Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Recruitment
2.2. Human Urinary Exosome Isolation
2.3. Assessing Human Urinary Particle Size and Number
2.4. Immunoblotting
2.5. Analyzing Human Urinary Exosome Appearance
3. Results
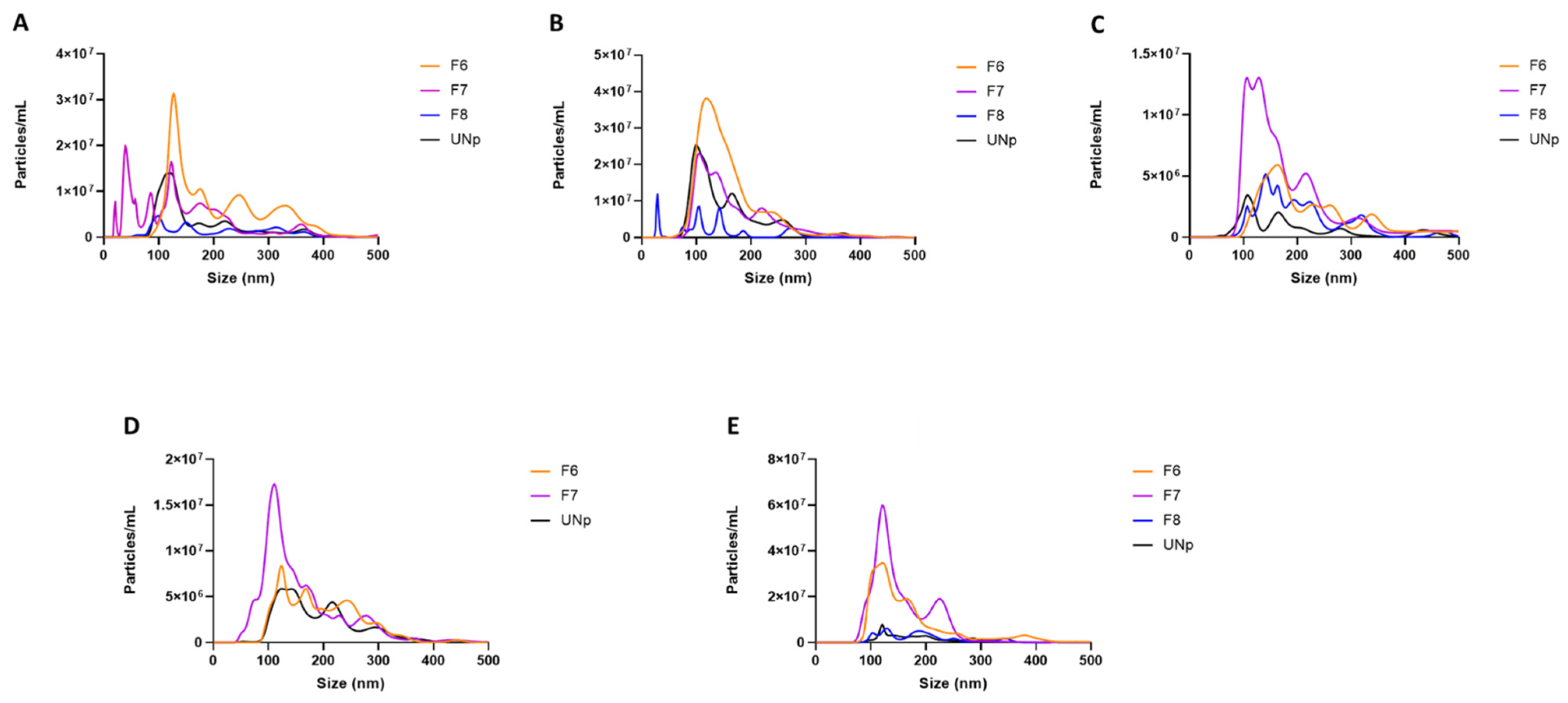
3.1. Particle Size Comparisons of Human Urinary Exosomal Preparations Isolated via Ultracentrifugation That Remain Unpurified versus SEC Column-Purified
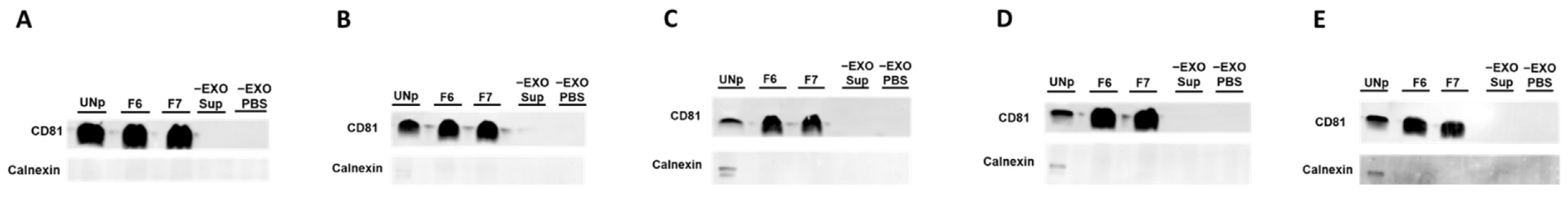
3.2. Human Urinary Exosomal Preparations Isolated Using Ultracentrifugation Followed-by SEC Column-Purification Are Devoid of Contamination
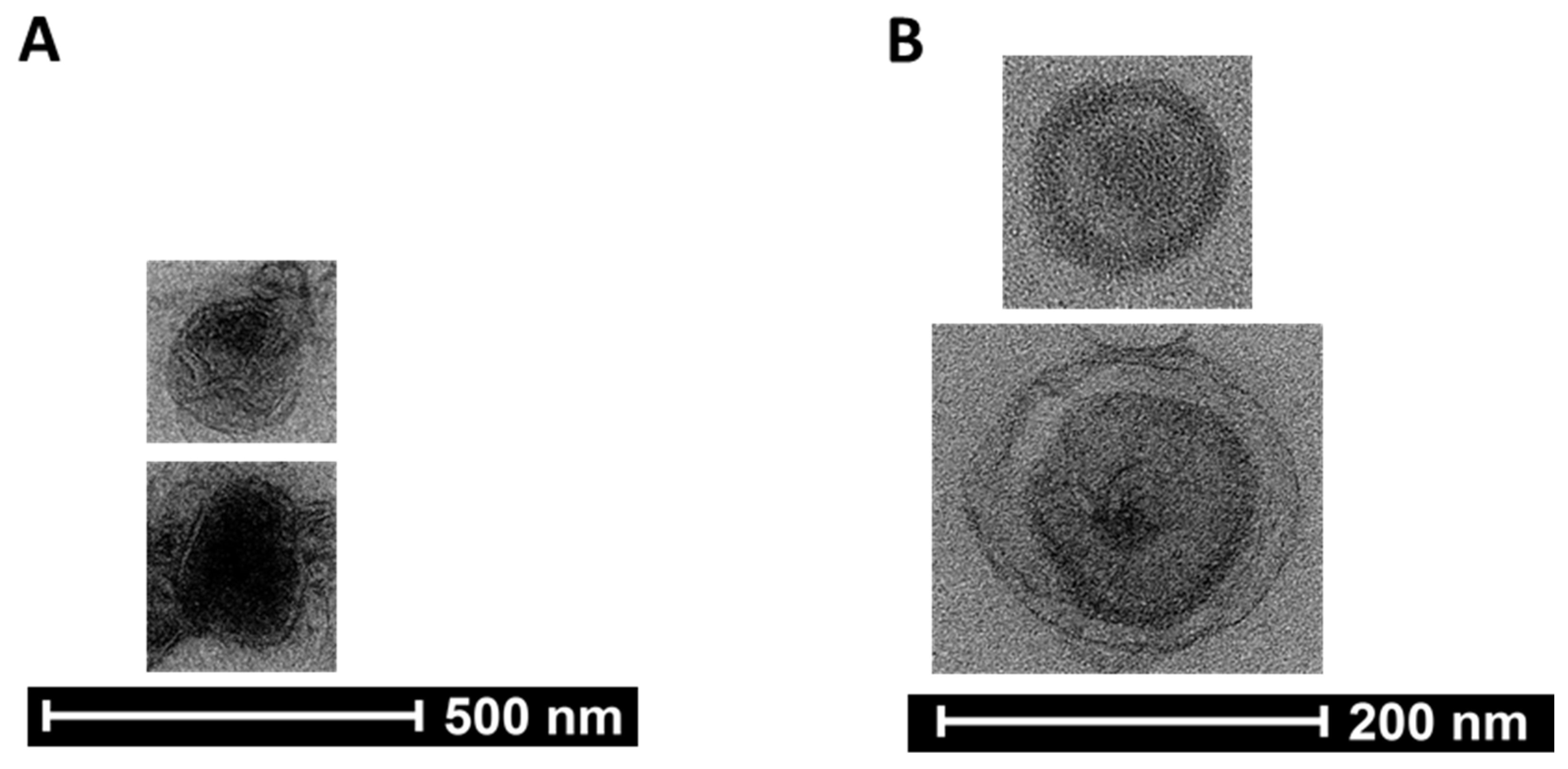
3.3. Assessing the Presence of Exosomes in Pooled SEC Fractionated Preparations by Transmission Electron Microscopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure. In StatPearls; EDN: Treasure Island, FL, USA, 2022. [Google Scholar]
- Benjamin, O.; Lappin, S.L. End-Stage Renal Disease. In StatPearls; EDN: Treasure Island, FL, USA, 2022. [Google Scholar]
- Davis, A.E.; Mehrotra, S.; McElroy, L.M.; Friedewald, J.J.; Skaro, A.I.; Lapin, B.; Kang, R.; Holl, J.L.; Abecassis, M.M.; Ladner, D.P. The extent and predictors of waiting time geographic disparity in kidney transplantation in the United States. Transplantation 2014, 97, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kianda, M.N.; Wissing, K.M.; Broeders, N.E.; Lemy, A.; Ghisdal, L.; Hoang, A.D.; Mikhalski, D.; Donckier, V.; Vereerstraeten, P.; Abramowicz, D. Ineligibility for renal transplantation: Prevalence, causes and survival in a consecutive cohort of 445 patients. Clin. Transplant. 2011, 25, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Masaki, N.; Iwadoh, K.; Kondo, A.; Koyama, I.; Nakajima, I.; Fuchinoue, S. Causes of Ineligibility for Recipients in Living Kidney Transplantation. Transplant. Proc. 2018, 50, 978–981. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Jameson, M.D.; Wiegmann, T.B. Principles, uses, and complications of hemodialysis. Med. Clin. N. Am. 1990, 74, 945–960. [Google Scholar] [CrossRef]
- Vadakedath, S.; Kandi, V. Dialysis: A Review of the Mechanisms Underlying Complications in the Management of Chronic Renal Failure. Cureus 2017, 9, e1603. [Google Scholar] [CrossRef] [Green Version]
- Kontodimopoulos, N.; Niakas, D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy 2008, 86, 85–96. [Google Scholar] [CrossRef]
- Md Yusop, N.B.; Yoke Mun, C.; Shariff, Z.M.; Beng Huat, C. Factors associated with quality of life among hemodialysis patients in Malaysia. PLoS ONE 2013, 8, e84152. [Google Scholar] [CrossRef] [Green Version]
- Schell, J.O.; Da Silva-Gane, M.; Germain, M.J. Recent insights into life expectancy with and without dialysis. Curr Opin Nephrol Hypertens 2013, 22, 185–192. [Google Scholar] [CrossRef]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Giacoman, S.; Madero, M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol. 2015, 4, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, N.; Craig, J.; Bauman, A.; Manera, K.; Saglimbene, V.; Tong, A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: A systematic review. BMJ Open 2019, 9, e031625. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H. Diet and Chronic Kidney Disease. Adv. Nutr. 2019, 10 (Suppl. 4), S367–S379. [Google Scholar] [CrossRef]
- Michishita, R.; Matsuda, T.; Kawakami, S.; Kiyonaga, A.; Tanaka, H.; Morito, N.; Higaki, Y. The accumulation of healthy lifestyle behaviors prevents the incidence of chronic kidney disease (CKD) in middle-aged and older males. Environ. Health Prev. Med. 2016, 21, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.L.; Khosroheidari, M.; Kanchi Ravi, R.; DiStefano, J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Khurana, R.; Ranches, G.; Schafferer, S.; Lukasser, M.; Rudnicki, M.; Mayer, G.; Huttenhofer, A. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA 2017, 23, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Magayr, T.A.; Song, X.; Streets, A.J.; Vergoz, L.; Chang, L.; Valluru, M.K.; Yap, H.L.; Lannoy, M.; Haghighi, A.; Simms, R.J.; et al. Global microRNA profiling in human urinary exosomes reveals novel disease biomarkers and cellular pathways for autosomal dominant polycystic kidney disease. Kidney Int. 2020, 98, 420–435. [Google Scholar] [CrossRef]
- Ong, S.G.; Lee, W.H.; Huang, M.; Dey, D.; Kodo, K.; Sanchez-Freire, V.; Gold, J.D.; Wu, J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: Role of exosomal microRNA transfer. Circulation 2014, 130 (Suppl. 1), S60–S69. [Google Scholar] [CrossRef] [Green Version]
- Hergenreider, E.; Heydt, S.; Treguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Stamatikos, A.; Knight, E.; Vojtech, L.; Bi, L.; Wacker, B.K.; Tang, C.; Dichek, D.A. Exosome-Mediated Transfer of Anti-miR-33a-5p from Transduced Endothelial Cells Enhances Macrophage and Vascular Smooth Muscle Cell Cholesterol Efflux. Hum. Gene Ther. 2020, 31, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell Vesicles 2014, 3, 24858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation-efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [Green Version]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Correia-Costa, L.; Azevedo, A.; Caldas Afonso, A. Childhood Obesity and Impact on the Kidney. Nephron 2019, 143, 8–11. [Google Scholar] [CrossRef]
- Ding, W.; Cheung, W.W.; Mak, R.H. Impact of obesity on kidney function and blood pressure in children. World J. Nephrol. 2015, 4, 223–229. [Google Scholar] [CrossRef]
- Yim, H.E.; Yoo, K.H. Obesity and chronic kidney disease: Prevalence, mechanism, and management. Clin. Exp. Pediatr. 2021, 64, 511–518. [Google Scholar] [CrossRef]
- Wright, M. Nanoparticle tracking analysis for the multiparameter characterization and counting of nanoparticle suspensions. Methods Mol. Biol. 2012, 906, 511–524. [Google Scholar] [PubMed]
- Huang, K.; Jo, H.; Echesabal-Chen, J.; Stamatikos, A. Combined LXR and RXR Agonist Therapy Increases ABCA1 Protein Expression and Enhances ApoAI-Mediated Cholesterol Efflux in Cultured Endothelial Cells. Metabolites 2021, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Olivares, D.; Forner, M.J.; Ortega, A.; Solaz, E.; Martinez, F.; Chaves, F.J.; Redon, J.; Cortes, R. Urinary exosome miR-146a is a potential marker of albuminuria in essential hypertension. J. Transl. Med. 2018, 16, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Kume, H.; Matsuzaki, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Uemura, M.; Miyagawa, Y.; Tomonaga, T.; Nonomura, N. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci. Rep. 2017, 7, 42961. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2021, 9, 811971. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Duong, P.; Chung, A.; Bouchareychas, L.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC) improves the isolation efficiency of extracellular vesicles. PLoS ONE 2019, 14, e0215324. [Google Scholar] [CrossRef]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned-Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes. Methods Mol. Biol. 2018, 1740, 69–83. [Google Scholar]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef]
- Prendergast, E.N.; de Souza Fonseca, M.A.; Dezem, F.S.; Lester, J.; Karlan, B.Y.; Noushmehr, H.; Lin, X.; Lawrenson, K. Optimizing exosomal RNA isolation for RNA-Seq analyses of archival sera specimens. PLoS ONE 2018, 13, e0196913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamdar, S.; Nitiyanandan, R.; Rege, K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng. Transl. Med. 2017, 2, 70–80. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls; EDN: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stevens, L.A.; Levey, A.S. Measured GFR as a confirmatory test for estimated GFR. J. Am. Soc. Nephrol. 2009, 20, 2305–2313. [Google Scholar] [CrossRef] [Green Version]
| Age | Sex | Race/Ethnicity | Weight | Height | BMI |
|---|---|---|---|---|---|
| 13 y 0 m | F | Black/African American | 85.5 | 1.627 | 32.30 |
| 11 y 6 m | M | Hispanic | 60.1 | 1.478 | 27.51 |
| 8 y 3 m | M | Black/African American | 94 | 1.334 | 52.82 |
| 7 y 6 m | M | Black/African American | 40.4 | 1.306 | 23.69 |
| 15 y 1 m | F | Black/African American | 106 | 1.645 | 39.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Garimella, S.; Clay-Gilmour, A.; Vojtech, L.; Armstrong, B.; Bessonny, M.; Stamatikos, A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J. Pers. Med. 2022, 12, 340. https://doi.org/10.3390/jpm12030340
Huang K, Garimella S, Clay-Gilmour A, Vojtech L, Armstrong B, Bessonny M, Stamatikos A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. Journal of Personalized Medicine. 2022; 12(3):340. https://doi.org/10.3390/jpm12030340
Chicago/Turabian StyleHuang, Kun, Sudha Garimella, Alyssa Clay-Gilmour, Lucia Vojtech, Bridget Armstrong, Madison Bessonny, and Alexis Stamatikos. 2022. "Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification" Journal of Personalized Medicine 12, no. 3: 340. https://doi.org/10.3390/jpm12030340
APA StyleHuang, K., Garimella, S., Clay-Gilmour, A., Vojtech, L., Armstrong, B., Bessonny, M., & Stamatikos, A. (2022). Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. Journal of Personalized Medicine, 12(3), 340. https://doi.org/10.3390/jpm12030340






