXS-101—Onasemnogene Abeparvovec | SMN gene therapy | Genomics | Multicenter, Open-Label, Single-Arm Study to Evaluate Long-Term Safety, Tolerability, and Effectiveness of 10 mg/kg BID Olesoxime in Patients with Spinal Muscular Atrophy | Belgium, France and others. | II |
| NCT04576494 | 24 January 2022 | Recruiting | SMA genetic diagnosis | Nusinersen—Spinraza | ASO designed to allow the SMN2 gene to produce the full-length protein that can function normally | Genomics | Study of the Functional Effects of Nusinersen in 5q-spinal Muscular Amyotrophy Adults (SMA Type 2 or 3 Forms): a Multicenter Single-case Experimental Design in Multiple Baselines Across Subjects, Randomized, Single-blinded Evaluation | France | Not Applicable | |
| NCT04851873 | 8 September 2021 | Recruiting | SMN1–SMN2 genetic diagnosis | OAV101 (AVXS-101) | SMN gene therapy | Genomics | A Phase IIIb, Open-label, Single-arm, Single-dose, Multicenter Study to Evaluate the Safety, Tolerability and Efficacy of Gene Replacement Therapy with Intravenous OAV101 (AVXS-101) in Pediatric Patients With Spinal Muscular Atrophy (SMA) | Australia | III | |
| NCT05089656 | 2 February 2022 | Recruiting | SMN1–SMN2 genetic diagnosis | OAV101 (AVXS-101) | SMN gene therapy | Genomics | A Randomized, Sham-controlled, Double-blind Study to Evaluate the Efficacy and Safety of Intrathecal OAV101 in Patients Type 2 Spinal Muscular Atrophy (SMA) Who Are ≥ 2 to < 18 Years of Age, Treatment Naive, Sitting, and Never Ambulatory | United States | III | |
| NCT03779334 | 7 August 2019 | Active, not recruiting | SMN2 genetic diagnosis | RO7034067- Risdiplam | A splice modifier of the pre-mRNA of the SMN2 gene | Genomics | An Open-Label Study of Risdiplam in Infants with Genetically Diagnosed and Presymptomatic Spinal Muscular Atrophy | United States | II | |
| NCT01671384 | August 2013 | Unknown | SMN1 genetic diagnosis | Valproate and levocarnitine | Valproic acid (VPA) = a histone deacetylase inhibitor (HDAC) | Genomics | Randomized Placebo Controlled Trial of Valproate and Levocarnitine in Children with Spinal Muscular Atrophy Aged 2–15 Years | India | III | |
| NCT00439569 | January 2008 | August 2008 | Terminated | SMA clinical diagnosis | Sodium phenylbutyrate | Histone deacetylase inhibitor and a chemical chaperone | Transcriptomics | Phase I/IIa Clinical Trial of Sodium Phenylbutyrate in Pediatric Subjects with Type II/III Spinal Muscular Atrophy | United States | I-II |
| NCT03032172 | 3 March 2017 | Active, not recruiting | SMN2 genetic diagnosis | RO7034067- Risdiplam | A splice modifier of the pre-mRNA of the SMN2 gene | Transcriptomics | An Open-Label Study to Investigate the Safety, Tolerability, and Pharmacokinetics/Pharmacodynamics of Risdiplam (RO7034067) in Adult and Pediatric Patients with Spinal Muscular Atrophy | United States | II | |
| NCT02908685 | 20 October 2016 | Active, not recruiting | SMA genetic diagnosis | RO7034067- Risdiplam | A splice modifier of the pre-mRNA of the SMN2 gene | Genomics and transcriptomics | A Two Part Seamless, Multi-Center Randomized, Placebo-Controlled, Double-Blind Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of Risdiplam (RO7034067) in Type 2 and 3 Spinal Muscular Atrophy Patients | United States | II-III | |
| NCT02913482 | 23 December 2016 | Active, not recruiting | SMA genetic diagnosis | RO7034067- Risdiplam | A splice modifier of the pre-mRNA of the SMN2 gene | Genomics and transcriptomics | A Two Part Seamless, Open-label, Multicenter Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of Risdiplam (RO7034067) in Infants with Type 1 Spinal Muscular Atrophy | United States | II-III | |
| NCT00485511 | June 2007 | June 2009 | Completed | SMN1–SMN2 genetic diagnosis | Hydroxyurea | SMN2 transcription pattern modified | Genomics and transcriptomics | A Randomized, Double-Blind, Placebo-Controlled Trial of Hydroxyurea in Spinal Muscular Atrophy | Taiwan | II-III |
| NCT02550691 | 15 December 2015 | 4 July 2016 | Terminated | SMN1–SMN2 genetic diagnosis | Genomics | Identification of a Biomarker Associated with Cis-duplication of the SMN1 Gene Aiming at Improving the Genetic Counseling in Spinal Muscular Atrophy Families | France | Not Applicable | ||
| NCT04833348 | August 2021 | Not yet recruiting | SMN1 genetic diagnosis | Genomics | Quantification of Motor Function in Infants with Spinal Muscular Atrophy Treated with Innovative Therapies, IMUSMA Project | France | Not Applicable | |||
| NCT00303446 | March 2006 | December 2009 | Completed | SBMA genetically confirmed | Dutasteride | Inhibitor of 5-alpha-reductase I and II | Genomics | Dutasteride to Treat Spinal and Bulbar Muscular Atrophy (SBMA) | United States | II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffo, P.; Cavallaro, S.; Conforti, F.L. The Advent of Omics Sciences in Clinical Trials of Motor Neuron Diseases. J. Pers. Med. 2022, 12, 758. https://doi.org/10.3390/jpm12050758
Ruffo P, Cavallaro S, Conforti FL. The Advent of Omics Sciences in Clinical Trials of Motor Neuron Diseases. Journal of Personalized Medicine. 2022; 12(5):758. https://doi.org/10.3390/jpm12050758
Chicago/Turabian StyleRuffo, Paola, Sebastiano Cavallaro, and Francesca Luisa Conforti. 2022. "The Advent of Omics Sciences in Clinical Trials of Motor Neuron Diseases" Journal of Personalized Medicine 12, no. 5: 758. https://doi.org/10.3390/jpm12050758
APA StyleRuffo, P., Cavallaro, S., & Conforti, F. L. (2022). The Advent of Omics Sciences in Clinical Trials of Motor Neuron Diseases. Journal of Personalized Medicine, 12(5), 758. https://doi.org/10.3390/jpm12050758









