Association between Smoking Status and Incident Non-Cystic Fibrosis Bronchiectasis in Young Adults: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Methods
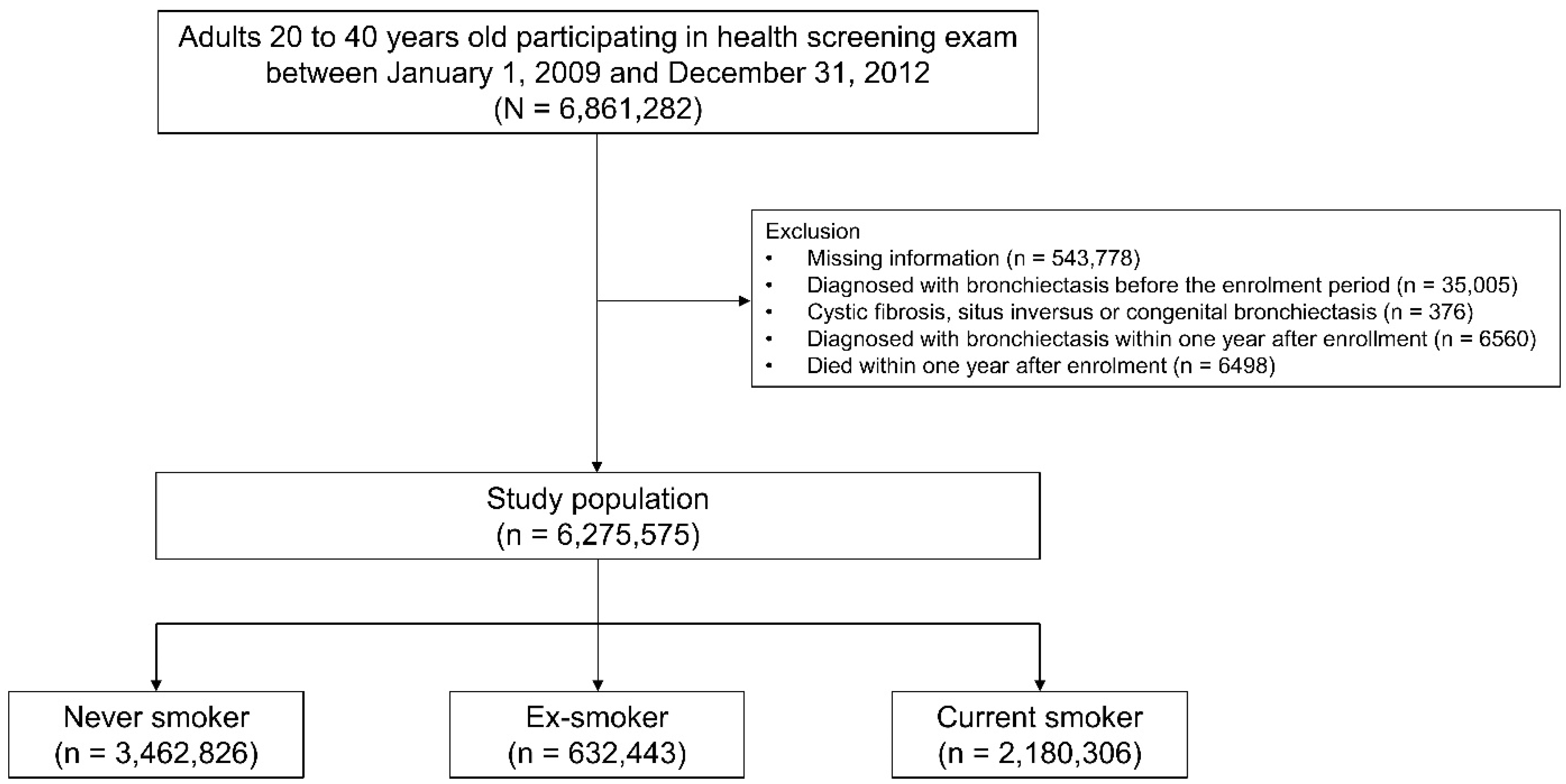
2.1. Study Population
2.2. Exposure: Smoking Status
2.3. Outcome: Incident Bronchiectasis
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
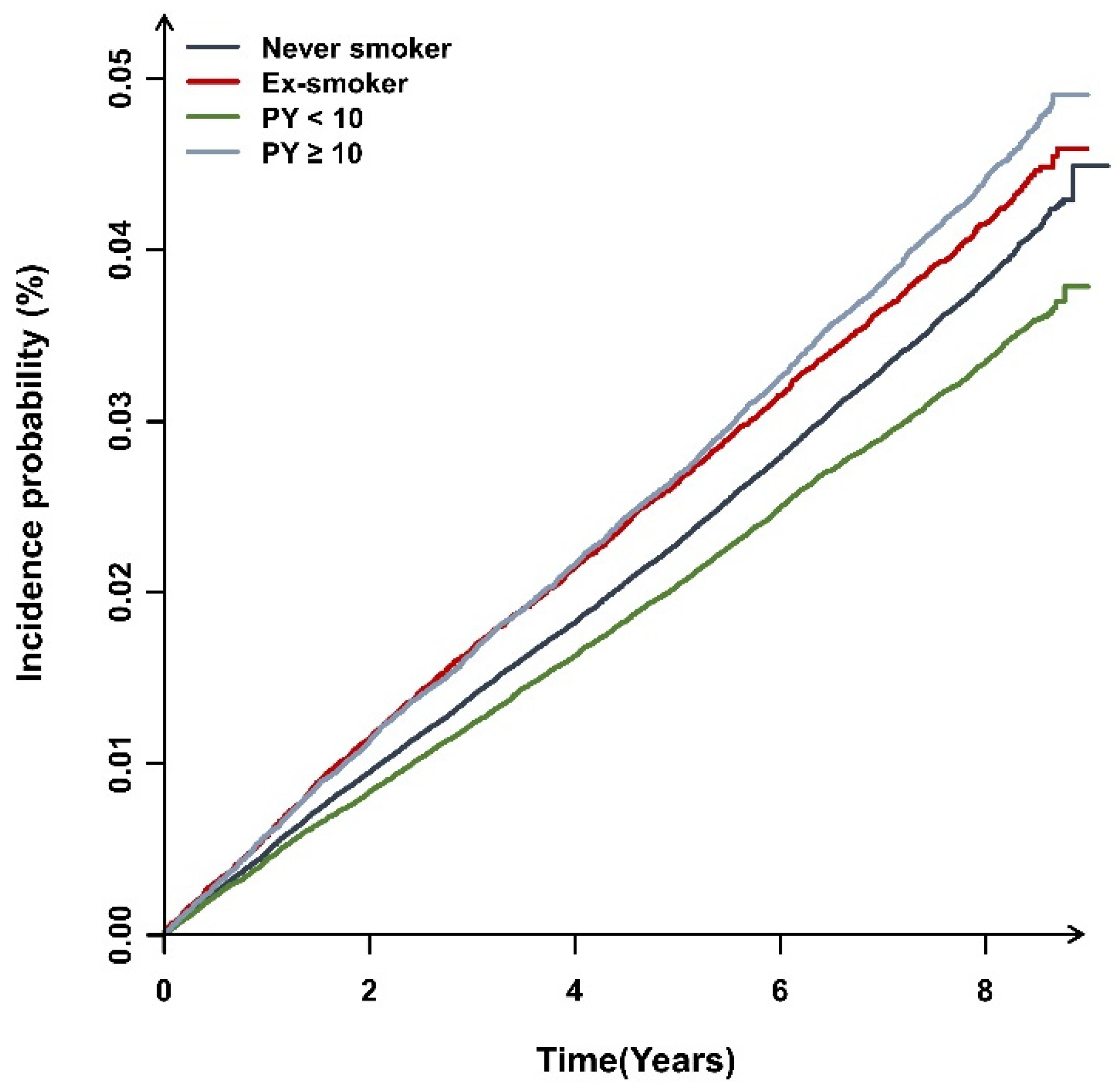
3.2. The Incidence of Bronchiectasis and Smoking
3.3. Effect of Comorbid Profiles on the Relationship between Smoking Status and Incident Bronchiectasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imam, J.S.; Duarte, A.G. Non-CF bronchiectasis: Orphan disease no longer. Respir. Med. 2020, 166, 105940. [Google Scholar] [CrossRef]
- King, P.T. The pathophysiology of bronchiectasis. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Millett, E.R.; Joshi, M.; Navaratnam, V.; Thomas, S.L.; Hurst, J.R.; Smeeth, L.; Brown, J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016, 47, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Choi, H.; Lim, J.H.; Park, H.Y.; Kang, D.; Cho, J.; Lee, J.S.; Lee, S.W.; Oh, Y.M.; Moon, J.Y.; et al. The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: A national database study in Korea. Ann. Transl. Med. 2019, 7, 770. [Google Scholar] [CrossRef]
- Choi, H.; Yang, B.; Kim, Y.J.; Sin, S.; Jo, Y.S.; Kim, Y.; Park, H.Y.; Ra, S.W.; Oh, Y.M.; Chung, S.J.; et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci. Rep. 2021, 11, 7126. [Google Scholar] [CrossRef]
- Diel, R.; Chalmers, J.D.; Rabe, K.F.; Nienhaus, A.; Loddenkemper, R.; Ringshausen, F.C. Economic burden of bronchiectasis in Germany. Eur. Respir. J. 2019, 53, 1802033. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Hill, T.A.; Sullivan, L.A.; Chalmers, D.J.; De Soyza, A.; Stuart Elborn, J.; Andres Floto, R.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, S.C.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, H.; Chalmers, J.D.; Dhar, R.; Nguyen, T.Q.; Visser, S.K.; Morgan, L.C.; Oh, Y.-M. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021, 26, 619–621. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef]
- Gupta, N.; Malhotra, N.; Ish, P. GOLD 2021 guidelines for COPD—What’s new and why. Adv. Respir. Med. 2021, 89, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Muñoz-Torrico, M.; Duarte, R.; Galvão, T.; Bonini, E.H.; Arbex, F.F.; Arbex, M.A.; Augusto, V.M.; Rabahi, M.F.; Mello, F.C.Q. Risk factors for tuberculosis: Diabetes, smoking, alcohol use, and the use of other drugs. J. Bras. Pneumol. Publ. Soc. Bras. Pneumol. Tisilogia 2018, 44, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, L.A.; Ernster, V.L.; Warner, K.E.; Abbotts, J.; Laszlo, J. Smoking and lung cancer: An overview. Cancer Res. 1984, 44, 5940–5958. [Google Scholar] [PubMed]
- Singleton, R.; Morris, A.; Redding, G.; Poll, J.; Holck, P.; Martinez, P.; Kruse, D.; Bulkow, L.R.; Petersen, K.M.; Lewis, C. Bronchiectasis in Alaska Native children: Causes and clinical courses. Pediatric Pulmonol. 2000, 29, 182–187. [Google Scholar] [CrossRef]
- Edwards, E.A.; Asher, M.I.; Byrnes, C.A. Paediatric bronchiectasis in the twenty-first century: Experience of a tertiary children’s hospital in New Zealand. J. Paediatr. Child Health 2003, 39, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Kovesi, T.A. Bronchiectasis in children from Qikiqtani (Baffin) Region, Nunavut, Canada. Ann. Am. Thorac. Soc. 2015, 12, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.Y.; Kwon, H.S.; Cha, B.S.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Lee, B.W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef]
- Choi, H.; Yang, B.; Nam, H.; Kyoung, D.-S.; Sim, Y.S.; Park, H.Y.; Lee, J.S.; Lee, S.W.; Oh, Y.-M.; Ra, S.W.; et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 2019, 54, 1900194. [Google Scholar] [CrossRef]
- Yang, B.; Han, K.; Kim, S.H.; Lee, D.H.; Park, S.H.; Yoo, J.E.; Shin, D.W.; Choi, H.; Lee, H. Being Underweight Increases the Risk of Non-Cystic Fibrosis Bronchiectasis in the Young Population: A Nationwide Population-Based Study. Nutrients 2021, 13, 3206. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.; Ryu, J.; Chung, S.J.; Park, D.W.; Sohn, J.W.; Yoon, H.J.; Kim, S.H. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: A nationwide population study. Ther. Adv. Respir. Dis. 2020, 14, 1753466620963030. [Google Scholar] [CrossRef]
- Yang, B.; Lee, D.-H.; Han, K.; Choi, H.; Kang, H.K.; Shin, D.W.; Lee, H. Female Reproductive Factors and the Risk of Bronchiectasis: A Nationwide Population-Based Longitudinal Study. Biomedicines 2022, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, W.Y.; Kang, J.H.; Kang, J.H.; Kim, B.T.; Kim, S.M.; Kim, E.M.; Suh, S.H.; Shin, H.J.; Lee, K.R.; et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. 2014, 29, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.E.; Kim, D.; Han, K.; Rhee, S.Y.; Shin, D.W.; Lee, H. Diabetes Status and Association With Risk of Tuberculosis Among Korean Adults. JAMA Netw. Open 2021, 4, e2126099. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Han, K.; Yang, B.; Shin, D.W.; Sohn, J.W.; Lee, H. Female reproductive factors and incidence of non-tuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, ciac134. [Google Scholar] [CrossRef]
- Sin, S.; Yun, S.Y.; Kim, J.M.; Park, C.M.; Cho, J.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, S.M.; Yoo, C.G.; et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir. Res. 2019, 20, 271. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Nawrot, T.S.; Ruttens, D.; Seys, S.; Dupont, L.J. Mortality in non-cystic fibrosis bronchiectasis: A prospective cohort analysis. Respir. Med. 2014, 108, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Flume, P.A.; Chalmers, J.D.; Olivier, K.N. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018, 392, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Martínez-García, M.; de la Rosa-Carrillo, D.; Soler-Cataluña, J.J.; Catalan-Serra, P.; Ballester, M.; Roca Vanaclocha, Y.; Agramunt, M.; Ballestin, J.; Garcia-Ortega, A.; Oscullo, G.; et al. Bronchial Infection and Temporal Evolution of Bronchiectasis in Patients With Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 403–410. [Google Scholar] [CrossRef]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef]
- Marcy, T.W.; Merrill, W.W. Cigarette smoking and respiratory tract infection. Clin. Chest Med. 1987, 8, 381–391. [Google Scholar] [CrossRef]
- Ishii, Y. Smoking and respiratory diseases. Nihon Rinsho. Jpn. J. Clin. Med. 2013, 71, 416–420. [Google Scholar]
- Gramegna, A.; Amati, F.; Terranova, L.; Sotgiu, G.; Tarsia, P.; Miglietta, D.; Calderazzo, M.A.; Aliberti, S.; Blasi, F. Neutrophil elastase in bronchiectasis. Respir. Res. 2017, 18, 211. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Wagner, B.D.; Anthony, M.M.; Emmett, P.; Zemanick, E.T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Finch, S. Sputum colour in non-CF bronchiectasis: The original neutrophil biomarker. Respirology 2014, 19, 153–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaillac, C.; Yong, V.F.L.; Jaggi, T.K.; Soh, M.M.; Chotirmall, S.H. Gender differences in bronchiectasis: A real issue? Breathe 2018, 14, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Stabile, L.P.; Siegfried, J.M. Estrogen receptor pathways in lung cancer. Curr. Oncol. Rep. 2004, 6, 259–267. [Google Scholar] [CrossRef]
- Tam, A.; Churg, A.; Wright, J.L.; Zhou, S.; Kirby, M.; Coxson, H.O.; Lam, S.; Man, S.F.; Sin, D.D. Sex Differences in Airway Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 825–834. [Google Scholar] [CrossRef]
- Wang, R.D.; Tai, H.; Xie, C.; Wang, X.; Wright, J.L.; Churg, A. Cigarette smoke produces airway wall remodeling in rat tracheal explants. Am. J. Respir. Crit. Care Med. 2003, 168, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, P. Obesity and lung inflammation. J. Appl. Physiol. 2010, 108, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Alexeeff, S.E.; Litonjua, A.A.; Suh, H.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Ozone exposure and lung function: Effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest 2007, 132, 1890–1897. [Google Scholar] [CrossRef]
- Bellmeyer, A.; Martino, J.M.; Chandel, N.S.; Scott Budinger, G.R.; Dean, D.A.; Mutlu, G.M. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2007, 175, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, W.D.; Hazucha, M.J.; Folinsbee, L.J.; Bromberg, P.A.; Kissling, G.E.; London, S.J. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal. Toxicol. 2007, 19, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Vaart, H.; Postma, D.S.; Timens, W.; Ten Hacken, N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Smoking Status | |||||
|---|---|---|---|---|---|
| Total (N = 6,275,575) | Never Smoker (n = 3,462,826) | Ex-Smoker (n = 632,443) | Current Smoker (n = 2,180,306) | p-Value | |
| Age, years | 30.8 ± 4.9 | 30.1 ± 5.1 | 32.5 ± 4.64 | 31.4 ± 4.74 | <0.001 |
| <30 | 2,655,026 (42.4) | 1,675,479 (48.4) | 175,548 (27.7) | 803,999 (36.8) | <0.001 |
| ≥30 | 3,620,549 (57.6) | 1,787,347 (51.6) | 456,895 (72.3) | 1,376,307 (63.2) | |
| Sex | <0.001 | ||||
| Male | 3,712,379 (59.2) | 1,135,390 (32.8) | 546,321 (86.4) | 2,030,668 (93.2) | |
| Female | 2,563,196 (40.8) | 2,327,436 (67.2) | 86,122 (13.6) | 149,638 (6.8) | |
| BMI (kg/m2) | 23.0 ± 3.6 | 22.2 ± 3.5 | 24.0 ± 3.3 | 24.0 ± 3.6 | <0.001 |
| <18.5 kg/m2 | 476,686 (7.6) | 371,396 (10.7) | 19,801 (3.1) | 85,489 (3.9) | <0.001 |
| 18.5–22.9 kg/m2 | 2,931,905 (46.7) | 1,885,080 (54.4) | 227,953 (36.0) | 818,872 (37.5) | |
| 23–24.9 kg/m2 | 1,203,883 (19.2) | 557,097 (16.1) | 158,130 (25.0) | 488,656 (22.4) | |
| ≥25 kg/m2 | 1,663,101 (26.5) | 649,253 (18.8) | 226,559 (35.8) | 787,289 (36.1) | |
| Alcohol consumption | <0.001 | ||||
| None | 2,367,631 (37.7) | 1,811,004 (52.3) | 141,565 (22.4) | 415,062 (19.1) | |
| Mild | 3,353,737 (53.5) | 1,557,776 (45.0) | 408,841 (64.6) | 1,387,120 (63.6) | |
| Heavy | 554,207 (8.8) | 94,046(2.7) | 82,037 (13.0) | 378,124 (17.3) | |
| Regular exercise | <0.001 | ||||
| No | 5,470,001 (87.2) | 3,069,862 (88.7) | 518,788 (82.0) | 1,881,351 (86.3) | |
| Yes | 805,574 (12.8) | 392,964 (11.3) | 113,655 (18.0) | 298,955 (13.7) | |
| Low income | <0.001 | ||||
| No | 5,276,741 (84.1) | 2,812,455 (81.2) | 567,943 (89.8) | 1,896,343 (87.0) | |
| Yes | 998,834 (15.9) | 650,371 (18.8) | 64,500 (10.2) | 283,963 (13.0) | |
| Residence | <0.001 | ||||
| Rural | 3,276,315 (52.2) | 1,767,983 (51.1) | 330,394 (52.2) | 1,177,938 (54.0) | |
| Urban | 2,999,260 (47.8) | 1,694,843 (48.9) | 302,049 (47.8) | 1,002,368 (46.0) | |
| Number of hospital visits | 3.6 ± 6.5 | 3.9 ± 6.9 | 4.2 ± 7.2 | 2.9 ± 5.7 | <0.001 |
| Admission | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | <0.001 |
| Outpatient | 3.6 ± 6.5 | 3.9 ± 6.8 | 4.1 ± 7.1 | 2.9 ± 5.6 | <0.001 |
| Comorbidities | |||||
| DM | 122,007 (1.9) | 44,005 (1.2) | 15,258 (2.4) | 62,744 (2.9) | <0.001 |
| CKD or ESRD | 2317 (0.1) | 1280 (0.1) | 483 (0.1) | 554 (0.1) | <0.001 |
| GERD | 674,566 (10.8) | 414,329 (11.9) | 74,335 (11.7) | 185,902 (8.5) | <0.001 |
| Respiratory disease | 328,703 (5.3) | 208,670 (6.1) | 34,419 (5.5) | 85,614 (4.0) | <0.001 |
| Asthma | 326,557 (5.2) | 207,418 (6.0) | 34,133 (5.4) | 85,006 (3.9) | <0.001 |
| TB | 2418 (0.1) | 1437 (0.1) | 320 (0.1) | 661 (0.1) | <0.001 |
| NTM disease | 104 (<0.1) | 63 (<0.1) | 17 (<0.1) | 24 (<0.1) | 0.013 |
| Others | |||||
| Connective tissue disease | 44,670 (0.7) | 27,211 (0.8) | 4878 (0.8) | 12,581 (0.6) | <0.001 |
| Solid cancer | 12,563 (0.2) | 9782 (0.3) | 1685 (0.3) | 1096 (0.1) | <0.001 |
| Hematologic malignancy | 14 (<0.1) | 8 (<0.1) | 6 (<0.1) | 0 (<0.1) | <0.001 |
| Transplantation status | 53 (<0.1) | 25 (<0.1) | 19 (<0.1) | 9 (<0.1) | <0.001 |
| HIV/AIDS | 441 (<0.1) | 176 (<0.1) | 60 (<0.1) | 205 (<0.1) | <0.001 |
| Immunodeficiency | 295 (<0.1) | 210 (<0.1) | 26 (<0.1) | 59 (<0.1) | <0.001 |
| Inflammatory bowel disease | 7906 (0.2) | 4539 (0.1) | 1275 (0.2) | 2092 (0.1) | <0.001 |
| HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Status | N | Bronchiectasis | Duration (PY) | IR per 1000 | Model 1 | Model 2 | Model 3 | |
| Overall | Never smoker | 3,462,826 | 12,884 | 25,333,8814 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 632,443 | 2639 | 4,731,442 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.03,1.13) | |
| Current smoker | 2,180,306 | 8086 | 16,133,463 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.01,1.09) | 1.06 (1.02,1.10) | |
| Pack years < 10 | 1,358,100 | 4453 | 9,963,491 | 0.446 | 0.87 (0.84,0.91) | 1.03 (0.98,1.06) | 1.03 (0.98,1.07) | |
| Pack years ≥ 10 | 822,206 | 3633 | 6,169,972 | 0.588 | 1.15 (1.11,1.19) | 1.11 (1.05,1.15) | 1.12 (1.06,1.16) | |
| p for trend | 0.035 | <0.001 | <0.001 | |||||
| Sex | ||||||||
| Male | Never smoker | 1,135,390 | 4029 | 8,376,524 | 0.480 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 546,321 | 2321 | 4,119,567 | 0.563 | 1.17 (1.11,1.23) | 1.07 (1.01,1.12) | 1.06 (1.01,1.12) | |
| Current smoker | 2,030,668 | 7494 | 15,077,177 | 0.497 | 1.03 (0.99,1.07) | 1.03 (0.99,1.07) | 1.038(0.99,1.08) | |
| Pack years < 10 | 1,216,241 | 3911 | 8,960,985 | 0.436 | 0.90 (0.86,0.94) | 0.98 (0.94,1.03) | 0.99 (0.94,1.03) | |
| Pack years ≥ 10 | 814,427 | 3583 | 6,116,192 | 0.585 | 1.21 (1.16,1.27) | 1.09 (1.04,1.14) | 1.10 (1.05,1.15) | |
| p for trend | <0.001 | 0.018 | 0.005 | |||||
| Female | Never smoker | 2,327,436 | 8855 | 16,957,357 | 0.522 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 86,122 | 318 | 611,874 | 0.519 | 0.99 (0.89,1.12) | 1.07 (0.96,1.20) | 1.06 (0.95,1.19) | |
| Current smoker | 149,638 | 592 | 1,056,285 | 0.560 | 1.07 (0.99,1.17) | 1.21 (1.11,1.31) | 1.20 (1.10,1.31) | |
| Pack years < 10 | 141,859 | 542 | 1,002,505 | 0.540 | 1.04 (0.95,1.13) | 1.18 (1.08,1.29) | 1.18 (1.08,1.29) | |
| Pack years ≥ 10 | 7779 | 50 | 53,780 | 0.929 | 1.79 (1.36,2.37) | 1.51 (1.14,2.00) | 1.49 (1.12,1.97) | |
| p for trend | 0.041 | <0.001 | <0.001 | |||||
| p value | <0.001 | <0.001 | <0.001 | |||||
| p for interaction | <0.001 | <0.001 | <0.001 | |||||
| Age, years | ||||||||
| <30 | Never smoker | 1,675,479 | 4484 | 12,126,911 | 0.369 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 175,548 | 505 | 1,276,722 | 0.395 | 1.06 (0.97,1.17) | 1.13 (1.02,1.24) | 1.12 (1.02,1.24) | |
| Current smoker | 803,999 | 2127 | 5,826,907 | 0.365 | 0.98 (0.93,1.03) | 1.10 (1.03,1.17) | 1.10 (1.03,1.17) | |
| Pack years < 10 | 672,670 | 1730 | 4,855,497 | 0.356 | 0.96 (0.91,1.01) | 1.09 (1.02,1.16) | 1.09 (1.01,1.16) | |
| Pack years ≥ 10 | 131,329 | 397 | 971,410 | 0.408 | 1.10 (0.99,1.22) | 1.18 (1.05,1.32) | 1.18 (1.05,1.32) | |
| p for trend | 0.904 | 0.001 | 0.001 | |||||
| ≥30 | Never smoker | 1,787,347 | 8400 | 13,206,970 | 0.636 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 456,895 | 2134 | 3,454,719 | 0.617 | 0.97 (0.92,1.01) | 1.06 (1.01,1.12) | 1.05 (1.01,1.11) | |
| Current smoker | 1,376,307 | 5959 | 10,306,556 | 0.578 | 0.91 (0.87,0.93) | 1.03 (0.98,1.07) | 1.03 (0.99,1.08) | |
| Pack years < 10 | 685,430 | 2723 | 5,107,993 | 0.533 | 0.83 (0.80,0.87) | 0.98 (0.93,1.03) | 0.98 (0.94,1.04) | |
| Pack years ≥ 10 | 690,877 | 3236 | 5,198,562 | 0.622 | 0.97 (0.93,1.01) | 1.08 (1.03,1.14) | 1.09 (1.04,1.15) | |
| p for trend | <0.001 | 0.029 | 0.001 | |||||
| p for interaction | <0.001 | 0.022 | 0.036 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | Never smoker | 371,396 | 1716 | 2,709,061 | 0.633 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 19,801 | 111 | 144,234 | 0.769 | 1.21 (1.01,1.47) | 0.98 (0.80,1.21) | 0.98 (0.80,1.20) | |
| Current smoker | 85,489 | 443 | 623,242 | 0.710 | 1.12 (1.01,1.24) | 0.85 (0.74,0.98) | 0.86 (0.75,0.99) | |
| Pack years < 10 | 64,695 | 292 | 468,357 | 0.623 | 0.98 (0.87,1.11) | 0.86 (0.74,1.01) | 0.86 (0.74,1.01) | |
| Pack years ≥ 10 | 20,794 | 151 | 154,884 | 0.974 | 1.53 (1.29,1.81) | 0.85 (0.69,1.04) | 0.85 (0.69,1.05) | |
| p for trend | 0.001 | 0.038 | 0.046 | |||||
| 18.5–22.9 | Never smoker | 1,885,080 | 7056 | 13,792,785 | 0.511 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 227,953 | 1080 | 1,698,419 | 0.635 | 1.24 (1.16,1.32) | 1.16 (1.08,1.24) | 1.15 (1.07,1.23) | |
| Current smoker | 818,872 | 3209 | 6,054,509 | 0.530 | 1.03 (0.99,1.07) | 1.04 (0.98,1.09) | 1.04 (0.99,1.10) | |
| Pack years < 10 | 552,628 | 1917 | 4,050,839 | 0.473 | 0.92 (0.87,0.97) | 1.02 (0.96,1.08) | 1.02 (0.96,1.09) | |
| Pack years ≥ 10 | 266,244 | 1292 | 2,003,669 | 0.644 | 1.25 (1.18,1.33) | 1.07 (1.01,1.15) | 1.08 (1.01,1.16) | |
| p for trend | <0.001 | 0.163 | 0.099 | |||||
| 23–24.9 | Never smoker | 557,097 | 1877 | 4,088,205 | 0.459 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 158,130 | 625 | 1,192,235 | 0.524 | 1.14 (1.04,1.24) | 1.08 (0.98,1.19) | 1.07 (0.97,1.19) | |
| Current smoker | 488,656 | 1680 | 3,633,804 | 0.462 | 1.01 (0.94,1.07) | 1.04 (0.96,1.12) | 1.04 (0.96,1.13) | |
| Pack years < 10 | 298,815 | 887 | 2,203,629 | 0.402 | 0.87 (0.80,0.94) | 0.99 (0.90,1.08) | 0.99 (0.90,1.08) | |
| Pack years ≥ 10 | 189,841 | 793 | 1,430,175 | 0.554 | 1.20 (1.11,1.31) | 1.11 (1.01,1.22) | 1.12 (1.01,1.23) | |
| p for trend | 0.064 | 0.151 | 0.110 | |||||
| ≥25 | Never smoker | 649,253 | 2235 | 4,743,830 | 0.471 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 226,559 | 823 | 1,696,552 | 0.485 | 1.02 (0.94,1.11) | 1.03 (0.94,1.12) | 1.02 (0.94,1.12) | |
| Current smoker | 787,289 | 2754 | 5,821,907 | 0.473 | 1.00 (0.94,1.06) | 1.10 (1.03,1.17) | 1.10 (1.03,1.18) | |
| Pack years < 10 | 441,962 | 1357 | 3,240,663 | 0.418 | 0.88 (0.83,0.95) | 1.05 (0.97,1.13) | 1.05 (0.97,1.13) | |
| Pack years ≥ 10 | 345,327 | 1397 | 2,581,243 | 0.541 | 1.14 (1.07,1.22) | 1.16 (1.07,1.25) | 1.17 (1.08,1.26) | |
| p for trend | 0.046 | <0.001 | <0.001 | |||||
| p for interaction | 0.064 | 0.015 | 0.023 | |||||
| HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Status | N | Bronchiectasis | Duration (PY) | IR per 1000 | Model 1 | Model 2 | Model 3 | |
| Respiratory disease | ||||||||
| No | Never smoker | 3,254,156 | 11,471 | 23,832,643 | 0.481 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 598,024 | 2380 | 4,477,618 | 0.531 | 1.10 (1.05,1.15) | 1.08 (1.03,1.14) | 1.08 (1.03,1.13) | |
| Current smoker | 2,094,692 | 7490 | 15,510,580 | 0.482 | 1.02 (0.93,1.03) | 1.06 (1.02,1.10) | 1.06 (1.02,1.10) | |
| Pack years < 10 | 1,304,433 | 4127 | 9,577,112 | 0.430 | 0.89 (0.86,0.92) | 1.03 (0.99,1.07) | 1.03 (0.99,1.07) | |
| Pack years ≥ 10 | 790,259 | 3363 | 5,933,468 | 0.566 | 1.17 (1.13,1.22) | 1.11 (1.06,1.17) | 1.11 (1.06,1.17) | |
| p for trend | 0.001 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 208,670 | 1413 | 1,501,238 | 0.941 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 34,419 | 259 | 253,823 | 1.020 | 1.08 (0.95,1.23) | 1.01 (0.87,1.17) | 1.01 (0.87,1.17) | |
| Current smoker | 85,614 | 596 | 622,883 | 0.956 | 1.01 (0.92,1.12) | 1.02 (0.91,1.15) | 1.03 (0.93,1.14) | |
| Pack years < 10 | 53,667 | 326 | 386,379 | 0.843 | 0.89 (0.79,1.01) | 0.98 (0.85,1.12) | 0.98 (0.85,1.12) | |
| Pack years ≥ 10 | 31,947 | 270 | 236,504 | 1.141 | 1.21 (1.06,1.38) | 1.09 (0.93,1.28) | 1.09 (0.93,1.27) | |
| p for trend | 0.197 | 0.435 | 0.448 | |||||
| p for interaction | 0.957 | 0.877 | 0.891 | |||||
| Connective tissue disease | ||||||||
| No | Never smoker | 3,435,615 | 12,688 | 25,135,861 | 0.504 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 627,565 | 2597 | 4,695,583 | 0.553 | 1.09 (1.04,1.14) | 1.07 (1.02,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,167,725 | 7995 | 16,041,918 | 0.498 | 0.98 (0.95,1.01) | 1.05 (1.02,1.09) | 1.05 (1.02,1.09) | |
| Pack years < 10 | 1,350,665 | 4411 | 9,909,679 | 0.445 | 0.88 (0.85,0.91) | 1.02 (0.98,1.06) | 1.02 (0.98,1.07) | |
| Pack years ≥ 10 | 817,060 | 3584 | 6,132,238 | 0.584 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.029 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 27,211 | 196 | 198,020 | 0.989 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 4878 | 42 | 35,858 | 1.171 | 1.18 (0.85,1.65) | 1.35 (0.92,1.98) | 1.34 (0.92,1.96) | |
| Current smoker | 12,581 | 91 | 91,545 | 0.994 | 1.01 (0.78,1.29) | 1.19 (0.86,1.64) | 1.19 (0.86,1.63) | |
| Pack years < 10 | 7435 | 42 | 53,811 | 0.780 | 0.78 (0.56,1.10) | 0.97 (0.66,1.42) | 0.97 (0.66,1.42) | |
| Pack years ≥ 10 | 5146 | 49 | 37,733 | 1.298 | 1.31 (0.96,1.80) | 1.57 (1.06,2.34) | 1.58 (1.06,2.35) | |
| p for trend | 0.483 | 0.114 | 0.111 | |||||
| p for interaction | 0.690 | 0.495 | 0.508 | |||||
| Inflammatory bowel disease | ||||||||
| No | Never smoker | 3,458,287 | 12,862 | 25,300,827 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 631,168 | 2625 | 4,721,944 | 0.555 | 1.09 (1.04,1.13) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,178,214 | 8067 | 16,118,195 | 0.500 | 0.98 (0.95,1.01) | 1.06 (1.01,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,356,726 | 4442 | 9,953,533 | 0.446 | 0.87 (0.84,0.90) | 1.02 (0.98,1.06) | 1.02 (0.98,1.06) | |
| Pack years ≥ 10 | 821,488 | 3625 | 6,164,661 | 0.588 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.042 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 4539 | 22 | 33,054 | 0.665 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 1275 | 14 | 9497 | 1.474 | 2.21 (1.13,4.33) | 1.80 (0.86,3.78) | 1.80 (0.85,3.78) | |
| Current smoker | 2092 | 19 | 15,268 | 1.244 | 1.86 (1.01,3.45) | 1.59 (0.79,3.19) | 1.55 (0.77,3.11) | |
| Pack years < 10 | 1374 | 11 | 9957 | 1.104 | 1.65 (0.80,3.42) | 1.46 (0.66,3.21) | 1.42 (0.64,3.14) | |
| Pack years ≥ 10 | 718 | 8 | 5310 | 1.506 | 2.26 (1.01,5.08) | 1.84 (0.74,4.55) | 1.83 (0.74,4.54) | |
| p for trend | 0.029 | 0.195 | 0.208 | |||||
| p for interaction | 0.110 | 0.138 | 0.149 | |||||
| Solid cancer or Hematologic malignancy | ||||||||
| No | Never smoker | 3,453,036 | 12,830 | 25,263,418 | 0.507 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 630,754 | 2631 | 4,718,957 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,179,210 | 8081 | 16,125,496 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.02,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,357,444 | 4448 | 9,958,767 | 0.446 | 0.87 (0.85,0.91) | 1.02 (0.98,1.06) | 1.02 (0.98,1.06) | |
| Pack years ≥ 10 | 821,766 | 3633 | 6,166,728 | 0.589 | 1.15 (1.11,1.20) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.027 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 9790 | 54 | 70,462 | 0.766 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 1689 | 8 | 12,484 | 0.640 | 0.82 (0.39,1.72) | 1.26 (0.49,3.18) | 1.26 (0.49,3.19) | |
| Current smoker | 1096 | 5 | 7967 | 0.627 | 0.81 (0.32,2.02) | 1.44 (0.48,4.28) | 1.44 (0.48,4.28) | |
| Pack years < 10 | 656 | 5 | 4724 | 1.058 | 1.36 (0.54,3.41) | 2.19 (0.77,6.23) | 2.18 (0.76,6.20) | |
| Pack years ≥ 10 | 440 | 0 | 3243 | 0 | NA | NA | NA | |
| p for trend | 0.362 | 0.807 | 0.814 | |||||
| p for interaction | 0.637 | 0.657 | 0.651 | |||||
| Immunocompromised disease * | ||||||||
| No | Never smoker | 3,462,415 | 12,880 | 25,330,891 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference)z |
| Ex-smoker | 632,338 | 2638 | 4,730,674 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,180,033 | 8085 | 16,131,533 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.01,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,357,914 | 4453 | 9,962,181 | 0.446 | 0.87 (0.84,0.90) | 1.02 (0.98,1.06) | 1.02 (0.98,1.07) | |
| Pack years ≥ 10 | 822,119 | 3632 | 6,169,352 | 0.588 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.034 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 411 | 4 | 2989 | 1.337 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 105 | 1 | 767 | 1.303 | 0.98 (0.11,8.78) | 2.24 (0.15,32.63) | 1.50 (0.07,28.54) | |
| Current smoker | 273 | 1 | 1929 | 0.518 | 0.38 (0.04,3.41) | 1.31 (0.08,20.82) | 2.18 (0.09,51.96) | |
| Pack years < 10 | 186 | 0 | 1309 | 0 | NA | NA | NA | |
| Pack years ≥ 10 | 87 | 1 | 620 | 1.612 | 1.19 (0.13,10.72) | 6.93 (0.24,199.33) | 9.62 (0.22,416.78) | |
| p for trend | 0.560 | 0.511 | 0.351 | |||||
| p for interaction | 0.992 | 0.987 | 0.863 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Han, K.; Kim, B.; Kang, H.K.; Kim, J.S.; Kim, E.-G.; Choi, H.; Lee, H. Association between Smoking Status and Incident Non-Cystic Fibrosis Bronchiectasis in Young Adults: A Nationwide Population-Based Study. J. Pers. Med. 2022, 12, 691. https://doi.org/10.3390/jpm12050691
Yang B, Han K, Kim B, Kang HK, Kim JS, Kim E-G, Choi H, Lee H. Association between Smoking Status and Incident Non-Cystic Fibrosis Bronchiectasis in Young Adults: A Nationwide Population-Based Study. Journal of Personalized Medicine. 2022; 12(5):691. https://doi.org/10.3390/jpm12050691
Chicago/Turabian StyleYang, Bumhee, Kyungdo Han, Bongseong Kim, Hyung Koo Kang, Jung Soo Kim, Eung-Gook Kim, Hayoung Choi, and Hyun Lee. 2022. "Association between Smoking Status and Incident Non-Cystic Fibrosis Bronchiectasis in Young Adults: A Nationwide Population-Based Study" Journal of Personalized Medicine 12, no. 5: 691. https://doi.org/10.3390/jpm12050691
APA StyleYang, B., Han, K., Kim, B., Kang, H. K., Kim, J. S., Kim, E.-G., Choi, H., & Lee, H. (2022). Association between Smoking Status and Incident Non-Cystic Fibrosis Bronchiectasis in Young Adults: A Nationwide Population-Based Study. Journal of Personalized Medicine, 12(5), 691. https://doi.org/10.3390/jpm12050691








