Dynamics of Soluble Factors and Double-Negative T Cells Associated with Response to Renal Denervation in Resistant Hypertension Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Renal Denervation
2.3. Follow-Up
2.4. Soluble Cytokine, Chemokine and Growth Factors Profiling
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Response to Renal Denervation
3.2. Low Levels of RANTES Are Associated with Response to Renal Denervation
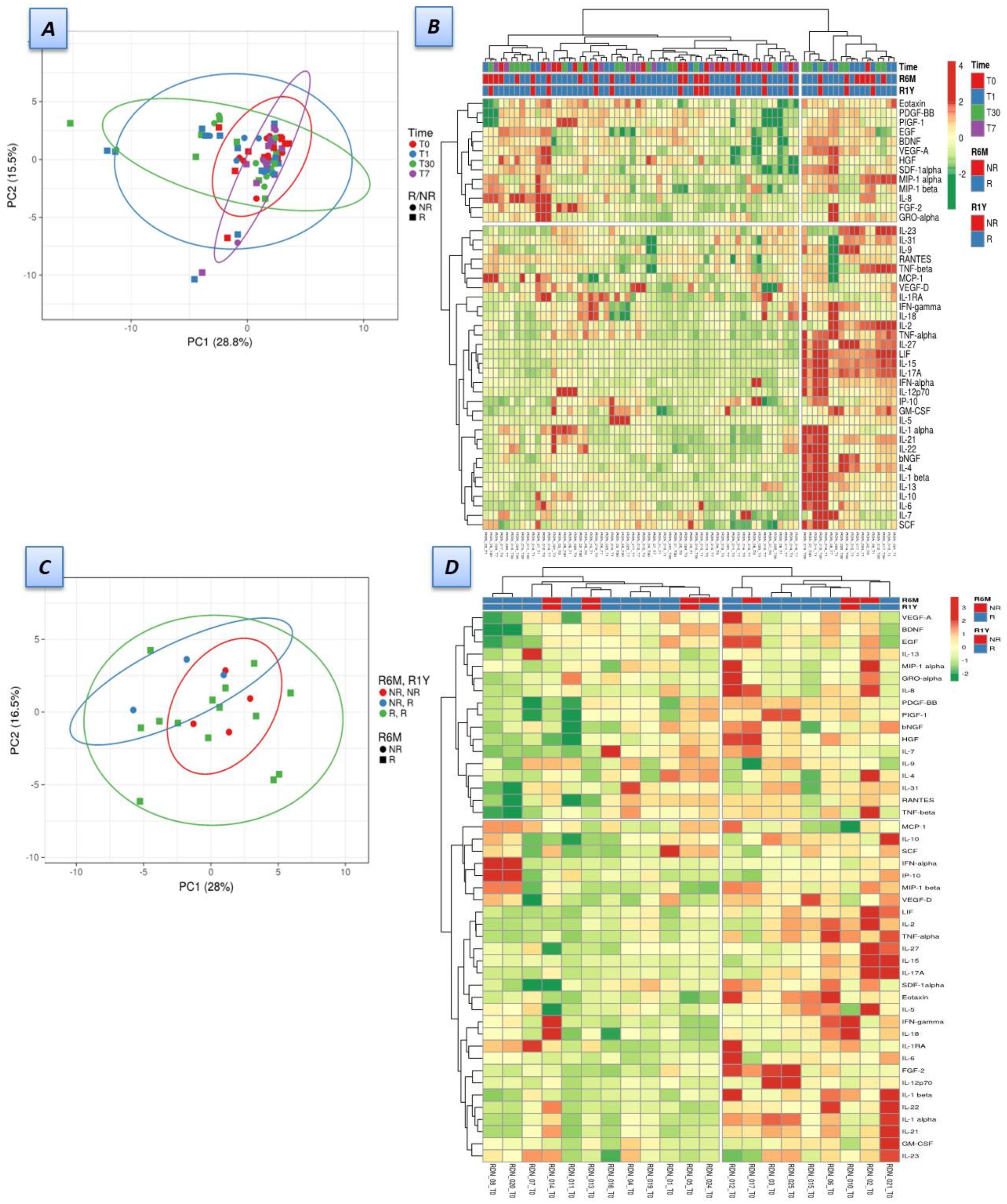
3.3. Dynamics of Soluble Factors in Renal Denervation Patients
3.4. Soluble Factors Were Correlated with Double-Negative T-Cells in Responders
4. Discussion
5. Limitations
6. Conclusions
7. Highlights
- The role of the immune system in the pathophysiology of hypertensive patients treated with renal denervation is not clear.
- RANTES is a potential predictor of response to renal denervation.
- IL-15, IL-27, IL-17A, and LIF increased 24 h after renal denervation in responders and decreased one week later.
- Pro-inflammatory cytokines negatively correlated with double-negative T cells (shown to be reduced in responders).
- Renal denervation effectively lowered blood pressure in the majority of patients.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RDN | renal denervation |
| BP | blood pressure |
| HT | hypertension |
| SNS | sympathetic nervous system |
| ABPM | ambulatory blood pressure monitoring |
References
- Deepak, L.B.; David, E.K.; William, W.O.’N.; Ralph, D.’A.; John, M.F.; Barry, T.K.; Martin, B.L.; Minglei, L.; Laura, M.; Manuela, N.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar]
- Böhm, M.; Kario, K.; Kandzari, D.E.; Mahfoud, F.; Weber, M.A.; Schmieder, R.E.; Tsioufis, K.; Pocock, S.; Konstantinidis, D.; Choi, J.W.; et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): A multicentre, randomised, sham-controlled trial. Lancet 2020, 395, 1444–1451. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Böhm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Lobo, M.D.; RADIANCE-HTN Investigators. Six-Month Results of Treatment-Blinded Medication Titration for Hypertension Control Following Randomization to Endovascular Ultrasound Renal Denervation or a Sham Procedure in the RADIANCE-HTN SOLO Trial. Circulation 2019, 139, 2542–2553. [Google Scholar] [CrossRef]
- Ram, C.V.S. Status of Renal Denervation Therapy for Hypertension. Circulation 2019, 139, 601–603. [Google Scholar] [CrossRef]
- Bautista, L.E.; Vera, L.M.; Arenas, I.A.; Gamarra, G. Independent association between infammatory markers (Creactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J. Hum. Hypertens. 2005, 19, 149–154. [Google Scholar] [CrossRef]
- Singh, M.; Chapleau, M.; Harwani, S.; Abboud, F.M. The immune system and hypertension. Immunol. Res. 2014, 59, 243–253. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Strawn, W.B. Role of the renin-angiotensin-aldosterone system and pro-inflammatory mediators in cardiovascular disease. Am. J. Cardiol. 2006, 98, 121–128. [Google Scholar] [CrossRef]
- Didion, S. Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Dikalova, A.E. Contribution of mitochondrial oxidative stress to hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 73–80. [Google Scholar] [CrossRef]
- Dörr, O.; Liebetrau, C.; Möllmann, H.; Gaed, L.; Troidl, C.; Rixe, J. Soluble fms-like tyrosine kinase-1 and endothelial adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1) as predictive markers for blood pressure reduction after renal sympathetic denervation. Hypertension 2014, 63, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Dörr, O.; Liebetrau, C.; Möllmann, H.; Gaede, L.; Troidl, C.; Haidner, V. Brain-derived neurotrophic factor as a marker for immediate assessment of the success of renal sympathetic denervation. J. Am. Coll. Cardiol. 2015, 65, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Eikelis, N.; Hering, D.; Marusic, P.; Walton, A.S.; Lambert, E.A.; Sata, Y. Soluble vascular endothelial growth factor receptor-1 is reduced in patients with resistant hypertension after renal denervation. J. Hum. Hypertens. 2017, 31, 248–252. [Google Scholar] [CrossRef]
- Delgado Silva, J.; Almeida, J.S.; Rodrigues-Santos, P.; Santos Rosa, M.; Gonçalves, L. Activated double-negative T cells (CD3 + CD4-CD8-HLA-DR +) define response to renal denervation for resistant hypertension. Clin. Immunol. 2020, 218, 108521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, G.J.; Han, L.N.; Bai, Y.Y.; He, M.; Liu, H.B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. 2017, 14, 135–150. [Google Scholar]
- Neumann, J.T.; Ewen, S.; Mortensen, K.; Nef, H.; Zeller, T.; Ojeda, F. Effects of renal denervation on heart failure biomarkers and blood pressure in patients with resistant hypertension. Biomark. Med. 2016, 10, 841–851. [Google Scholar] [CrossRef]
- Gear, A.R.; Camerini, D. Platelet chemokines and chemokine receptors: Linking hemostasis, inflammation, and host defense. Microcirculation 2003, 10, 335–350. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Petersen, F.; Brandt, E. Platelet-derived chemokines in vascular biology. Thromb. Haemost. 2007, 97, 704–713. [Google Scholar] [CrossRef]
- Weber, C.; Schober, A.; Zernecke, A. Chemokines: Key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1997–2008. [Google Scholar] [CrossRef]
- Nomura, S.; Uehata, S.; Saito, S.; Osumi, K.; Ozeki, Y.; Kimura, Y. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb. Haemost. 2003, 89, 506–512. [Google Scholar]
- Cavusoglu, E.; Eng, C.; Chopra, V.; Clark, L.T.; Pinsky, D.J.; Marmur, J.D. Low Plasma RANTES Levels Are an Independent Predictor of Cardiac Mortality in Patients Referred for Coronary Angiography. Arter. Thromb. Vasc. Biol. 2007, 27, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Kim, H.Y.; Do, B.S.; Kim, H.S. Angiotensin II inhibits chemokine CCL5 expression in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens. Res. 2011, 34, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Delgado Silva, J.; Costa, M.; Gersh, B.J.; Gonçalves, L. Renal denervation in the era of HTN-3. Comprehensive review and glimpse into the future. J. Am. Soc. Hypertens. 2016, 10, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Rudemiller, N.; Patel, M.B.; Zhang, J.-D.; Jeffs, A.D.; Karlovich, N.S.; Griffiths, R.; Kan, M.J.; Buckley, A.F.; Gunn, M.D.; Crowley, S.D. C-C Motif Chemokine 5 Attenuates Angiotensin II–Dependent Kidney Injury by Limiting Renal Macrophage Infiltration. Am. J. Pathol. 2016, 186, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Silva, J.; Fernandes, R.; Pita, I.R.; Pereira, F.C.; Jaguszewski, M.; Gutiérrez-Chico, J.L.; Ribeiro-Rodrigues, T.M.; Girao, H.; Ioannou, A.; Goncalves, L. Intravascular imaging, histopathological analysis, and catecholamine quantification following catheter-based renal denervation in a swine model: The impact of prebifurcation energy delivery. Hypertens. Res. 2018, 41, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kim, J.S.; Kim, I.Y.; Kim, H.S.; Kim, J.Y.; Rhee, H. Catheter-based renal sympathetic denervation induces acute renal inflammation through activation of caspase-1 and NLRP3 inflammasome. Anatol. J. Cardiol 2019, 21, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ueba, T.; Nomura, S.; Inami, N.; Yokoi, T.; Inoue, T. Elevated RANTES Level Is Associated with Metabolic Syndrome and Correlated with Activated Platelets Associated Markers in Healthy Younger Men. Clin. Appl. Thromb. 2014, 20, 813–818. [Google Scholar] [CrossRef]
- Mendall, M.A.; Patel, P.; Asante, M.; Ballam, L.; Morris, J.; Strachan, D.P. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart 1997, 78, 273–277. [Google Scholar] [CrossRef]
- Lang, D.; Nahler, A.; Lambert, T.; Grund, M.; Kammler, J.; Kellermair, J. Anti-Inflammatory Effects and Prediction of Blood Pressure Response by Baseline Inflammatory State in Catheter-Based Renal Denervation. J. Clin. Hypertens. 2016, 18, 1173–1179. [Google Scholar] [CrossRef]
- Dörr, O.; Liebetrau, C.; Möllmann, H.; Mahfoud, F.; Ewen, S.; Gaede, L.; Troidl, C.; Hoffmann, J.; Busch, N.; Laux, G.; et al. Beneficial effects of renal sympathetic denervation on cardiovascular inflammation and remodeling in essential hypertension. Clin. Res. Cardiol. 2015, 104, 175–184. [Google Scholar] [CrossRef]
- Hilderman, M.; Qureshi, A.R.; Abtahi, F.; Witt, N.; Jägren, C.; Olbers, J.; Delle, M.; Lindecrantz, K.; Bruchfeld, A. The cholinergic anti-inflammatory pathway in resistant hypertension treated with renal denervation. Mol. Med. 2019, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaibe, M.; Ohishi, M.; Ito, N.; Yuan, M.; Takagi, T.; Terai, M.; Tatara, Y.; Komai, N.; Rakugi, H.; Ogihara, T. Serum Interleukin-15 Concentration in Patients with Essential Hypertension. Am. J. Hypertens. 2005, 18, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Saita, E.; Suzuki-Sugihara, N.; Miyata, K.; Ikemura, N.; Ohmori, R.; Ikegami, Y.; Kishimoto, Y.; Kondo, K.; Momiyama, Y. Plasma interleukin-27 levels in patients with coronary artery disease. Medicine 2017, 96, e8260. [Google Scholar] [CrossRef] [PubMed]
- von Vietinghoff, S.; Ley, K. Interleukin 17 in vascular inflammation. Cytokine Growth Factor Rev. 2010, 21, 463–469. [Google Scholar] [CrossRef]
- Zouein, F.A.; Kurdi, M.; Booz, G.W. LIF and the heart: Just another brick in the wall? Eur. Cytokine Netw. 2013, 24, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, Z.; Li, Y.; Wang, J.; Hu, J.; Yin, Y. Renal denervation restrains the inflammatory response in myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 2020, 115, 15. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Gan, X.B.; Fan, Z.D.; Zhang, F.; Zhou, Y.B.; Gao, X.Y.; De, W.; Zhu, G.Q. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol. 2011, 203, 289–297. [Google Scholar] [CrossRef]
- Wei, S.G.; Zhang, Z.H.; Yu, Y.; Felder, R.B. Central SDF-1/CXCL12 expression and its cardiovascular and sympathetic effects: The role of angiotensin II, TNF-α, and MAP kinase signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1643–H1654. [Google Scholar] [CrossRef][Green Version]
- Grassi, G. Renal denervation in cardiometabolic disease: Concepts, achievements and perspectives. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 77–83. [Google Scholar] [CrossRef]
- Krum, H.; Sobotka, P.; Mahfoud, F. Device-based antihypertensive therapy: Therapeutic modulation of the autonomic nervous system. Circulation 2011, 123, 209–215. [Google Scholar] [CrossRef]
- Verloop, W.L.; Spiering, W.; Vink, E.E. Denervation of the renal arteries in metabolic syndrome: The DREAMS-Study. Hypertension 2015, 65, 751–757. [Google Scholar] [CrossRef] [PubMed]



| R (n = 14) | NR (n = 7) | p Value | R1Y (n = 17) | NR1Y (n = 4) | p Value | |
|---|---|---|---|---|---|---|
| Age in Y (mean ± SD) | 61 ± 10 | 55 ± 13.5 | ns | 59.8 ± 11.8 | 55.5 ± 9.5 | ns |
| Diagnosis of HT in Y (mean ± SD) | 16.3 ± 9.3 | 17 ± 5.9 | ns | 16.6 ± 8.6 | 16.2 ± 7.5 | ns |
| Female sex (%) | 21.4 | 57.1 | ns | 35.3 | 25 | ns |
| Dyslipidemia (%) | 85.7 | 100 | ns | 88.2 | 100 | ns |
| Type 2 diabetes (%) | 42.9 | 57.1 | ns | 47.1 | 50 | ns |
| Active smoking (%) | 14.3 | 42.9 | ns | 11.8 | 75 | 0.008 |
| Sleep apnea (%) | 57.1 | 57.1 | ns | 58.8 | 50 | ns |
| Number of HT drugs (n ± SD) | 5.1 ± 1.4 | 5.4 ± 0.5 | ns | 5.2 ± 1.3 | 5.2 ± 0.5 | ns |
| On spironolactone (%) | 57.1 | 42.9 | ns | 52.9 | 50 | ns |
| Isolated HT (%) | 14.3 | 14.3 | ns | 17.6 | 0 | ns |
| BMI (Kg/m2) | 29.4 ± 3.8 | 30.2 ± 4.3 | ns | 30.1 ± 3.8 | 27.8 ± 4.3 | ns |
| Biochemical profile at baseline | ||||||
| HBA1c (%) | 5.9 ± 0.75 | 7 ± 2.5 | 0.02 | 5.9 ± 0.7 | 7.7 ± 3.4 | <0.001 |
| NT pro-BNP (pg/mL) | 165 ± 265 | 1037 ± 2478 | 0.005 | 541 ± 1594 | 94.7 ± 62.2 | ns |
| LpA (mg/dL) | 42.3 ± 46 | 30 ± 45.2 | ns | 37.6 ± 42.7 | 40.6 ± 60.9 | ns |
| Fibrinogen (mg/dL) | 278.1 ± 59.1 | 271.2 ± 76.5 | ns | 282 ± 65 | 243.7 ± 44.8 | ns |
| Creatinin (mg/dL) | 0.97 ± 0.2 | 0.76 ± 0.2 | ns | 0.9 ± 0.2 | 0.76 ± 0.2 | ns |
| Cystatin C (mg/L) | 0.97 ± 0.2 | 0.85 ± 0.2 | ns | 0.95 ± 0.2 | 0.8 ± 0.1 | ns |
| LDL col (mg/dL) | 98 ± 23 | 118 ± 26 | ns | 102 ± 25 | 116 ± 28 | ns |
| PCR (mg/dL) | 0.83 ± 0.97 | 1.2 ± 2.2 | ns | 1.1 ± 1.6 | 0.4 ± 0.3 | ns |
| Baseline | ||||||
| ABPM systolic BP (mmHg) | 154 ± 12.3 | 153.4 ± 12.9 | ns | 154.8 ± 12.5 | 149.7 ± 11.6 | ns |
| ABPM diastolic BP (mmHg) | 87.1 ± 14.3 | 92.7 ± 15.6 | ns | 89 ± 15.3 | 88.7 ± 13.3 | ns |
| HR (bpm) | 70.6 ± 9.1 | 73.3 ± 13.9 | ns | 72.3 ± 9.8 | 68 ± 15 | ns |
| E/E’ ratio | 10.6 ± 4.8 | 15 ± 3.6 | ns | 11.4 ± 5.2 | 13.8 ± 3.5 | ns |
| Six Months | ||||||
| ABPM systolic BP (mmHg) | 133 ± 11 | 161 ± 9.7 | <0.001 | 138.6 ± 16.6 | 158.2 ± 7.4 | 0.004 |
| ABPM diastolic BP (mmHg) | 77.1 ± 11.2 | 95.1 ± 15.8 | 0.007 | 81.3 ± 15 | 90.5 ± 13.7 | ns |
| HR (bpm) | 68.8 ± 7.3 | 75.8 ± 10.8 | 0.09 | 71.3 ± 8.7 | 70.5 ± 11.7 | ns |
| E/E’ ratio | 12.8 ± 6.3 | 12.3 ± 2.8 | ns | 12.6 ± 5.2 | 12.5 ± 3.4 | ns |
| One year | ||||||
| ABPM systolic BP (mmHg) | 135.9 ± 16.2 | 149.3 ± 20.2 | ns | 135.6 ± 15.8 | 160.5 ± 15 | 0.03 |
| ABPM diastolic BP (mmHg) | 77.1 ± 11.2 | 88.4 ± 14.3 | ns | 77.5 ± 10.5 | 94.5 ± 15.8 | ns |
| HR (bpm) | 67.8 ± 8.1 | 74.4 ± 12 | ns | 69.4 ± 8.3 | 72.5 ± 16.3 | ns |
| Total ablations (n) | 23.8.6 ± 8.3 | 22.4 ± 7.3 | ns | 23.9 ± 8.5 | 20.7 ± 4 | ns |
| Drop in ABPM systolic BP at 6 months (mmHg) | 21.1 ± 13.3 | −7.6 ± 10.6 | <0.001 | 16.2 ± 16.6 | −8.5 ± 11.9 | 0.013 |
| Drop in ABPM systolic BP at 1 year (mmHg) | 18.1 ± 11.5 | 4 ± 21.5 | ns | 19.2 ± 12.2 | −10.7 ± 6.7 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Silva, J.; Rodrigues-Santos, P.; Almeida, J.-S.; Santos-Rosa, M.; Gonçalves, L. Dynamics of Soluble Factors and Double-Negative T Cells Associated with Response to Renal Denervation in Resistant Hypertension Patients. J. Pers. Med. 2022, 12, 343. https://doi.org/10.3390/jpm12030343
Delgado-Silva J, Rodrigues-Santos P, Almeida J-S, Santos-Rosa M, Gonçalves L. Dynamics of Soluble Factors and Double-Negative T Cells Associated with Response to Renal Denervation in Resistant Hypertension Patients. Journal of Personalized Medicine. 2022; 12(3):343. https://doi.org/10.3390/jpm12030343
Chicago/Turabian StyleDelgado-Silva, Joana, Paulo Rodrigues-Santos, Jani-Sofia Almeida, Manuel Santos-Rosa, and Lino Gonçalves. 2022. "Dynamics of Soluble Factors and Double-Negative T Cells Associated with Response to Renal Denervation in Resistant Hypertension Patients" Journal of Personalized Medicine 12, no. 3: 343. https://doi.org/10.3390/jpm12030343
APA StyleDelgado-Silva, J., Rodrigues-Santos, P., Almeida, J.-S., Santos-Rosa, M., & Gonçalves, L. (2022). Dynamics of Soluble Factors and Double-Negative T Cells Associated with Response to Renal Denervation in Resistant Hypertension Patients. Journal of Personalized Medicine, 12(3), 343. https://doi.org/10.3390/jpm12030343







